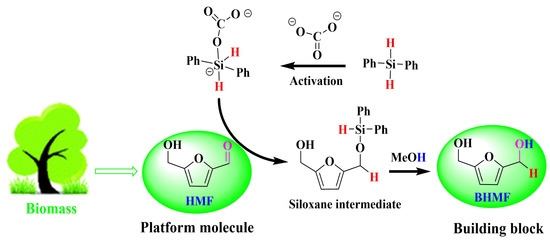
Carbonate-Catalyzed Room-Temperature Selective Reduction of Biomass-Derived 5-Hydroxymethylfurfural into 2,5-Bis(hydroxymethyl)furan
Abstract
:1. Introduction
2. Results and Discussions
2.1. Effect of Different Catalysts
2.2. Influence of Reaction Time
2.3. Influence of Various Solvents
2.4. Influence of Different Hydrosilanes
2.5. Influence of Ph2SiH2 Dosage
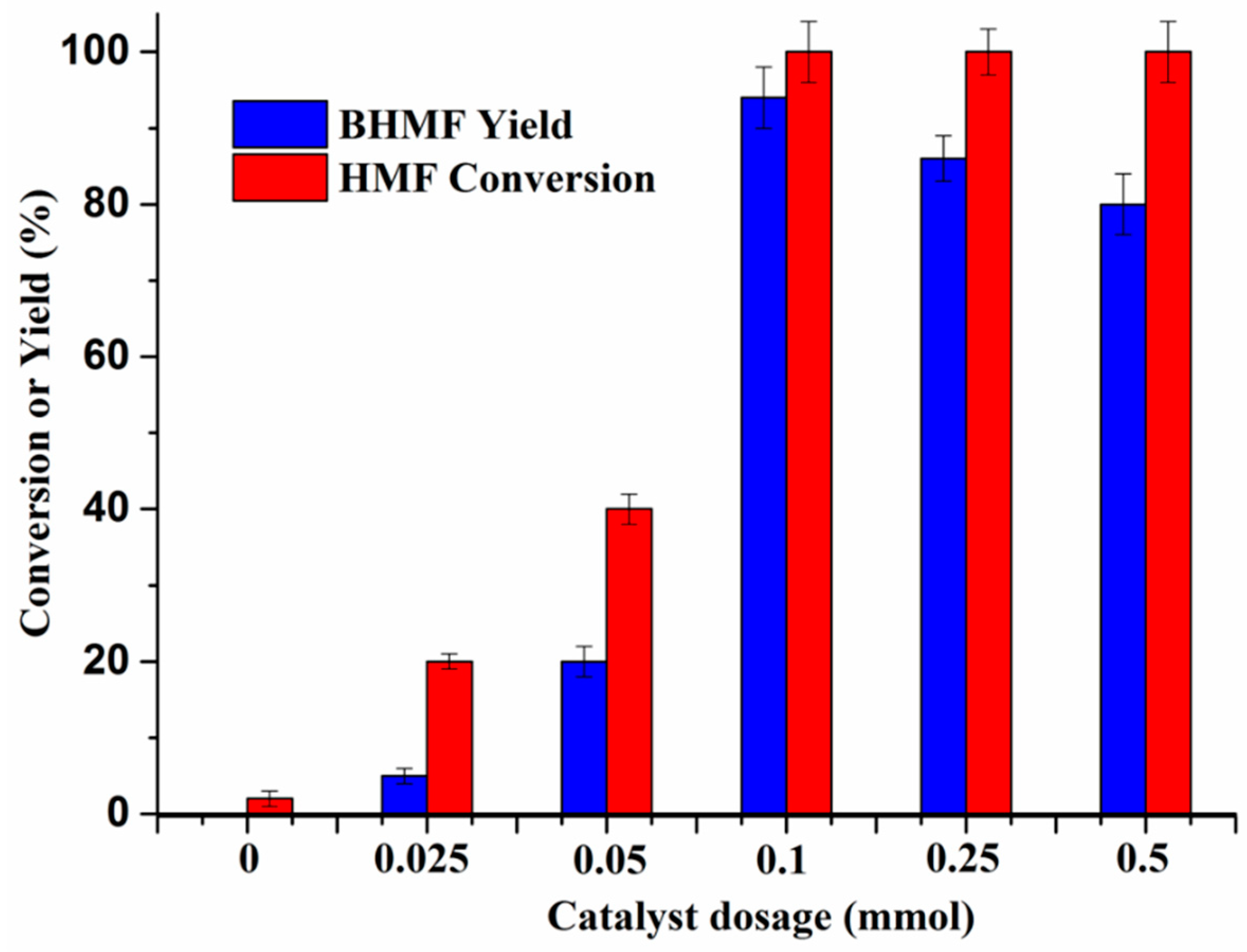
2.6. Influence of Catalyst Dosage
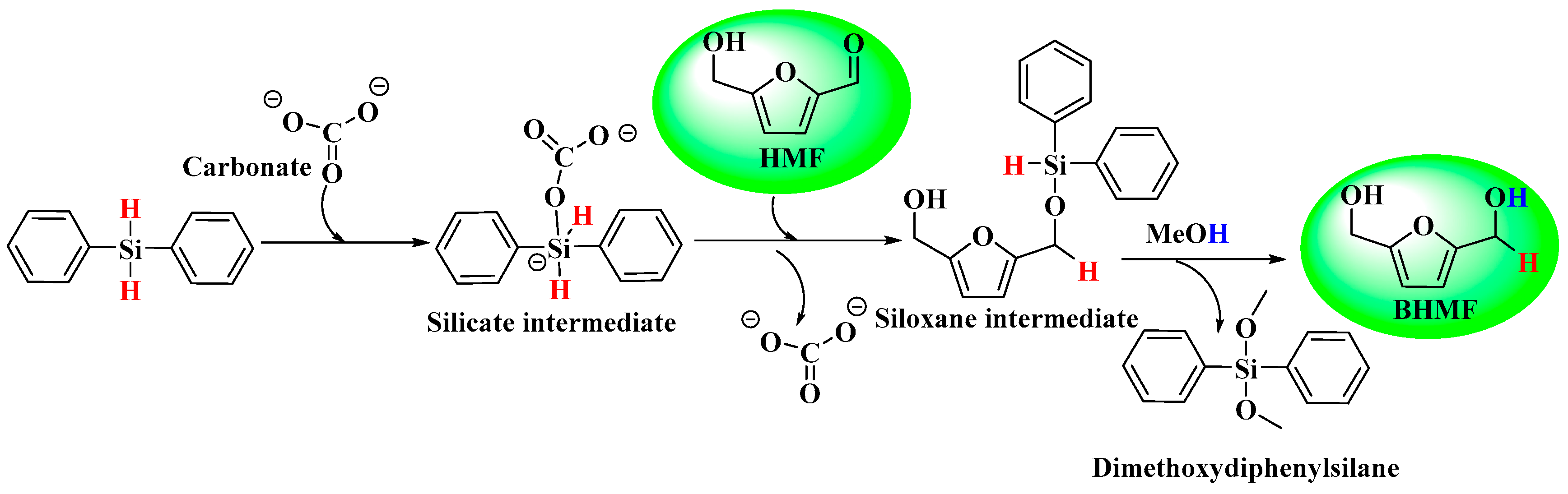
2.7. Reaction Mechanism for HMF-to-BHMF Hydrogenation
3. Materials and Experiments
3.1. Materials
3.2. Procedures for Catalytic Reduction of HMF into BHMF
3.3. Analytical Methods 1100 (Agilent Technologies) system
3.4. Reaction Time-Course Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Li, H.; Fang, Z.; Luo, J.; Yang, S. Direct conversion of biomass components to the biofuel methyl levulinate catalyzed by acid-based bifunctional zirconia-zeolites. Appl. Catal. B. Environ. 2017, 200, 182–191. [Google Scholar] [CrossRef]
- Hu, L.; Xu, J.; Zhou, S.; He, A.; Tang, X.; Lin, L.; Zhao, Y. Catalytic advances in the production and application of biomass-derived 2,5-dihydroxymethylfuran. ACS Catal. 2018, 8, 2959–2980. [Google Scholar] [CrossRef]
- Patel, M.; Kumar, A. Production of renewable diesel through the hydroprocessing of lignocellulosic biomass-derived bio-oil: A review. Renew. Sustain. Energy Rev. 2016, 58, 1293–1307. [Google Scholar] [CrossRef]
- Qin, Y.; Yu, L.; Wu, R.; Yang, D.; Qiu, X.; Zhu, J.Y. Biorefinery lignosulfonates from sulfite-pretreated softwoods as dispersant for graphite. ACS Sustain. Chem. Eng. 2016, 4, 2200–2205. [Google Scholar] [CrossRef]
- Li, H.; Fang, Z.; Smith Jr, R.L.; Yang, S. Efficient valorization of biomass to biofuels with bifunctional solid catalytic materials. Proc. Energy Combust. 2016, 55, 98–194. [Google Scholar] [CrossRef]
- Li, H.; Riisager, A.; Saravanamurugan, S.; Pandey, A.; Sangwan, R.S.; Yang, S.; Luque, R. Carbon-increasing catalytic strategies for upgrading biomass into energy-intensive fuels and chemicals. ACS Catal. 2017, 8, 148–187. [Google Scholar] [CrossRef]
- Román-Leshkov, Y.; Chheda, J.N.; Dumesic, J.A. Phase modifiers promote efficient production of hydroxymethylfurfural from fructose. Science 2006, 312, 1933–1937. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Watanabe, M.; Aida, T.M.; Smith, R.L., Jr. Efficient process for conversion of fructose to 5-hydroxymethylfurfural with ionic liquids. Green Chem. 2009, 11, 1327–1331. [Google Scholar] [CrossRef]
