Combining Carbon Fibers with Ni/γ–Al2O3 Used for Syngas Production: Part A: Preparation and Evaluation of Complex Carrier Catalysts
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characteristic of Catalysts
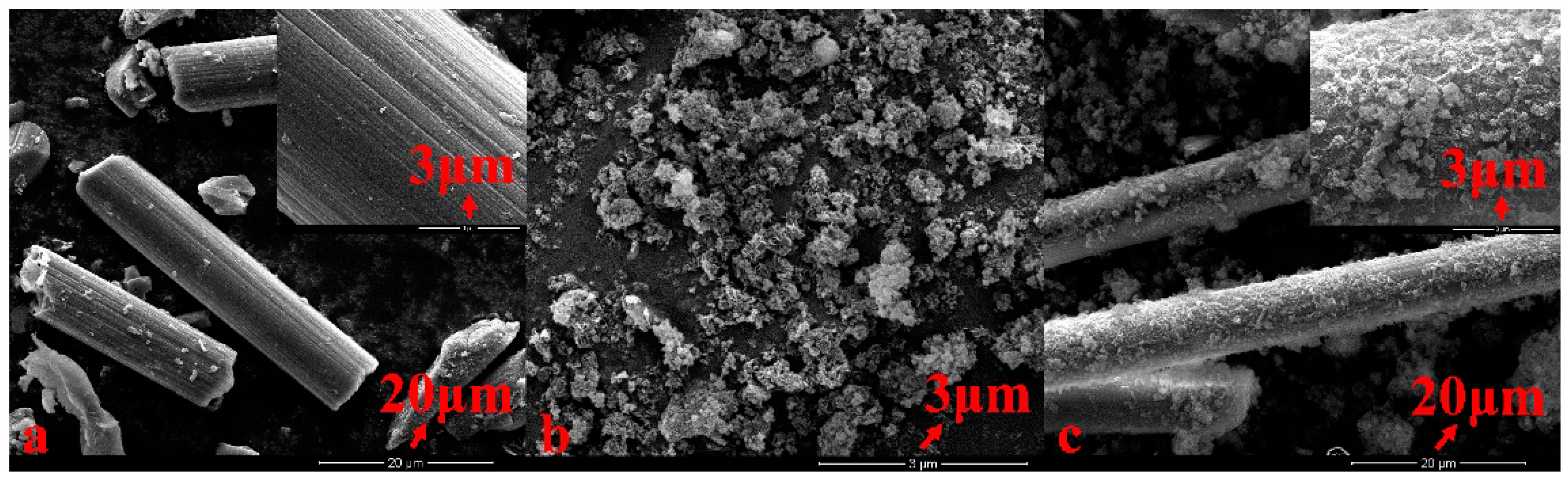
2.1.1. Surface Characterization
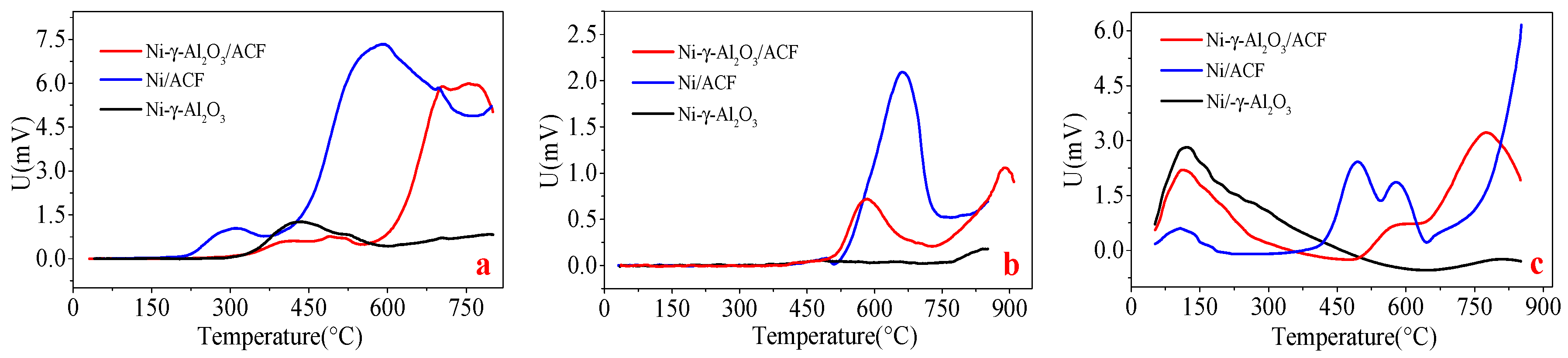
2.1.2. Chemisorption Characterization
2.1.3. X-Ray Diffraction
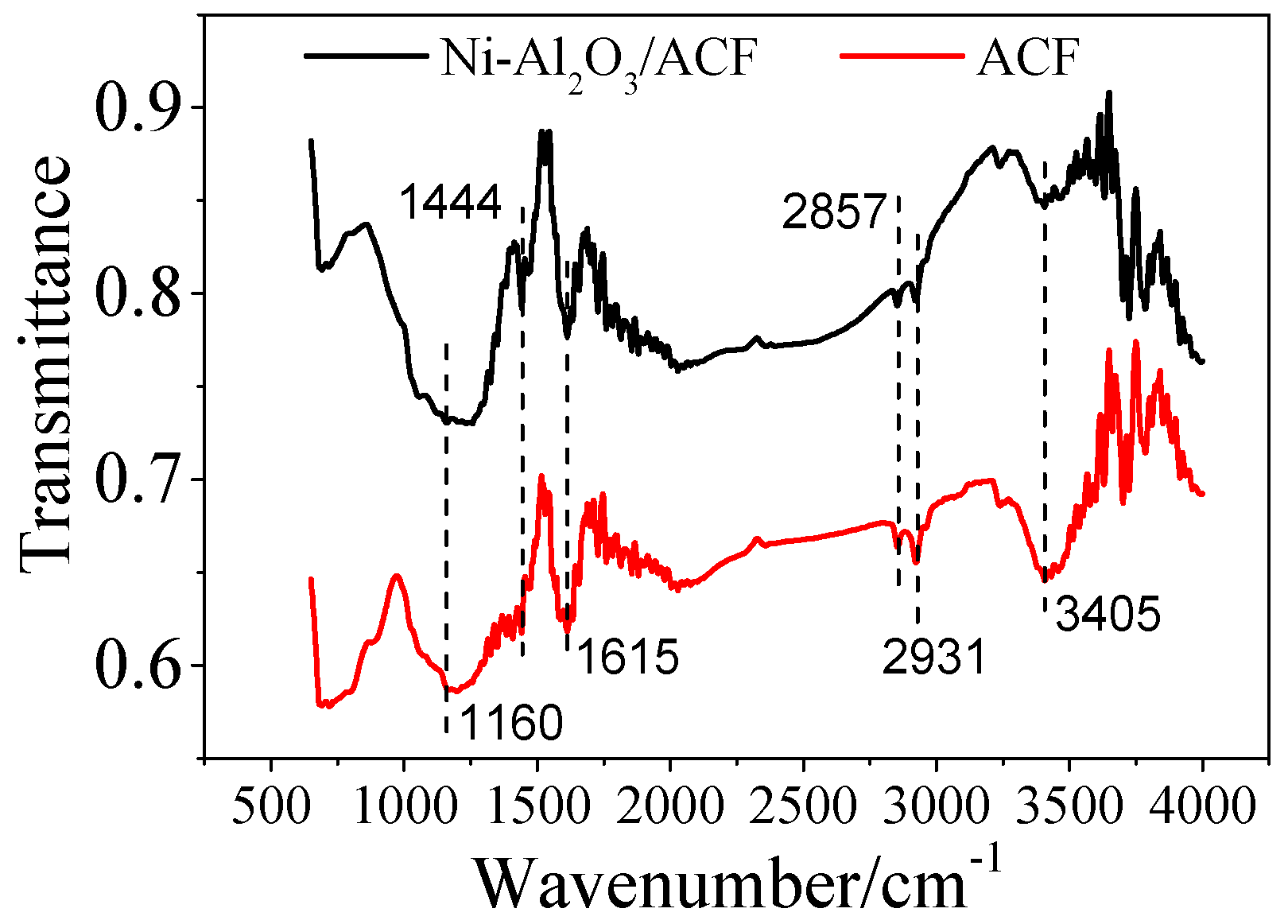
2.1.4. Fourier Transform Infrared
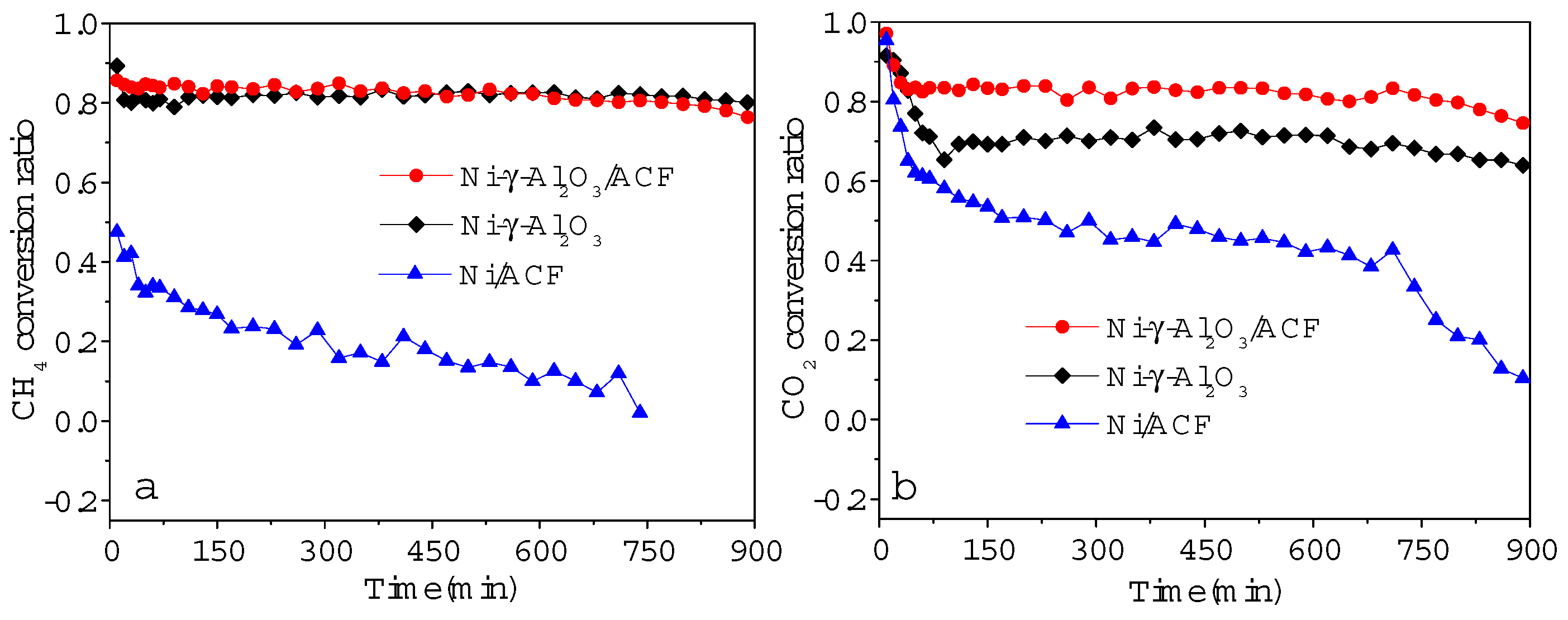
2.2. Evaluation of Catalytic Performance
2.2.1. Catalyst Stability Test
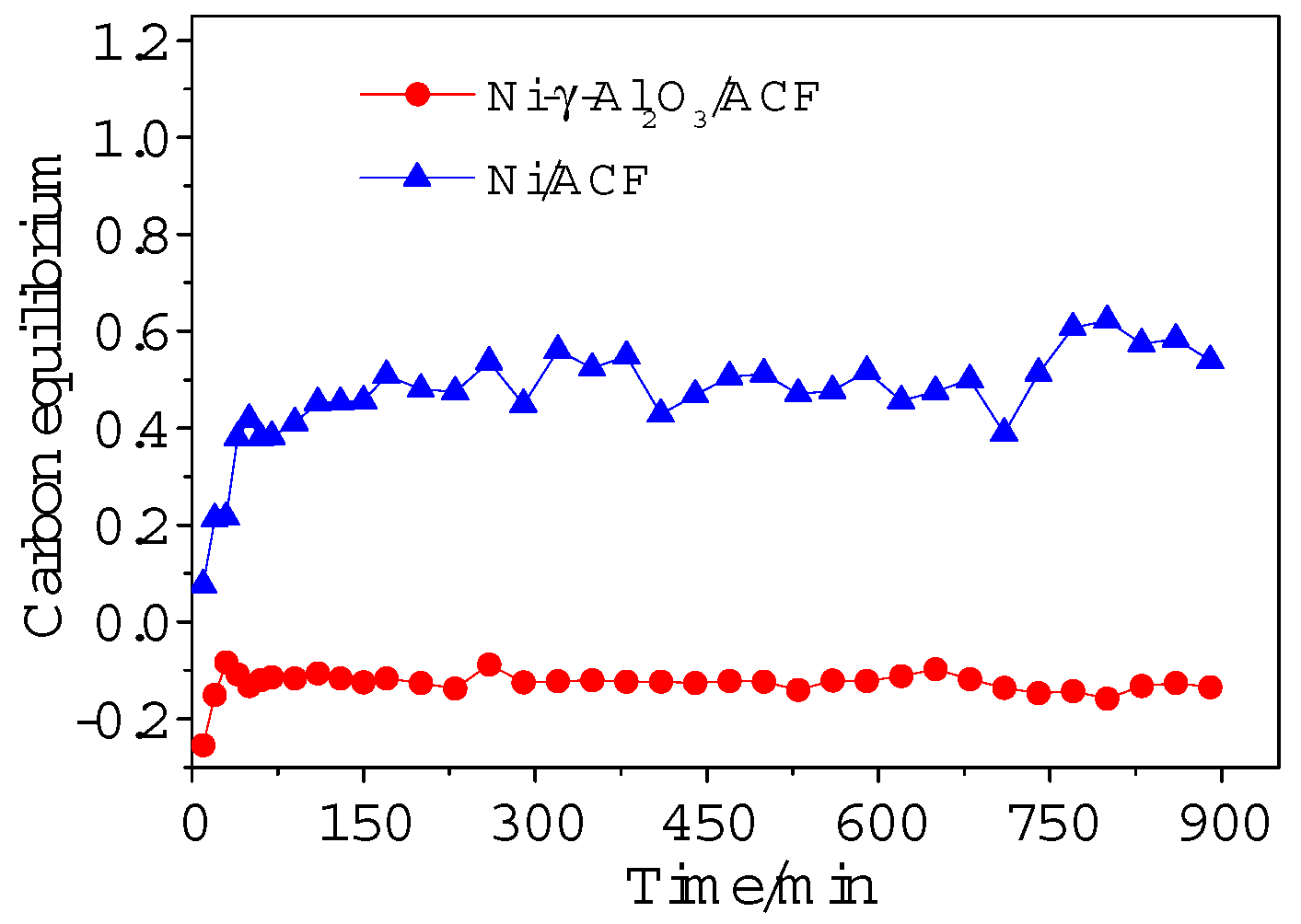
2.2.2. Carbon Equilibrium in Gas Phase
2.2.3. Catalytic Performance at Different Temperatures
2.3. Mechanism Analysis for Reforming
3. Materials and Methods
3.1. Preparation of Catalyst
3.2. Catalysts Evaluation
3.3. Catalyst Characterization and Product Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Li, D.; Li, R.; Lu, M.; Lin, X.; Zhan, Y.; Jiang, L. Carbon dioxide reforming of methane over Ru catalysts supported on Mg-Al oxides: A highly dispersed and stable Ru/Mg(Al)O catalyst. Appl. Catal. B-Environ. 2017, 200, 566–577. [Google Scholar] [CrossRef]
- Khavarian, M.; Chai, S.; Mohamed, A.R. The effects of process parameters on carbon dioxide reforming of methane over Co-Mo-MgO/MWCNTs nanocomposite catalysts. Fuel 2015, 158, 129–138. [Google Scholar] [CrossRef]
