Catalytic Wet Oxidation of Pharmaceutical Sludge by Molecular Sieve Loaded with Cu/Ce
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
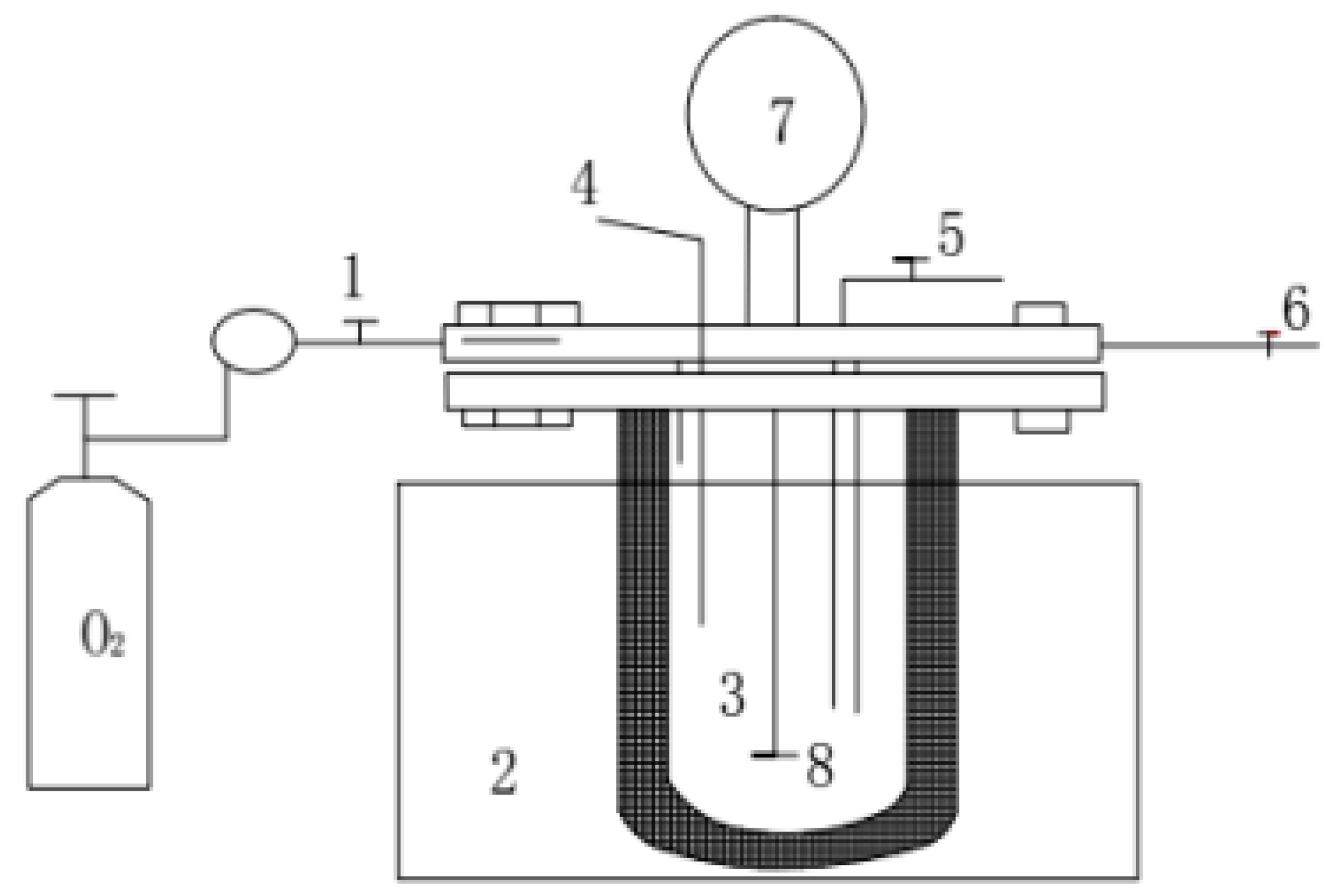
2.2. WO Reaction System
2.3. Synthesis of Catalyst
2.4. Analysis
3. Results and Discussion
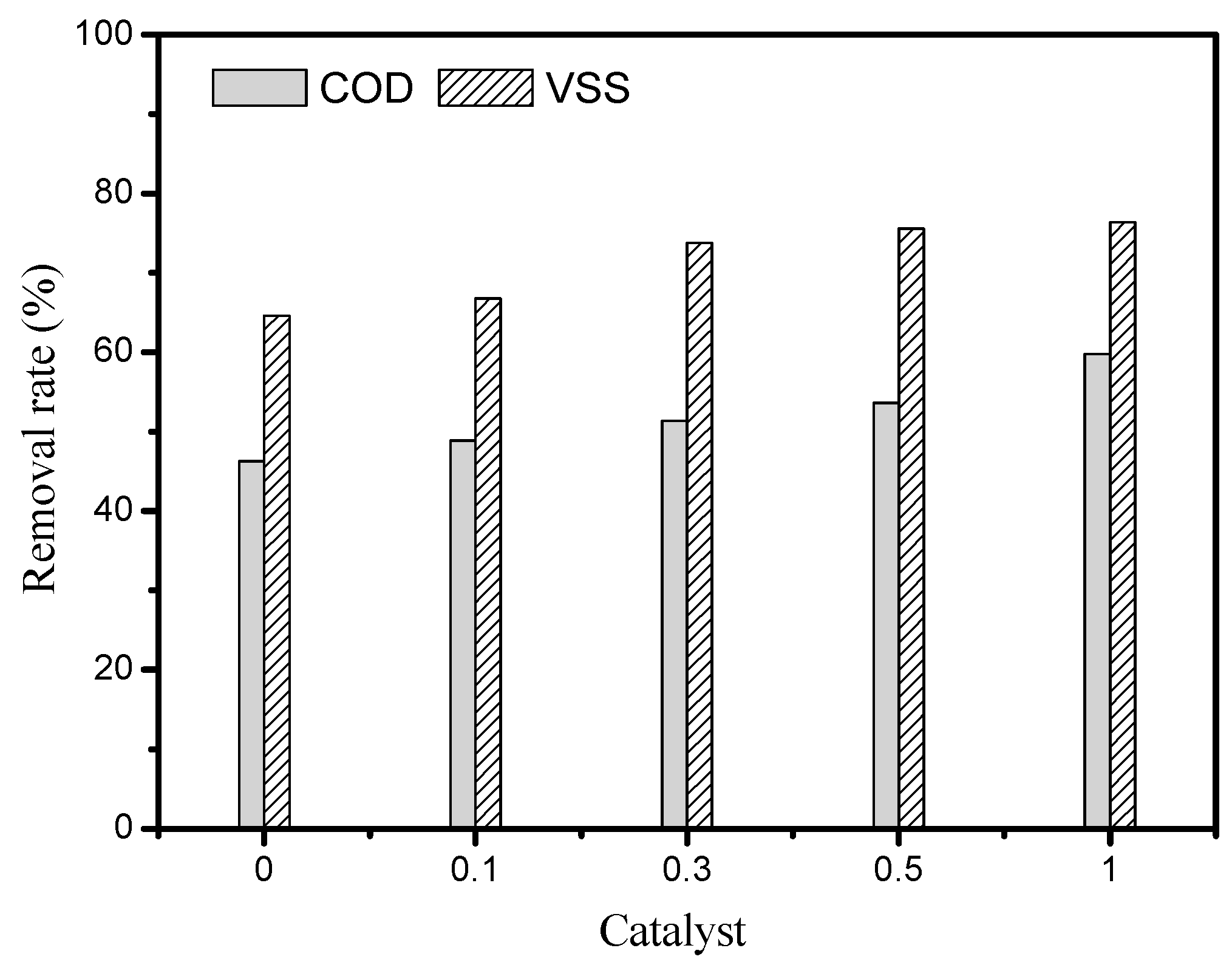
3.1. Effect of Catalyst Amount on the Sludge Degradation
3.2. Effect of Temperature on the Sludge Degradation
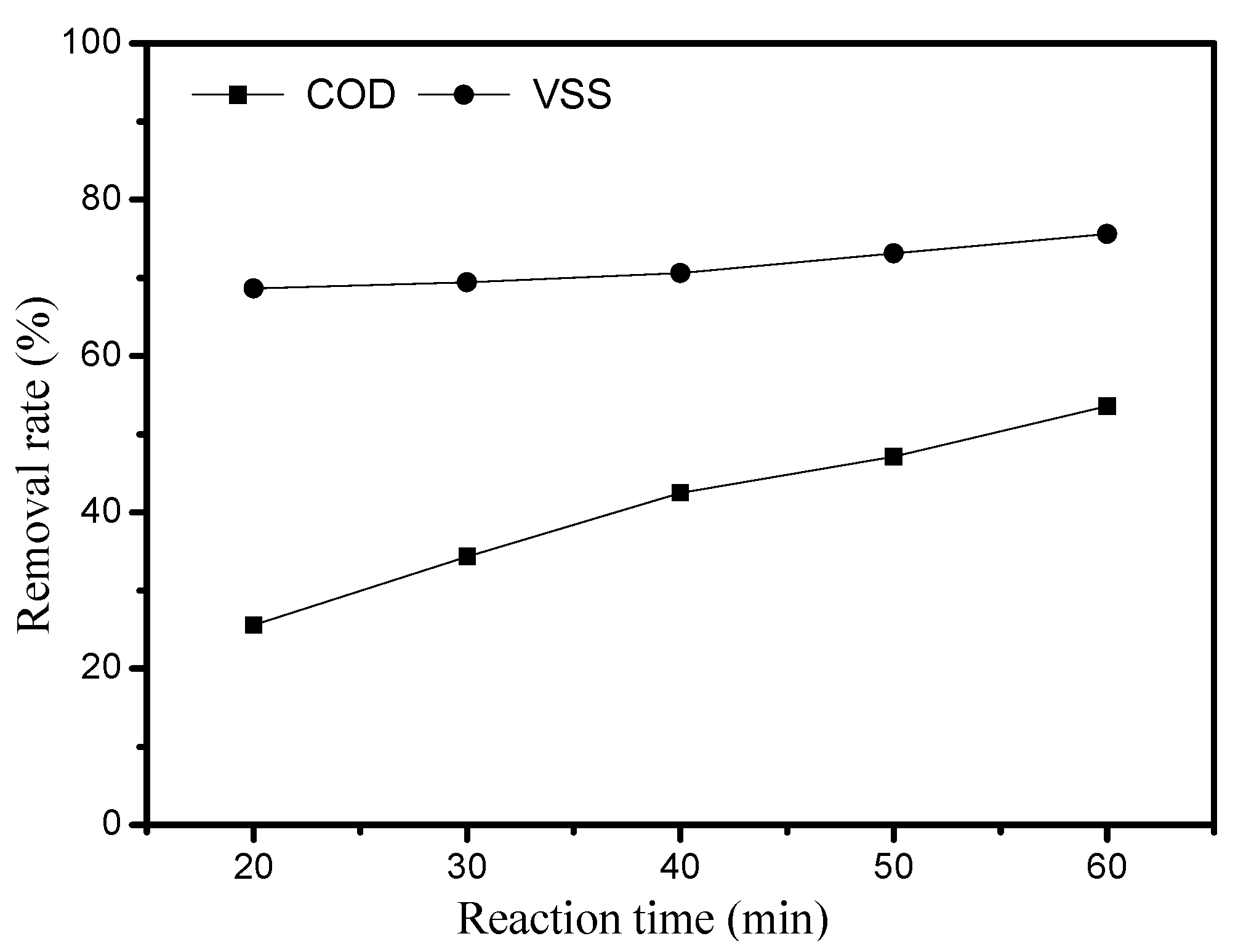
3.3. Effect of Reaction Time on the Sludge Degradation
3.4. Effect of Initial Oxygen Pressure on the Sludge Degradation
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pike, P.; Wilson, D.I.; Baroutian, S.; Andrews, J.; Gapes, D.J. A kinetic model of municipal sludge degradation during non-catalytic wet oxidation. Water Res. 2015, 87, 225–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robert, R.; Barbati, S.; Ricq, N.; Ambrosio, M. Intermediates in wet oxidation of cellulose: Identification of hydroxyl radical and characterization of hydrogen peroxide. Water Res. 2002, 36, 4821–4829. [Google Scholar] [CrossRef]
