Switchable Intrinsic Defect Chemistry of Titania for Catalytic Applications
Abstract
:1. Introduction
2. Defects in Titania
- (i)
- Point defects preferably gather as clusters rather than distributed randomly on the crystal structure and, hence, lead to line defects, planar and volume defects [45].
- (ii)
- Depending on the location of defects in the crystal structure, the activity varies as tabulated in Table 1.
- (iii)
- Point defects are generally represented using Kroger-Vink notation (Table 2).
- (iv)
- As charge-neutrality demands, point defects usually form as a charge-neutral complexes [Schottky defects (e.g., + 2, 2 + and Frenkel defects (e.g., + , )]. Their formation defect equilibria are given below [48,49]:
- (1)
- Defect equilibria of oxygen vacancy (VO) formation: Oo
Vo●● + 2e′ + ½ O2
- (2)
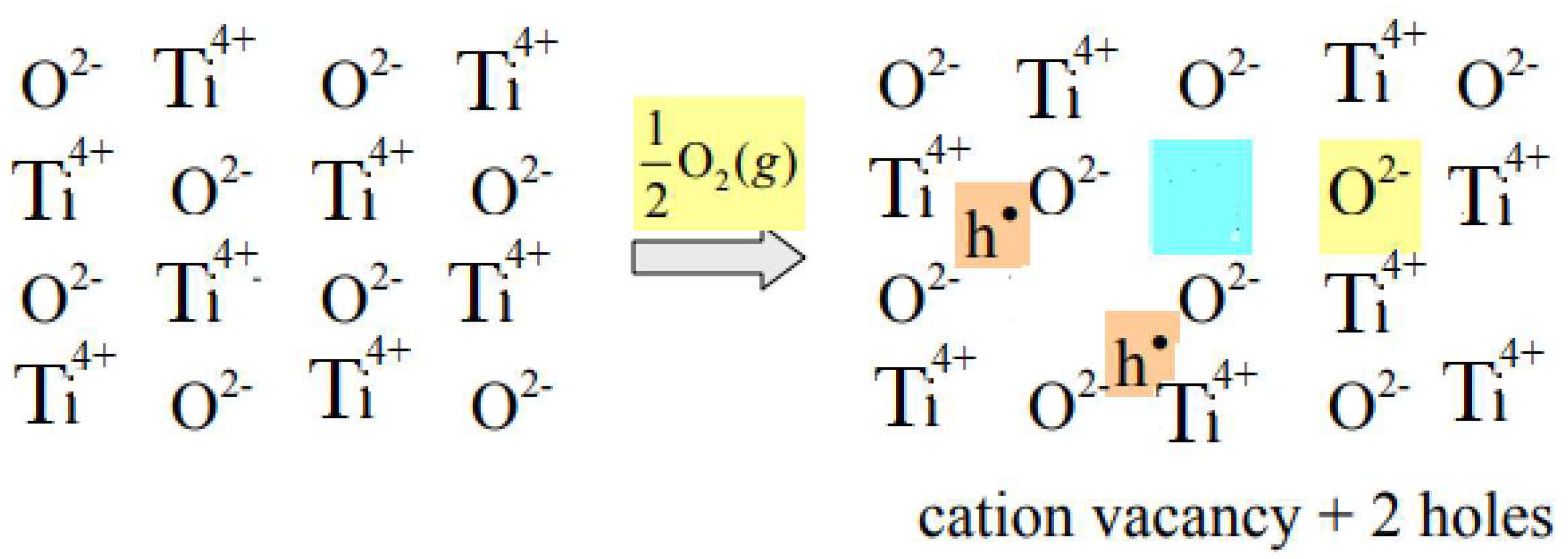
- Defect equilibria of Titanium vacancies (VTi): O2
2Oo + + 4h●
- (3)
- Defect equilibria of Titanium interstitials (Tii): 2Oo +
Tii●●● + 3e′ + O2
- (4)
- Defect equilibria of oxygen interstitials (Oi): + ½ O2
Oi′′ + 2h●
- (V)
- Intuitively, ionization of these ionic defects leads to the formation of electronic defects in the crystal system, which are called color centers [50,51]. There are two types of color center-based on their charge (i) electron center (F) and (ii) electron-hole center (V) and classified further as F, F+, F++ and V, V+, V++, respectively.