- Fan, C.; Zhu, Y.A.; Yang, M.L.; Sui, Z.J.; Zhou, X.G.; Chen, D. Density functional theory-assisted microkinetic analysis of methane dry reforming on Ni catalyst. Ind. Eng. Chem. Res. 2015, 54, 5901–5913. [Google Scholar] [CrossRef]
- Daza, C.E.; Moreno, S.; Molina, R. Co-precipitated Ni-Mg-Al catalysts containing Ce for CO2 reforming of methane. Int. J. Hydrog. Energy 2011, 36, 3886–3894. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, C.; Zhang, Y.; Xu, Y.; Shang, S.; Yin, Y. Ni-Co catalyst derived from layered double hydroxides for dry reforming of methane. Int. J. Hydrog. Energy 2015, 40, 16115–16126. [Google Scholar] [CrossRef]
- Rogers, J.L.; Mangarella, M.C.; D’Amico, A.D.; Gallagher, J.R.; Dutzer, M.R.; Stavitski, E.; Miller, J.T.; Sievers, C. Differences in the Nature of Active Sites for Methane Dry Reforming and Methane Steam Reforming over Nickel Aluminate Catalysts. ACS Catal. 2016, 6, 5873–5886. [Google Scholar] [CrossRef]
- Lertwittayanon, K.; Youravong, W.; Lau, W.J. Enhanced catalytic performance of Ni/α-Al2O3 catalyst modified with CaZrO3 nanoparticles in steam-methane reforming. Int. J. Hydrog. Energy 2017, 42, 28254–28265. [Google Scholar] [CrossRef]
- Yao, L.; Galvez, M.E.; Hu, C.; Costa, P.D. Mo-promoted Ni/Al2O3 catalyst for dry reforming of methane. Int. J. Hydrog. Energy 2017, 42, 23500–23507. [Google Scholar] [CrossRef]
- Sepehri, S.; Rezaei, M. Ce promoting effect on the activity and coke formation of Ni catalysts supported on mesoporous nanocrystalline γ-Al2O3 in autothermal reforming of methane. Int. J. Hydrog. Energy 2017, 42, 11130–11138. [Google Scholar] [CrossRef]