- Chen, J.; Li, K.; Chen, L.; Liu, R.; Huang, X.; Ye, D. Conversion of fructose into 5-hydroxymethylfurfural catalyzed by recyclable sulfonic acid-functionalized metal–organic frameworks. Green Chem. 2014, 16, 2490–2499. [Google Scholar] [CrossRef]
- Zhao, H.; Holladay, J.E.; Brown, H.; Zhang, Z.C. Metal chlorides in ionic liquid solvents convert sugars to 5-hydroxymethylfurfural. Science 2007, 316, 1597–1600. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Zhang, Z.; Song, J.; Zhou, Y.; Han, B. Efficient conversion of glucose into 5-hydroxymethylfurfural catalyzed by a common Lewis acid SnCl4 in an ionic liquid. Green Chem. 2009, 11, 1746–1749. [Google Scholar] [CrossRef]
- Choudhary, V.; Mushrif, S.H.; Ho, C.; Anderko, A.; Nikolakis, V.; Marinkovic, N.S.; Vlachos, D.G. Insights into the interplay of Lewis and Brønsted acid catalysts in glucose and fructose conversion to 5-(hydroxymethyl) furfural and levulinic acid in aqueous media. J. Am. Chem. Soc. 2013, 135, 3997–4006. [Google Scholar] [CrossRef] [PubMed]
- Teong, S.P.; Yi, G.; Zhang, Y. Hydroxymethylfurfural production from bioresources: Past, present and future. Green Chem. 2014, 16, 2015–2026. [Google Scholar] [CrossRef]
- Rout, P.K.; Nannaware, A.D.; Prakash, O.; Kalra, A.; Rajasekharan, R. Synthesis of hydroxymethylfurfural from cellulose using green processes: A promising biochemical and biofuel feedstock. Chem. Eng. Sci. 2016, 142, 318–346. [Google Scholar] [CrossRef]
- Zhang, J.; Li, J.; Tang, Y.; Lin, L.; Long, M. Advances in catalytic production of bio-based polyester monomer 2,5-furandicarboxylic acid derived from lignocellulosic biomass. Carbohyd. Polym. 2015, 130, 420–428. [Google Scholar] [CrossRef] [PubMed]
- van Putten, R.J.; Van Der Waal, J.C.; De Jong, E.D.; Rasrendra, C.B.; Heeres, H.J.; de Vries, J.G. Hydroxymethylfurfural, a versatile platform chemical made from renewable resources. Chem. Rev. 2013, 113, 1499–1597. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Lin, L.; Liu, S. Chemoselective hydrogenation of biomass-derived 5-hydroxymethylfurfural into the liquid biofuel 2,5-dimethylfuran. Ind. Eng. Chem. Res. 2014, 53, 9969–9978. [Google Scholar] [CrossRef]
- Román-Leshkov, Y.; Barrett, C.J.; Liu, Z.Y.; Dumesic, J.A. Production of dimethylfuran for liquid fuels from biomass-derived carbohydrates. Nature 2007, 447, 982–985. [Google Scholar] [CrossRef] [PubMed]