2.1. Electron (F, F+, F++) Center
2.1.1. F Center
2.1.2. F+ Center
2.1.3. F++ Center
2.2. Electron Holes
3. Experimental Approaches to Generate Defects in Titania
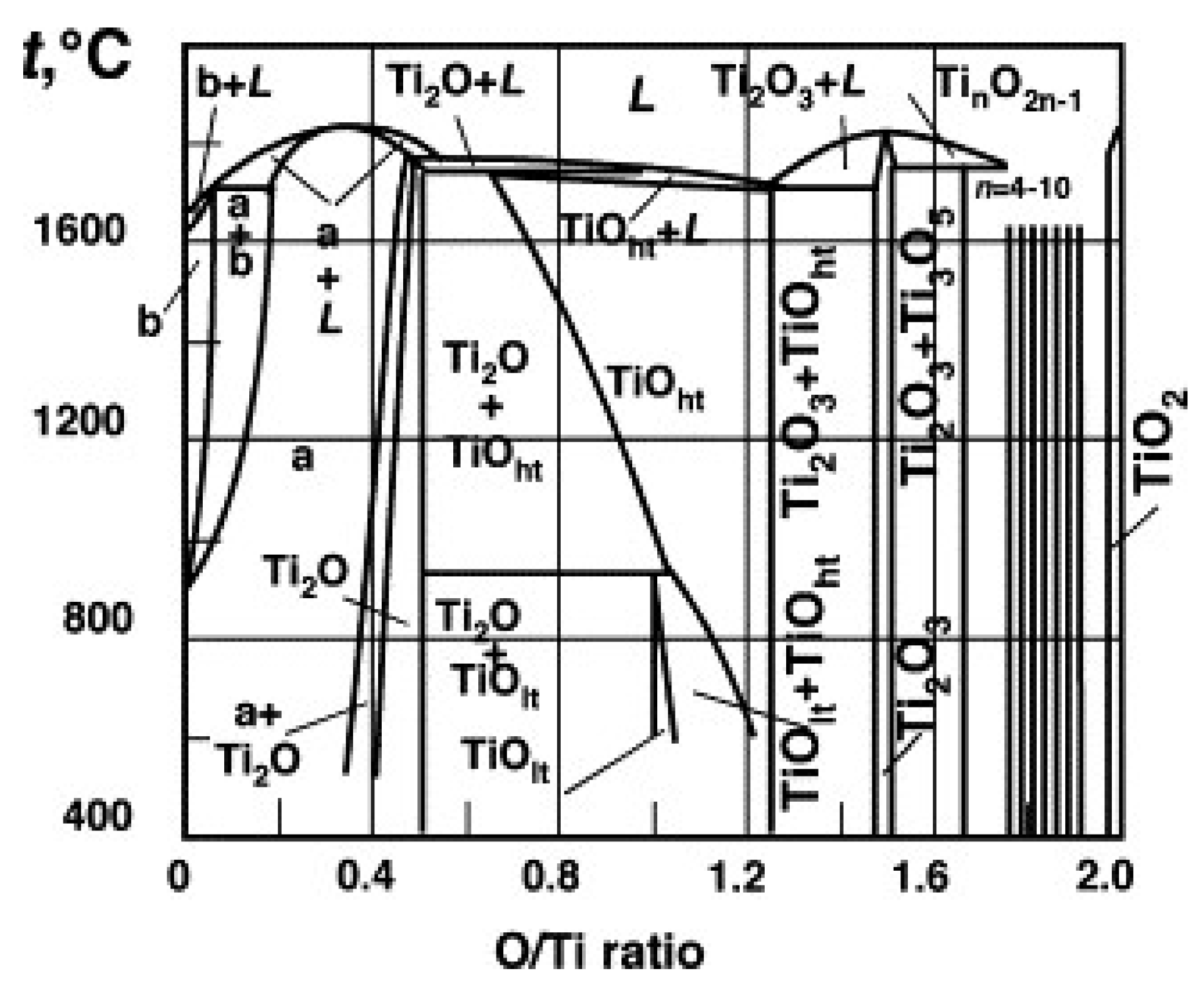
3.1. Effect of Defects on Crystal Structure of Titania
3.2. Crystal Structures of Various Titanium Oxides
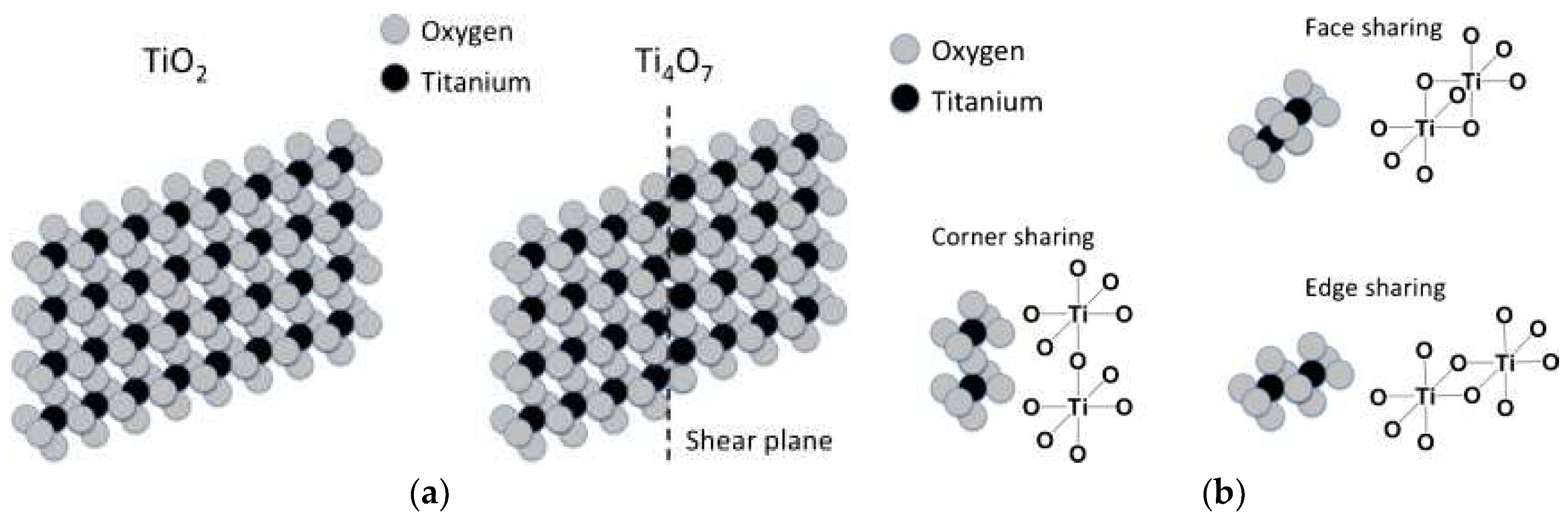
- ◊
- The structure of magneli phase can be regarded as made of stacked alternating shear slabs and the family members of magneli phase differ in many shear planes. Thus, they also diverge in length of the pseudo-rutile chain segments.