- Wang, S.; Lu, G.Q.; Millar, G.J. Carbon Dioxide Reforming of Methane to Produce Synthesis Gas over Metal-Supported Catalysts: State of the Art. Energy Fuels 1996, 10, 896–904. [Google Scholar] [CrossRef]
- Meshkani, F.; Rezaei, M. Nanocrystalline MgO supported nickel-based bimetallic catalysts for carbon dioxide reforming. Int. J. Hydrog. Energy 2010, 35, 10295–10301. [Google Scholar] [CrossRef]
- Bachiller-Baeza, B.; Mateos-Pedrero, C.; Soria, M.A.; Guerrero-Ruiz, A.; Rodemerck, U.; Rodríguez-Ramos, I. Transient studies of low-temperature dry reforming of methane over Ni-CaO-ZrO2-La2O3. Appl. Catal. B-Environ. 2013, 129, 450–459. [Google Scholar] [CrossRef]
- Nair, M.M.; Kaliaguine, S.; Kleitz, F. Coke resistant nanostructured Ni/La2O3 catalyst for dry reforming of methane. High Surface Area Mesoporous Perovskites for Catalytic Applications. ACS Catal. 2014, 4, 3837–3846. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Q.; Liu, Y.; Zhang, Y. Dry reforming of methane over Ni/MgO-Al2O3 catalysts prepared by two-step hydrothermal method. Appl. Surf. Sci. 2016, 389, 25–33. [Google Scholar] [CrossRef]
- Alipour, Z.; Rezaei, M.; Meshkani, F. Effect of alkaline earth promoters (MgO, CaO, and BaO) on the activity and coke formation of Ni catalysts supported on nanocrystalline Al2O3 in dry reforming of methane. J. Ind. Eng. Chem. 2014, 20, 2858–2863. [Google Scholar] [CrossRef]