- Saeid, B.; Daniel, J.G.; Ajit, K.S.; Mohammed, M.F.; Brent, R.Y. Formation and degradation of valuable intermediate products during wet oxidation of municipal sludge. Bioresour. Technol. 2016, 205, 280–285. [Google Scholar]
- Strong, P.J.; McDonald, B.; Gapes, D.J. Combined thermochemical and fermentative destruction of municipal biosolids: A comparison between thermal hydrolysis and wet oxidative pre-treatment. Bioresour. Technol. 2011, 102, 5520–5526. [Google Scholar] [CrossRef] [PubMed]
- Reddy, B.M.; Saikia, P.; Bharali, P. Highly dispersed CexZr1−xO2nano-oxides over alumina, silica and titania supports for catalytic applications. Catal. Surv. Asia 2008, 12, 214–228. [Google Scholar] [CrossRef]
- Moretti, E.; Lenarda, M.; Storaro, L.; Talon, A.; Montanari, T.; Busca, G.; Rodríguez-Castellón, E.; Jiménez-López, A.; Turco, M.; Bagnasco, G.; et al. One-step synthesis of a structurally organized mesoporous CuO-CeO2/Al2O3 system for the preferential CO oxidation. Appl. Catal. A 2008, 335, 46–55. [Google Scholar] [CrossRef]