- ◊
- Magneli phases with higher n value possess greater shear plane interval. The shear planes provides a pathway for electrons transport, and hence, magneli oxides are an excellent electrical conductor [93].
- ◊
- Magneli phase is a mixed valence compound of (n−2) Ti4+ (3d0) and two Ti3+ (3d1) configurations. The presence of both Ti3+ and Ti4+ ions offer several possible configurations of Ti in the crystal, leading to various charge-ordered states and viewed as electron-doped (oxygen deficient) TiO2 [94].
- ◊
- The value of n assures the crystal structure as well the electronic structure of the Magneli phase.
- ∆
- ∆
- ∆
- It is metallic in nature due to the high content of vacancy channels [107].
- ∆
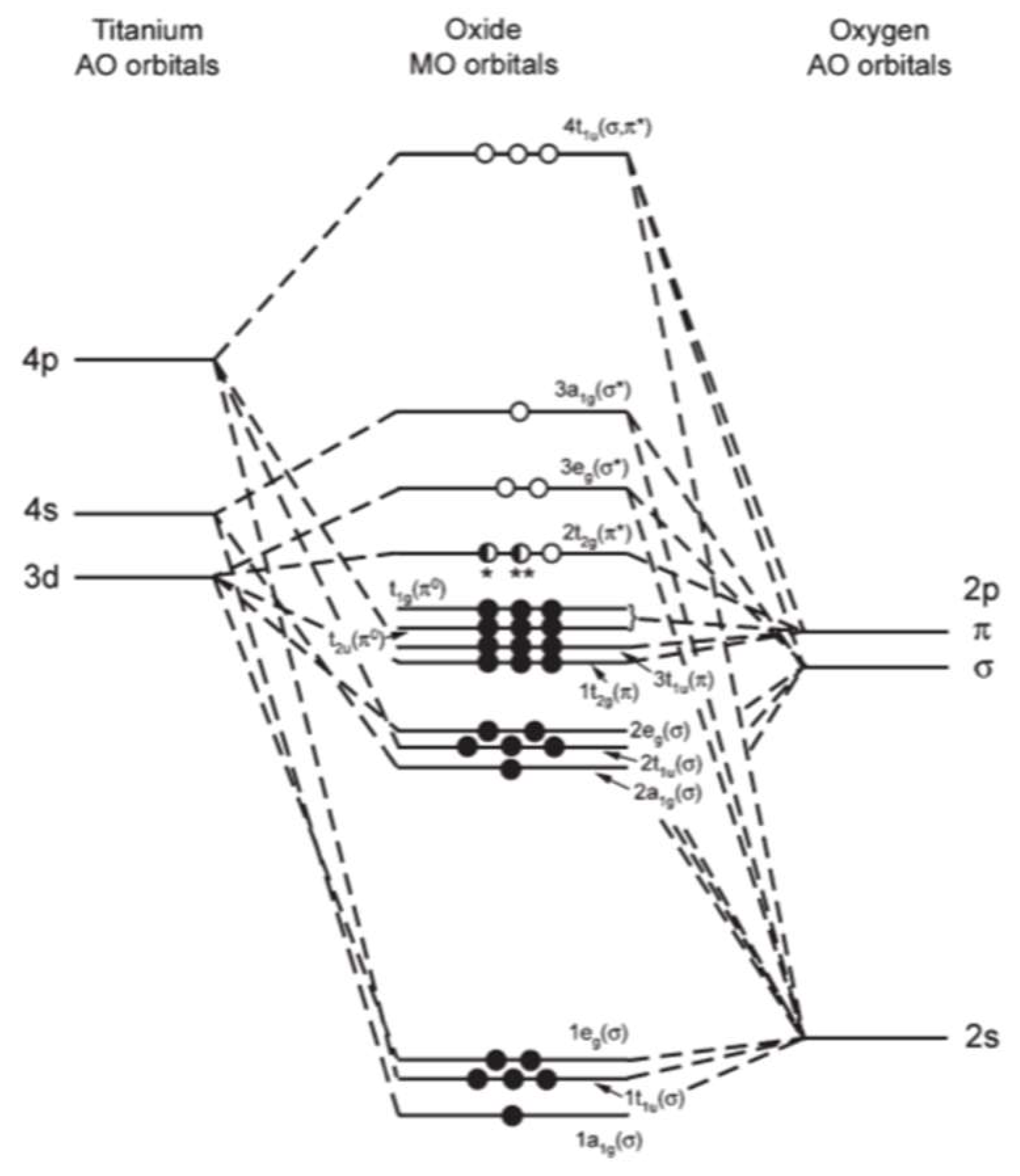
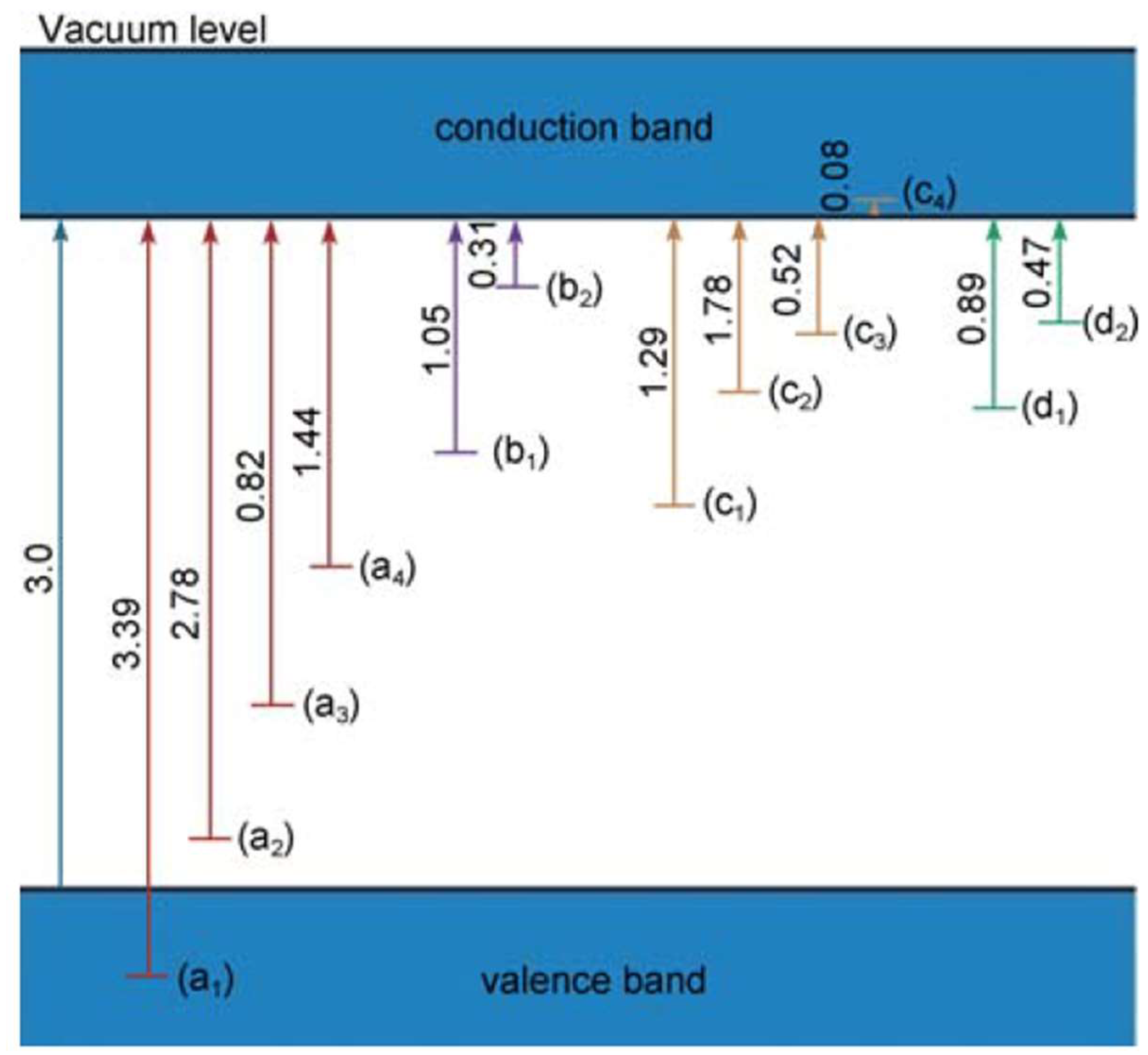
4. Effect of Defects on Optical Behavior of Titania
- the systematic decrease in crystal field splitting between t2g and, eg levels.
- the covalent bonding between Ti and O becomes ionic.