- Singha, R.K.; Yadav, A.; Agrawal, A.; Shukla, A.; Adak, S.; Sasaki, T.; Bal, R. Synthesis of highly coke resistant Ni nanoparticles supported MgO/ZnO catalyst for reforming of methane with carbon dioxide. Appl. Catal. B-Environ. 2016, 191, 165–178. [Google Scholar] [CrossRef]
- Liu, Z.; Grinter, D.C.; Lustemberg, P.G.; Nguyen-Phan, T.D.; Zhou, Y.; Luo, S.; Waluyo, I.; Crumlin, E.J.; Stacchiola, D.J.; Zhou, J.; et al. Dry Reforming of Methane on a Highly-Active Ni-CeO2 Catalyst: Effects of Metal-Support Interactions on C-H Bond Breaking. Angew. Chem. Int. Ed. 2016, 55, 7455–7459. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Luo, Y.; Tao, Y.; Strezov, V.; Nelson, P.; Jiang, Y. Promoter Effects on Nickel-Supported Magnesium Oxide Catalysts for the Carbon Dioxide Reforming of Methane. Energy Fuels 2017, 31, 2353–2359. [Google Scholar] [CrossRef]
- Van Keulen, A.N.J.; Seshan, K.; Hoebink, J.H.B.J.; Ross, J.R.H. TAP Investigations of the CO2 Reforming of CH4 over Pt/ZrO2. J. Catal. 1997, 166, 306–314. [Google Scholar] [CrossRef]
- Song, M.; Zhang, W.; Chen, Y.; Luo, J.; John, C.C. The preparation and performance of lignin-based activated carbon fiber adsorbents for treating gaseous streams. Front. Chem. Sci. Eng. 2017, 11, 328–337. [Google Scholar] [CrossRef]
- Song, M.; Yu, L.; Wang, K.; Jin, B. The preparation and adsorption performance of sewage sludge based carbon adsorbents by one step fluidized bed pyrolysis methods. Fresenius Environ. Bull. 2017, 26, 1883-U4. [Google Scholar]