- Hu, X.; Lam, F.; Cheung, L.; Chan, K.; Hao, X.; Lu, G. Copper/MCM-41 as catalyst for photochemically enhanced oxidation of phenol by hydrogen peroxide. Catal. Today 2001, 68, 129–133. [Google Scholar] [CrossRef]
- Parvulescu, V.; Su, B.L. Iron, cobalt or nickel substituted MCM-41 molecular sieves for oxidation of hydrocarbons. Catal. Today 2001, 69, 315–322. [Google Scholar] [CrossRef]
- Zhan, W.Q.; Guo, Y.L.; Wang, Y.Q.; Liu, X.H.; Guo, Y.; Wang, Y.S.; Zhang, Z.G.; Lu, G.Z. Synthesis of lanthanum-doped MCM-48 molecular sieves and its catalytic performance for the oxidation of styrene. J. Phys. Chem. B 2007, 111, 12103–12112. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Z.; Fan, Z.Y.; Shi, J.W.; Liu, Z.Y.; Zhou, J.W.; Shangguan, W.F. Catalytic oxidation of low concentration formaldehyde with the assist of ozone over supported cobalt-manganese composite oxides. J. Mol. Catal. 2014, 1, 60–66. [Google Scholar]
- Chou, B.; Tsai, J.L.; Cheng, S. Cu-substituted molecular sieves as liquid phase oxidation catalysts. Microp. Mesop. Mater. 2001, 48, 309–317. [Google Scholar] [CrossRef]
- Taran, O.P.; Zagoruiko, A.N.; Ayusheev, A.B.; Yashnik, S.A.; Prihod’Ko, R.V. Cu and Fe-containing ZSM-5 zeolites as catalysts for wet peroxide oxidation of organic contaminants: Reaction kinetics. Res. Chem. Intermed. 2015, 41, 9521–9537. [Google Scholar] [CrossRef]
- TOPAS. General Profile and Structure Analysis Software for Powder Diffraction Data, V4.2; Bruker AXS GmbH: Karlsruhe, Germany, 2009. [Google Scholar]
- Cheary, R.W.; Coelho, A. A fundamental parameters approach to X-ray line-profile fitting. J. Appl. Crystallogr. 1992, 25, 109–121. [Google Scholar] [CrossRef]
- Liu, W.M.; Hu, Y.Q.; Tu, S.T. Ruthenium supported on active carbon-ceramic sphere as catalysts for catalytic wet air oxidation (CWAO) of resin effluent. J. Hazard. Mater. 2010, 179, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.Y.; Meng, X.; Chen, G.X.; Zhao, P.Q. Catalytic wet air oxidation of high concentration organic pollutants by upflow packed-bed reactor using a Ru-Ce catalyst derived from Ru3(CO)12 precursor. RSC Adv. 2016, 6, 22633–22638. [Google Scholar] [CrossRef]