- Audemar, M.; Vigier, K.D.O.; Clacens, J.M.; De Campo, F.; Jérôme, F. Combination of Pd/C and Amberlyst-15 in a single reactor for the acid/hydrogenating catalytic conversion of carbohydrates to 5-hydroxy-2,5-hexanedione. Green Chem. 2014, 16, 4110–4114. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, J.; Xie, W.; Tang, Y.; Guo, D.; Ni, Y. Catalytic transfer hydrogenation of biobased HMF to 2,5-bis-(hydroxymethyl) furan over Ru/Co3O4. Catalysts 2017, 7, 92. [Google Scholar] [CrossRef]
- Revunova, K.; Nikonov, G.I. Main group catalysed reduction of unsaturated bonds. Dalton Trans. 2015, 44, 840–866. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.; de Jong, E.; Raoufmoghaddam, S.; Koper, M.T. Electrocatalytic hydrogenation of 5-hydroxymethylfurfural in the absence and presence of glucose. ChemSusChem 2013, 6, 1659–1667. [Google Scholar] [CrossRef] [PubMed]
- Alamillo, R.; Tucker, M.; Chia, M.; Pagán-Torres, Y.; Dumesic, J. The selective hydrogenation of biomass-derived 5-hydroxymethylfurfural using heterogeneous catalysts. Green Chem. 2012, 14, 1413–1419. [Google Scholar] [CrossRef]
- Chen, B.; Li, F.; Huang, Z.; Yuan, G. Carbon-coated Cu-Co bimetallic nanoparticles as selective and recyclable catalysts for production of biofuel 2,5-dimethylfuran. Appl. Catal. B-Environ. 2017, 200, 192–199. [Google Scholar] [CrossRef]
- Aellig, C.; Jenny, F.; Scholz, D.; Wolf, P.; Giovinazzo, I.; Kollhoff, F.; Hermans, I. Combined 1,4-butanediol lactonization and transfer hydrogenation/hydrogenolysis of furfural-derivatives under continuous flow conditions. Catal. Sci. Technol. 2014, 4, 2326–2331. [Google Scholar] [CrossRef]
- Upare, P.P.; Hwang, Y.K.; Hwang, D.W. An integrated process for the production of 2,5-dihydroxymethylfuran and its polymer from fructose. Green Chem. 2018, 20, 879–885. [Google Scholar] [CrossRef]
- Li, Q.; Man, P.; Yuan, L.; Zhang, P.; Li, Y.; Ai, S. Ruthenium supported on CoFe layered double oxide for selective hydrogenation of 5-hydroxymethylfurfural. Mol. Catal. 2017, 431, 32–38. [Google Scholar] [CrossRef]
- Shen, R.; Zhang, M.; Xiao, J.; Dong, C.; Han, L.B. Ph3P-Mediated Highly Selective C(α)-P Couplings of Quinone Monoacetals with R2P(O)H: Convenient and Practical Synthesis of ortho-Phosphinyl Phenols. Green Chem. 2018, 20, 5111–5116. [Google Scholar] [CrossRef]
- Hao, W.; Li, W.; Tang, X.; Zeng, X.; Sun, Y.; Liu, S.; Lin, L. Catalytic transfer hydrogenation of biomass-derived 5-hydroxymethyl furfural to the building block 2,5-bishydroxymethyl furan. Green Chem. 2016, 18, 1080–1088. [Google Scholar] [CrossRef]