- The degree of electron occupancy in the t2g level increases. TiO2 has none, Ti2O3 has one and TiO has two t2g electrons.
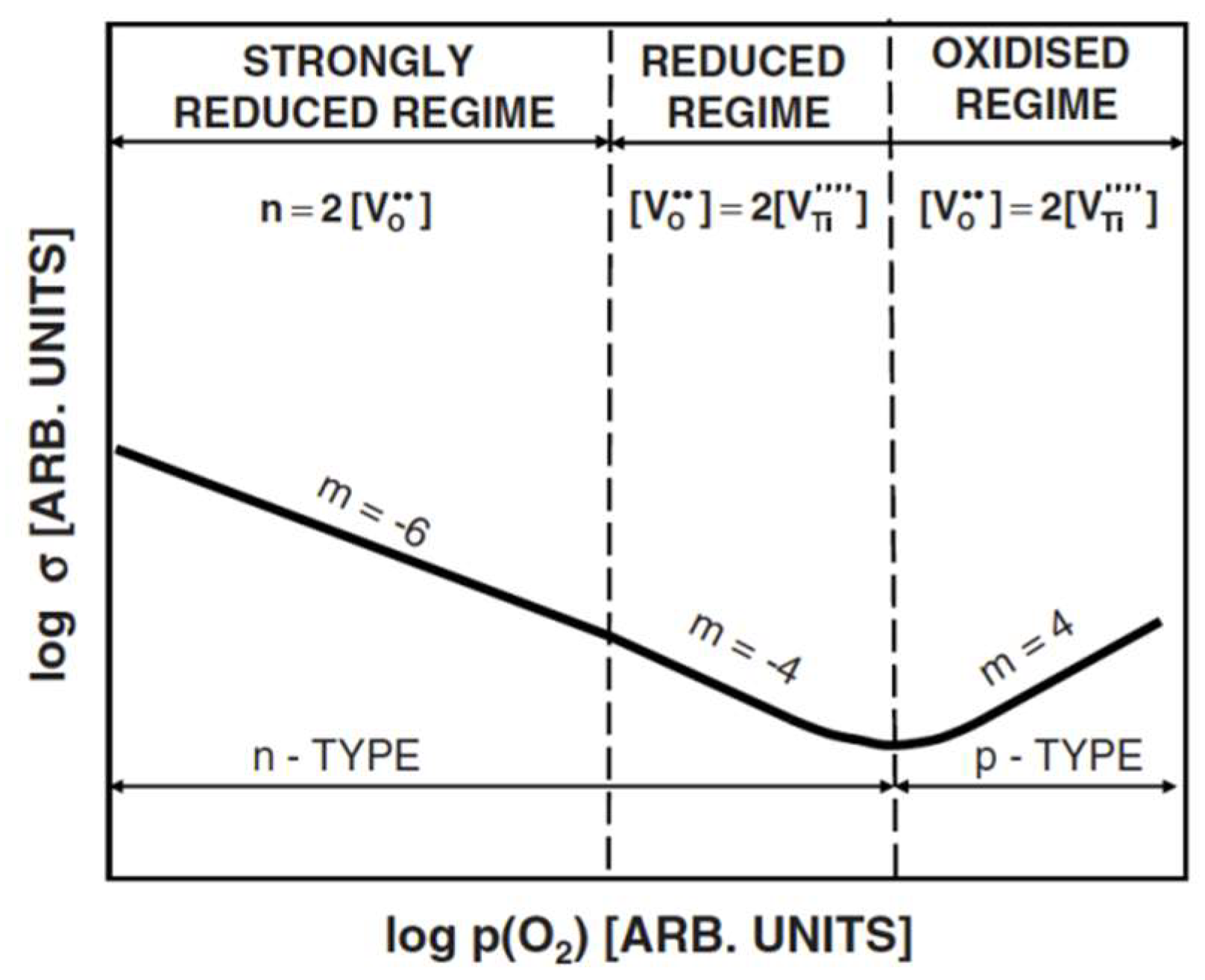
5. Effect of Defects on Electrical Behavior of Titania
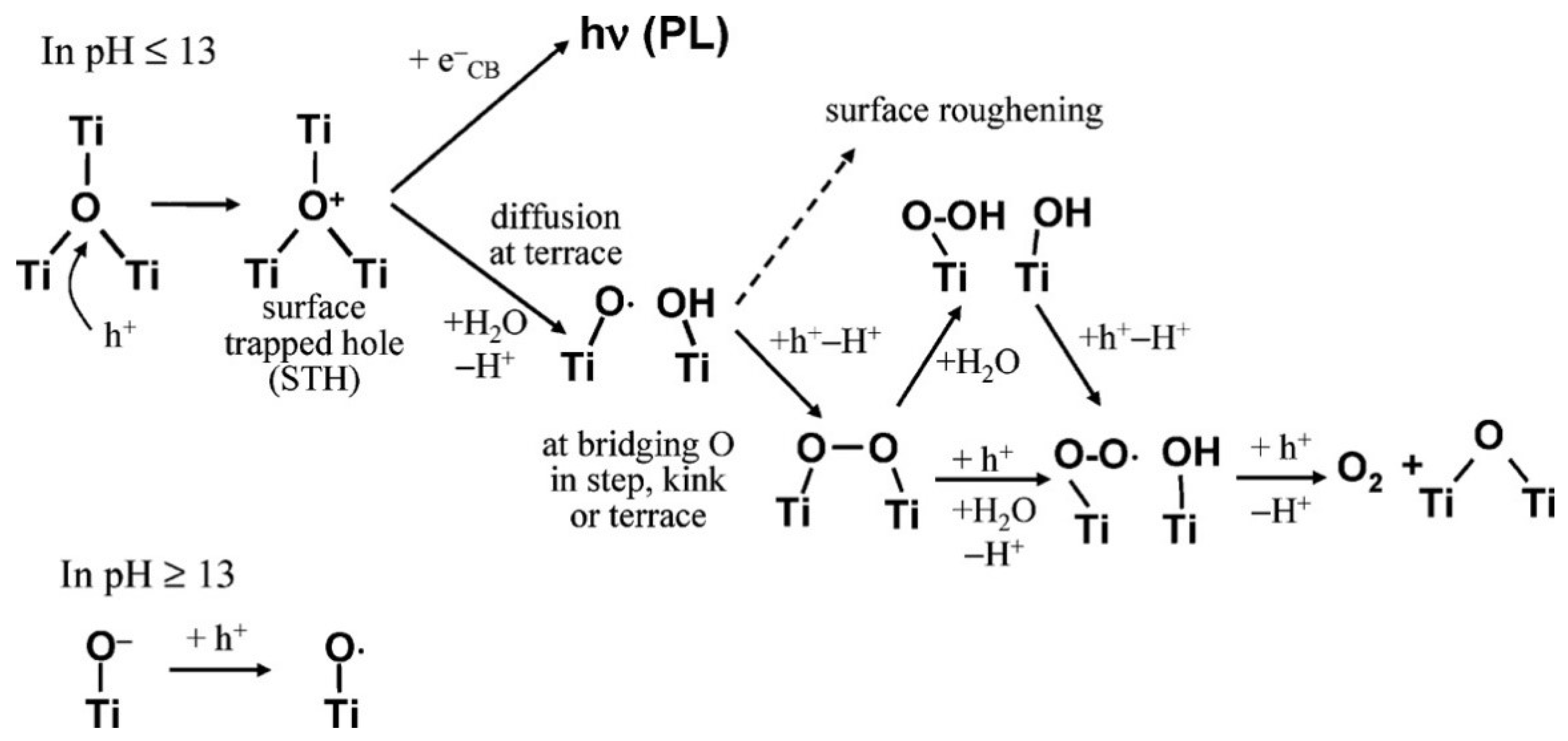
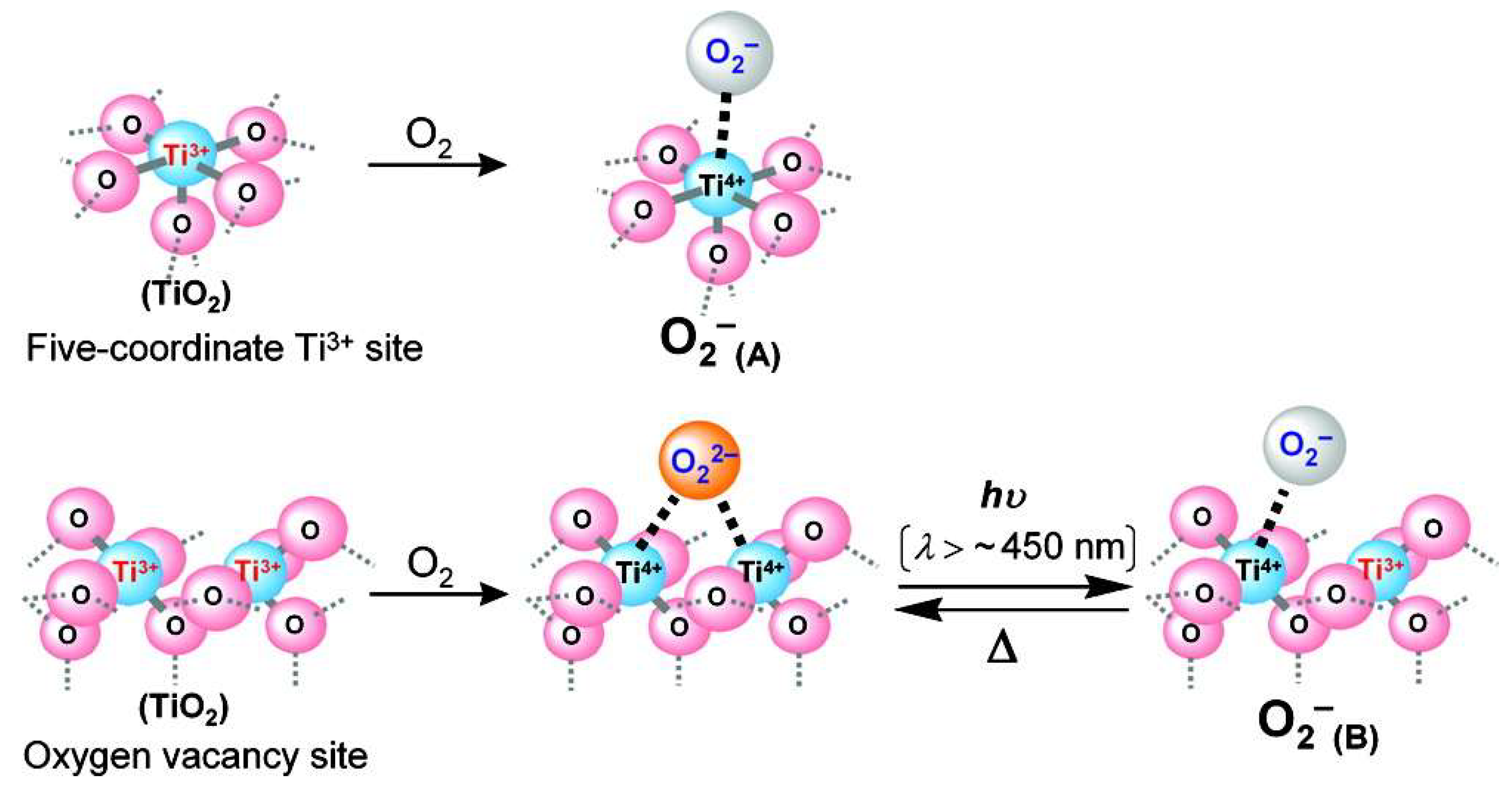
6. Effect of Defects on the Catalytic Activity of Titania
7. Role of Defects towards Electro-Catalytic Water Splitting
8. Role of Defects towards Photoelectrochemical Water Splitting
9. Challenges in Reduced Titania
10. Applications
11. Towards Commercial Development
12. Conclusions
Acknowledgments
Conflicts of Interest
References
- Askeland, D.R.; Pradeep, P.F.; Wright, W.J. Imperfections in the atomic and Ionic Arrangements. In The Science and Engineering of Materials, 6th ed.; Global Engineering: Boston, MA, USA, 2014; pp. 113–145. ISBN 978-0-495-29602-7. [Google Scholar]
- Heather, S. “DNA and Evolution” ThoughtCo. Available online: Thoughtco.com/dna-and-evolution-1224567 (accessed on 20 October 2018).