- Tang, X.; Song, M.; Yu, L.; Wang, X. Comparison and application of different component municipal solid wastes based carbon on adsorption of carbon dioxide. Int. J. Green Energy 2017, 14, 135–140. [Google Scholar] [CrossRef]
- Song, Q.; Xiao, R.; Li, Y.; Shen, L. Catalytic carbon dioxide reforming of methane to synthesis gas over activated carbon catalyst. Ind. Eng. Chem. Res. 2008, 47, 4349–4357. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, G.; Zhang, B.; Guo, F.; Sun, Y. Effects of pressure on CO2 reforming of CH4 over carbonaceous catalyst. Chem. Eng. J. 2011, 173, 592–597. [Google Scholar] [CrossRef]
- Bradford, M.C.; Vannice, M.A. CO2 reforming of CH4 over supported Ru catalysts. J. Catal. 1999, 183, 69–75. [Google Scholar] [CrossRef]
- Ferreira-Aparicio, P.; Marquez-Alvarez, C.; Rodrıguez-Ramos, I.; Schuurman, Y.; Guerrero-Ruiz, A.; Mirodatos, C. A transient kinetic study of the carbon dioxide reforming of methane over supported Ru catalysts. J. Catal. 1999, 184, 202–212. [Google Scholar] [CrossRef]
- Wei, Y.; Song, M.; Yu, L.; Tang, X. Preparation of ZnO-Loaded Lignin-Based Carbon Fiber for the Electrocatalytic Oxidation of Hydroquinone. Catalysts 2017, 7, 180. [Google Scholar] [CrossRef]
- Yu, L.; Song, M.; Gao, R.; Wei, Y. Preparation and application of nickel based carbon fibers for the steam reforming of methane. React. Kinet. Mech. Catl. 2017, 120, 477–488. [Google Scholar] [CrossRef]