- Ricq, N.; Barbati, S.; Ambrosio, M. Optimization of the degradation of sewage sludge by wet air oxidation. Study of the reaction mechanism on a cellulose model compound. Analusis 2001, 29, 872–877. [Google Scholar] [CrossRef]

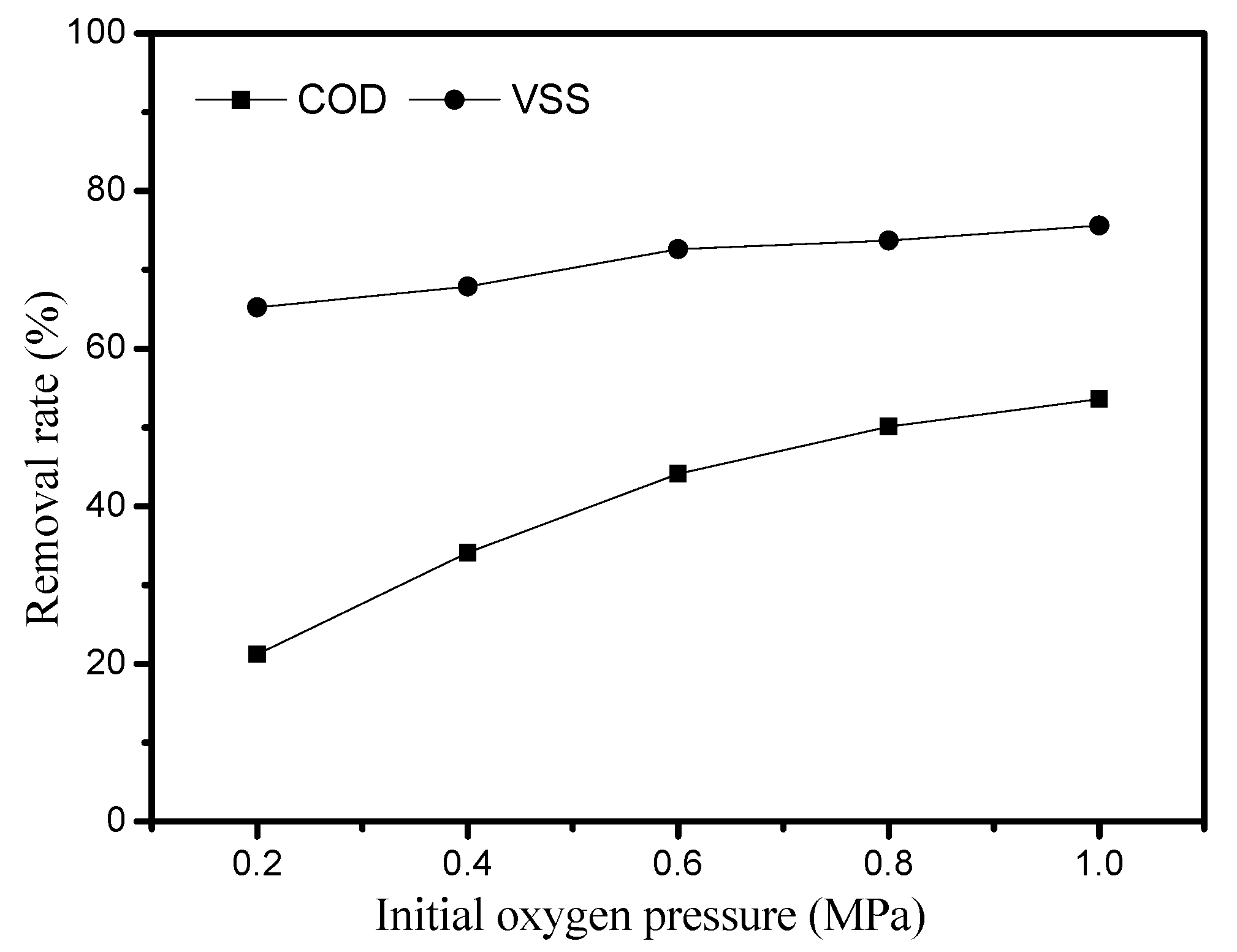
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, X.; Liu, J.; Zhao, J. Catalytic Wet Oxidation of Pharmaceutical Sludge by Molecular Sieve Loaded with Cu/Ce. Catalysts 2018, 8, 67. https://doi.org/10.3390/catal8020067
Zeng X, Liu J, Zhao J. Catalytic Wet Oxidation of Pharmaceutical Sludge by Molecular Sieve Loaded with Cu/Ce. Catalysts. 2018; 8(2):67. https://doi.org/10.3390/catal8020067
Chicago/Turabian StyleZeng, Xu, Jun Liu, and Jianfu Zhao. 2018. "Catalytic Wet Oxidation of Pharmaceutical Sludge by Molecular Sieve Loaded with Cu/Ce" Catalysts 8, no. 2: 67. https://doi.org/10.3390/catal8020067