- Hu, W.; Liu, Y.; Withers, R.L.; Frankcombe, T.J.; Norén, L.; Snashall, A.; Kitchin, M.; Smith, P.; Gong, B.; Chen, H.; et al. Electron-pinned defect-dipoles for high-performance colossal permittivity materials. Nat. Mater. 2013, 12, 821–826. [Google Scholar] [CrossRef] [PubMed]
- Repp, S.; Weber, S.; Erdem, E. Defect evolution of nonstoichiometric ZnO Quantum Dots. J. Phys. Chem. C 2016, 120, 25124–25130. [Google Scholar] [CrossRef]
- Hersam, M.C. Defects at the Two-Dimensional Limit. J. Phys. Chem. Lett. 2015, 6, 2738–2739. [Google Scholar] [CrossRef] [PubMed]
- Martinez, U.; Hansen, J.; Lira, E.; Kristoffersen, H.H.; Huo, P.; Bechstein, R.; Lægsgaard, E.; Besenbacher, F.; Hammer, B.; Wendt, S. Reduced step edges on rutile TiO2(110) as competing defects to oxygen vacancies on the terraces and reactive sites for ethanol dissociation. Phys. Rev. Lett. 2012, 109, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Cremer, T.; Jensen, S.C.; Friend, C.M. Enhanced photo-oxidation of formaldehyde on highly reduced o-TiO2(110). J. Phys. Chem. C 2014, 118, 29242–29251. [Google Scholar] [CrossRef]
- Sivula, K. Defects Give New Life to an Old Material: Electronically Leaky Titania as a Photoanode Protection Layer. ChemCatChem 2014, 6, 2796–2797. [Google Scholar] [CrossRef]
- Gai-Boyes, P.L. Defects in Oxide Catalysts: Fundamental Studies of Catalysis in Action. Catal. Rev. 1992, 34, 1–54. [Google Scholar] [CrossRef]
- Attariani, H.; Momeni, K.; Adkins, K. Defect Engineering: A Path toward Exceeding Perfection. ACS Omega 2017, 2, 663–669. [Google Scholar] [CrossRef]
- Barzan, C.; Groppo, E.; Bordiga, S.; Zecchina, A. Defect Sites in H2—Reduced TiO2 Convert Ethylene to High Density Polyethylene without Activator. ACS Catal. 2014, 4, 986–989. [Google Scholar] [CrossRef]
- Seo, H.; Park, C.J.; Cho, Y.J.; Kim, Y.B.; Choi, D.K. Correlation of band edge native defect state evolution to bulk mobility changes in ZnO thin films. Appl. Phys. Lett. 2010, 96, 65–68. [Google Scholar] [CrossRef]
- Myung, S.-T.; Kikuchi, M.; Yoon, C.S.; Yashiro, H.; Kim, S.-J.; Sun, Y.-K.; Scrosati, B. Black anatase titania enabling ultra high cycling rates for rechargeable lithium batteries. Energy Environ. Sci. 2013, 6, 2609. [Google Scholar] [CrossRef]
- Wang, X.; Huang, K.; Ma, W.; Cong, Y.; Ge, C.; Feng, S. Defect Engineering, Electronic Structure, and Catalytic Properties of Perovskite Oxide La0.5Sr0.5CoO3−δ. Chem. A Eur. J. 2017, 23, 1093–1100. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Li, X.; Ye, H.; Chen, S.; Ju, H.; Liu, D.; Lin, Y.; Ye, W.; Wang, C.; Xu, Q.; et al. Oxide Defect Engineering Enables to Couple Solar Energy into Oxygen Activation. J. Am. Chem. Soc. 2016, 138, 8928–8935. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Carvalho, B.R.; Kahn, E.; Lv, R.; Rao, R.; Terrones, H.; Pimenta, M.A.; Terrones, M. Defect engineering of two-dimensional transition metal dichalcogenides. 2D Mater. 2016, 3, 1–21. [Google Scholar] [CrossRef]
- Feng, H.; Xu, Z.; Ren, L.; Liu, C.; Zhuang, J.; Hu, Z.; Xu, X.; Chen, J.; Wang, J.; Hao, W.; et al. Activating Titania for Efficient Electrocatalysis by Vacancy Engineering. ACS Catal. 2018, 8, 4288–4293. [Google Scholar] [CrossRef]
- Zhang, H.; Lv, R. Defect engineering of two-dimensional materials for efficient electrocatalysis. J. Mater. 2018, 4, 95–107. [Google Scholar] [CrossRef]
- Chen, J.; Iyemperumal, S.K.; Fenton, T.; Carl, A.D.; Grimm, R.L.; Li, G.; Deskins, N.A. Synergy between Defects, Photoexcited Electrons, and Supported Single Atom Catalysts for CO2 Reduction. ACS Catal. 2018. [Google Scholar] [CrossRef]
- Swaminathan, J.; Subbiah, R.; Singaram, V. Defect-Rich Metallic Titania (TiO1.23)—An Efficient Hydrogen Evolution Catalyst for Electrochemical Water Splitting. ACS Catal. 2016, 6. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, A.Y.; Pei, D.N.; Yu, H.Q. Efficient Electrochemical Reduction of Nitrobenzene by Defect-Engineered TiO2−x Single Crystals. Environ. Sci. Technol. 2016, 50, 5234–5242. [Google Scholar] [CrossRef]
- Li, Y.H.; Liu, P.F.; Pan, L.F.; Wang, H.F.; Yang, Z.Z.; Zheng, L.R.; Hu, P.; Zhao, H.J.; Gu, L.; Yang, H.G. Local atomic structure modulations activate metal oxide as electrocatalyst for hydrogen evolution in acidic water. Nat. Commun. 2015, 6. [Google Scholar] [CrossRef]
- Bak, T.; Nowotny, J.; Nowotny, M.K.; Sheppard, L.R. Defect engineering of titanium dioxide. J. Aust. Ceram. Soc. 2008, 44, 63–67. [Google Scholar] [CrossRef]
- Lee, H.; Clark, S.J.; Robertson, J. Calculation of point defects in rutile TiO2 by the Screened Exchange Hybrid Functional. Phys. Rev. B 2012, 86, 075209. [Google Scholar] [CrossRef]
- Lu, T.C.; Wu, S.Y.; Lin, L.B.; Zheng, W.C. Defects in the reduced rutile single crystal. Phys. B Condens. Matter 2001, 304, 147–151. [Google Scholar] [CrossRef]
- Eyert, V.; Schwingenschlogl, U.; Eckern, U. Charge order, orbital order, and electron localization in the Magneli phase Ti4O7. Chem. Phys. Lett. 2004, 390, 151–156. [Google Scholar] [CrossRef]