- Frontera, P.; Aloise, A.; Macario, A.; Antonucci, P.L.; Crea, F.; Giordano, G.; Nagy, J.B. Bimetallic Zeolite Catalyst for CO2 Reforming of Methane. Top. Catal. 2010, 53, 265–272. [Google Scholar] [CrossRef]
- Tantis, I.; Dozzi, M.V.; Bettini, L.G.; Chiarello, G.L.; Dracopoulos, V.; Selli, E.; Lianos, P. Highly functional titania nanoparticles produced by flame spray pyrolysis. Photoelectrochemical and solar cell applications. Appl. Catal. B-Environ. 2016, 182, 369–374. [Google Scholar] [CrossRef]
- Ma, Y.; Yan, C.; Xu, H.; Liu, D.; Shi, P.; Zhu, Y.; Liu, J. Enhanced interfacial properties of carbon fiber reinforced polyamide 6 composites by grafting graphene oxide onto fiber surface. Appl. Surf. Sci. 2018, 452, 286–298. [Google Scholar] [CrossRef]
- Chen, H.; Cai, Q.; Wu, J.; Xia, X.; Liu, H.; Luo, Z. Interfacial enhancement of carbon fiber/nylon 12 composites by grafting nylon 6 to the surface of carbon fiber. Appl. Surf. Sci. 2018, 441, 538–545. [Google Scholar]
- Fei, J.; Duan, X.; Luo, L.; Zhang, C.; Qi, Y.; Li, H.; Feng, Y.; Huang, J. Grafting methyl acrylic onto carbon fiber via Diels-Alder reaction for excellent mechanical and tribological properties of phenolic composites. Appl. Surf. Sci. 2018, 433, 349–357. [Google Scholar] [CrossRef]
- Ruiz-Rosas, R.; Bedia, J.; Lallave, M.; Loscertales, I.G.; Barrero, A.; Rodríguez-Mirasol, J.; Cordero, T. The production of submicron diameter carbon fibers by the electrospinning of lignin. Carbon 2010, 48, 696–705. [Google Scholar] [CrossRef]