- Wei, J.; Cao, X.; Wang, T.; Liu, H.; Tang, X.; Zeng, X.; Lin, L. Catalytic transfer hydrogenation of biomass-derived 5-hydroxymethylfurfural into 2,5-bis (hydroxymethyl) furan over tunable Zr-based bimetallic catalysts. Catal. Sci. Technol. 2018, 8, 4474–4484. [Google Scholar] [CrossRef]
- Thananatthanachon, T.; Rauchfuss, T.B. Efficient route to hydroxymethylfurans from sugars via transfer hydrogenation. ChemSusChem 2010, 3, 1139–1141. [Google Scholar] [CrossRef] [PubMed]
- Thananatthanachon, T.; Rauchfuss, T.B. Efficient production of the liquid fuel 2,5-dimethylfuran from fructose using formic acid as a reagent. Angew. Chem. 2010, 122, 6766–6768. [Google Scholar] [CrossRef]
- Wu, W.; Zhao, W.; Fang, C.; Wang, Z.; Yang, T.; Li, H.; Yang, S. Quantitative hydrogenation of furfural to furfuryl alcohol with recyclable KF and hydrosilane at room temperature in minutes. Catal. Commun. 2018, 105, 6–10. [Google Scholar] [CrossRef]
- Roy, A.K. A review of recent progress in catalyzed homogeneous hydrosilation (hydrosilylation). Adv. Organomet. Chem. 2007, 55. [Google Scholar] [CrossRef]
- Oestreich, M.; Hermeke, J.; Mohr, J. A unified survey of Si-H and H-H bond activation catalysed by electron-deficient boranes. Chem. Soc. Rev. 2015, 44, 2202–2220. [Google Scholar] [CrossRef] [PubMed]
- Muthukumar, A.; Mamillapalli, N.C.; Sekar, G. Potassium phosphate-catalyzed chemoselective reduction of α-keto amides: Route to synthesize passerini adducts and 3-phenyloxindoles. Adv. Synth. Catal. 2016, 358, 643–652. [Google Scholar] [CrossRef]
- Xie, W.; Zhao, M.; Cui, C. Cesium carbonate-catalyzed reduction of amides with hydrosilanes. Organometallics 2013, 32, 7440–7444. [Google Scholar] [CrossRef]
- Li, H.; Zhao, W.; Dai, W.; Long, J.; Watanabe, M.; Meier, S.; Saravanamurugan, S.; Yang, S.; Riisager, A. Noble metal-free upgrading of multi-unsaturated biomass derivatives at room temperature: Silyl species enable reactivity. Green Chem. 2018. [Google Scholar] [CrossRef]
- Li, H.; Zhao, W.; Saravanamurugan, S.; Dai, W.; He, J.; Meier, S.; Yang, S.; Riisager, A. Control of selectivity in hydrosilane-promoted heterogeneous palladium-catalysed reduction of furfural and aromatic carboxides. Commun. Chem. 2018, 1, 32. [Google Scholar] [CrossRef]
- Zhao, W.; Yang, T.; Li, H.; Wu, W.; Wang, Z.; Fang, C.; Saravanamurugan, S.; Yang, S. Highly recyclable fluoride for enhanced cascade hydrosilylation-cyclization of levulinates to γ-valerolactone at low temperatures. ACS Sustain. Chem. Eng. 2017, 5, 9640–9644. [Google Scholar] [CrossRef]