- Li, L.; Zhang, L.; Shi, X.; Zhang, Y.; Li, J. Carbon dioxide reforming of methane over nickel catalysts supported on mesoporous MgO. J. Porous Mater. 2014, 21, 217–224. [Google Scholar] [CrossRef]
- Pakhare, D.; Spivey, J. A review of dry (CO2) reforming of methane over noble metal catalysts. Chem. Soc. Rev. 2014, 43, 7813–7837. [Google Scholar] [CrossRef] [PubMed]



| Samples | 2-Theta (°) | FWHM (°) | Crystallite Size of NiAl2O4 (nm) | Average Crystallite Size of NiAl2O4 (nm) |
|---|---|---|---|---|
| 5% Ni–Al2O3 | 37.16 | 0.898 | 9.333 | 10.5 |
| 45.279 | 0.749 | 11.492 | ||
| 66.7 | 0.894 | 10.638 | ||
| 5% Ni–Al2O3/ACF | 37.381 | 0.873 | 9.607 | 9.1 |
| 45.086 | 1.018 | 8.449 | ||
| 66.482 | 1.036 | 9.168 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, L.; Song, M.; Wei, Y.; Xiao, J. Combining Carbon Fibers with Ni/γ–Al2O3 Used for Syngas Production: Part A: Preparation and Evaluation of Complex Carrier Catalysts. Catalysts 2018, 8, 658. https://doi.org/10.3390/catal8120658
Yu L, Song M, Wei Y, Xiao J. Combining Carbon Fibers with Ni/γ–Al2O3 Used for Syngas Production: Part A: Preparation and Evaluation of Complex Carrier Catalysts. Catalysts. 2018; 8(12):658. https://doi.org/10.3390/catal8120658
Chicago/Turabian StyleYu, Lei, Min Song, Yuexing Wei, and Jun Xiao. 2018. "Combining Carbon Fibers with Ni/γ–Al2O3 Used for Syngas Production: Part A: Preparation and Evaluation of Complex Carrier Catalysts" Catalysts 8, no. 12: 658. https://doi.org/10.3390/catal8120658