- Ding, K.; Zannat, F.; Morris, J.C.; Brennessel, W.W.; Holland, P.L. Coordination of N-methylpyrrolidone to iron(II). J. Organomet. Chem. 2009, 694, 4204–4208. [Google Scholar] [CrossRef]
- Bisz, E.; Szostak, M. 2-Methyltetrahydrofuran: A green solvent for iron-catalyzed cross-coupling reactions. ChemSusChem 2018, 11, 1290–1294. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.; Wu, G.; Lafleur, T.; Jarvis, C. Production, properties and catalytic hydrogenation of furfural to fuel additives and value-added chemicals. Renew. Sustain. Energy Rev. 2014, 38, 663–676. [Google Scholar] [CrossRef]
- Sheldon, R.A. Fundamentals of green chemistry: Efficiency in reaction design. Chem. Soc. Rev. 2012, 41, 1437–1451. [Google Scholar] [CrossRef] [PubMed]
- Byrne, F.P.; Jin, S.; Paggiola, G.; Petchey, T.H.M.; Clark, J.H.; Farmer, T.; Hunt, A.J.; McElroy, C.R.; Sherwood, J. Tools and techniques for solvent selection: Green solvent selection guides. Sustain. Chem. Process 2016, 4, 7. [Google Scholar] [CrossRef] [Green Version]
- Alder, C.M.; Hayler, J.D.; Henderson, R.K.; Redman, A.M.; Shukla, L.; Shuster, L.E.; Sneddon, H.F. Updating and further expanding GSK’s solvent sustainability guide. Green Chem. 2016, 18, 3879–3890. [Google Scholar] [CrossRef]
- Kumar, G.; Muthukumar, M.A.; Sekar, G. A Mild and chemoselective hydrosilylation of α-keto amides using Cs2CO3/PMHS/2-MeTHF System. J. Org. Chem. 2017, 33, 4883–4890. [Google Scholar] [CrossRef]
- Fulajtárova, K.; Soták, T.; Hronec, M.; Vávra, I.; Dobročka, E.; Omastová, M. Aqueous phase hydrogenation of furfural to furfuryl alcohol over Pd-Cu catalysts. Appl. Catal. A 2015, 502, 78–85. [Google Scholar] [CrossRef]
- Li, H.; Smith, R.L. Solvents take control. Nat. Catal. 2018, 1, 176–177. [Google Scholar] [CrossRef]
- Bornschein, C.; Werkmeister, S.; Junge, K.; Beller, M. TBAF-catalyzed hydrosilylation for the reduction of aromatic nitriles. New J. Chem. 2013, 37, 2061–2065. [Google Scholar] [CrossRef]
- Zhao, W.; Wu, W.; Li, H.; Fang, C.; Yang, T.; Wang, Z.; Yang, S. Quantitative synthesis of 2,5-bis(hydroxymethyl)furan from biomass-derived 5-hydroxymethylfurfural and sugars over reusable solid catalysts at low temperatures. Fuel 2018, 217, 365–369. [Google Scholar] [CrossRef]
- Kira, M.; Sato, K.; Sakurai, H. Reduction of carbonyl compounds with pentacoordinate hydridosilicates. J. Org. Chem. 1987, 52, 948–949. [Google Scholar] [CrossRef]
- Ryoichi, K.; Masatoshi, T.; Yoshihiko, I. Reduction of amides to amines via catalytic hydrosilylation by a rhodium complex. Tetrahedron Lett. 1998, 39, 1017–1020. [Google Scholar] [CrossRef]
- Rendler, S.; Oestreich, M. Hypervalent Silicon as a reactive site in selective bond-forming processes. Synthesis 2005, 17, 1727–1747. [Google Scholar] [CrossRef]





| Entry | Catalyst | Temperature (°C) | Time (h) | Mole/Pressure of H-Donor | Yield (%) | Conversion (%) | Reference |
|---|---|---|---|---|---|---|---|
| 1 | Pd/C | 80 | 20 | 10 MPa H2 | 82 | 97 | [19] |
| 2 | Ru/CeOx | 130 | 2 | 2.7 MPa H2 | 81 | 100 | [23] |
| 3 | Ru-Mg–Zr | 130 | 2 | 2.72 MPa H2 | 94 | [23] | |
| 4 | Cu/C | 180 | 8 | 5 MPa H2 | 53.3 | 70 | [24] |
| 5 | Ru/CoNi-LDO | 180 | 4 | 1 MPa H2 | 49.9 | 100 | [27] |
| 6 | Cu(50)-SiO2 | 100 | 4 | 1.5 MPa H2 | 93 | 95 | [28] |
| 7 | ZrO(OH)2 | 150 | 2.5 | ethanol | 83.7 | 94.1 | [29] |
| 8 | ZrBa(3)-SBA | 150 | 2.5 | isopropanol | 55.4 | 58.8 | [30] |
| 9 | Cu/AlOx | 220 | CF a | 1,4-butanediol | 93 | 94 | [25] |
| 10 | Ru/Co3O4 | 190 | 6 | isopropanol | 82.8 | 100 | [20] |
| 11 | Cm*Ru(HTsDPEN) | 40 | 2 | formic acid | 99 | 100 | [31] |
| 12 | Pd/C | 70 | 4 | formic acid | 94 | - | [32] |
| 13 | K2CO3 | 25 | 2 | Ph2SiH2 | 94 | 100 | This work |
| Entry | Catalyst | Yield (%) | Conversion (%) |
|---|---|---|---|
| 1 | none | 0 | 0 |
| 2 | K2CO3 | 70.1 | 100 |
| 3 a | K2CO3 | 94.2 | 100 |
| 4 | KHCO3 | 40.0 | 53.1 |
| 5 | CH3COOK | 35.0 | 50.2 |
| 6 | Li2CO3 | 0 | <1 |
| 7 | Na2CO3 | 0 | <1 |
| 8 | KCl | 0 | 0 |
| 9 | CaCO3 | 0 | 0 |
| 10 | SrCO3 | 0 | <1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Long, J.; Zhao, W.; Xu, Y.; Li, H.; Yang, S. Carbonate-Catalyzed Room-Temperature Selective Reduction of Biomass-Derived 5-Hydroxymethylfurfural into 2,5-Bis(hydroxymethyl)furan. Catalysts 2018, 8, 633. https://doi.org/10.3390/catal8120633
Long J, Zhao W, Xu Y, Li H, Yang S. Carbonate-Catalyzed Room-Temperature Selective Reduction of Biomass-Derived 5-Hydroxymethylfurfural into 2,5-Bis(hydroxymethyl)furan. Catalysts. 2018; 8(12):633. https://doi.org/10.3390/catal8120633
Chicago/Turabian StyleLong, Jingxuan, Wenfeng Zhao, Yufei Xu, Hu Li, and Song Yang. 2018. "Carbonate-Catalyzed Room-Temperature Selective Reduction of Biomass-Derived 5-Hydroxymethylfurfural into 2,5-Bis(hydroxymethyl)furan" Catalysts 8, no. 12: 633. https://doi.org/10.3390/catal8120633