- Wang, Z.W.; Shu, D.J.; Wang, M.; Ming, N. Ben Strain effect on diffusion properties of oxygen vacancies in bulk and subsurface of rutile TiO2. Surf. Sci. 2012, 606, 186–191. [Google Scholar] [CrossRef]
- Boonchun, A.; Reunchan, P.; Umezawa, N. Energetics of native defects in anatase TiO2: A hybrid density functional study. Phys. Chem. Chem. Phys. 2016, 18, 30040–30046. [Google Scholar] [CrossRef]
- Yagi, E.; Hasiguti, R.R.; Aono, M. Electronic conduction above 4 K of slightly reduced oxygen-deficient rutile TiO2−x. Phys. Rev. B 1996, 54, 7945–7956. [Google Scholar] [CrossRef]
- Finazzi, E.; Di Valentin, C.; Pacchioni, G.; Selloni, A. Excess electron states in reduced bulk anatase TiO2: Comparison of standard GGA, GGA+U, and hybrid DFT calculations. J. Chem. Phys. 2008, 129. [Google Scholar] [CrossRef]
- Diebold, U. The surface science of titanium dioxide. Surf. Sci. Rep. 2003, 48, 53–229. [Google Scholar] [CrossRef]
- Bak, T.; Nowotny, J.; Rekas, M.; Sorrell, C.C. Defect chemistry and semiconducting properties of titanium dioxide: II. Defect diagrams. J. Phys. Chem. Solids 2003, 64, 1057–1067. [Google Scholar] [CrossRef]
- Xiao, P.; Fang, H.; Cao, G.; Zhang, Y.; Zhang, X. Effect of Tin+ defects on electrochemical properties of highly-ordered titania nanotube arrays. Thin Solid Films 2010, 518, 7152–7155. [Google Scholar] [CrossRef]
- Agrawal, A.; Lin, J.; Barth, M.; White, R.; Zheng, B.; Chopra, S.; Gupta, S.; Wang, K.; Gelatos, J.; Mohney, S.E.; et al. Fermi level depinning and contact resistivity reduction using a reduced titania interlayer in n-silicon metal-insulator-semiconductor ohmic contacts. Appl. Phys. Lett. 2014, 104, 8–12. [Google Scholar] [CrossRef]
- Nowotny, M.K.; Bak, T.; Nowotny, J. Electrical properties and defect chemistry of TiO2 single crystal. II. Thermoelectric power. J. Phys. Chem. B 2006, 110, 16283–16291. [Google Scholar] [CrossRef] [PubMed]
- Nowotny, J. Titanium dioxide-based semiconductors for solar-driven environmentally friendly applications: Impact of point defects on performance. Energy Environ. Sci. 2008, 1, 565–572. [Google Scholar] [CrossRef]
- Santo, V.D.; Naldoni, A. The Effect of Point Defects on the Ordered/Disorderd Morphology on the Electronic and Structural Features in the Black TiO2 Nanomaterials; World Scientific: Kansas city, KS, USA, 2017; Chapter 4; pp. 49–75. ISBN 978-1-78634-165-5. [Google Scholar]
- Nowotny, J.; Alim, M.A.; Bak, T.; Idris, M.A.; Ionescu, M.; Prince, K.; Sahdan, M.Z.; Sopian, K.; Mat Teridi, M.A.; Sigmund, W. Defect chemistry and defect engineering of TiO2-based semiconductors for solar energy conversion. Chem. Soc. Rev. 2015, 44, 8424–8442. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Zou, X.; Chen, J.-S. Self-modification of titanium dioxide materials by Ti3+ and/or oxygen vacancies: New insights into defect chemistry of metal oxides. RSC Adv. 2014, 4, 13979–13988. [Google Scholar] [CrossRef]
- Pan, X.; Yang, M.Q.; Fu, X.; Zhang, N.; Xu, Y.J. Defective TiO2 with oxygen vacancies: Synthesis, properties and photocatalytic applications. Nanoscale 2013, 5, 3601–3614. [Google Scholar] [CrossRef]
- Chen, X.; Liu, L.; Huang, F. Black titanium dioxide (TiO2) nanomaterials. Chem. Soc. Rev. 2015, 44, 1861–1885. [Google Scholar] [CrossRef]
- Fang, W.; Xing, M.; Zhang, J. Modifications on reduced titanium dioxide photocatalysts: A review. J. Photochem. Photobiol. C Photochem. Rev. 2017, 32, 21–39. [Google Scholar] [CrossRef]
- Easteal, A.J.; Udy, D.J. Extraction of Titanium Dioxide (TiO2) from Ilmenite and Titaniferous Slag. J. Appl. Chem. Biotechnol. 1973, 23, 865–870. [Google Scholar] [CrossRef]
- Buettner, K.M.; Valentine, A.M. Bioinorganic chemistry of Titanium. Chem. Rev. 2012, 112, 1863–1881. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Chen, X. Titanium dioxide nanomaterials: Self-structural modifications. Chem. Rev. 2014, 114, 9890–9918. [Google Scholar] [CrossRef] [PubMed]
- Seebauer, E.G.; Kratzer, M.C. Charged point defects in semiconductors. Mater. Sci. Eng. R Rep. 2006, 55, 57–149. [Google Scholar] [CrossRef]
- Nowotny, J.; Bak, T.; Ali, M.A. Semiconducting Properties and Defect Disorder of Titanium Dioxide. ECS Trans. 2015, 64, 11–28. [Google Scholar] [CrossRef]
- Bak, T.; Nowotny, J.; Nowotny, M.K.; Sheppard, L.R. Defect chemistry of titanium dioxide effect of interfaces. J. Aust. Ceram. Soc. 2007, 43, 49–55. [Google Scholar]
- Nowotny, J.; Bak, T.; Burg, T. Electrical properties of polycrystalline TiO2 at elevated temperatures. Electrical conductivity. Phys. Status Solidi Basic Res. 2007, 244, 2037–2054. [Google Scholar] [CrossRef]
- Serpone, N. Is the band gap of pristine TiO2 narrowed by anion- and cation-doping of titanium dioxide in second-generation photocatalysts? J. Phys. Chem. B 2006, 110, 24287–24293. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsov, V.N.; Serpone, N. On the Origin of the Spectral Bands in the Visible Absorption Spectra of Visible-Light-Active TiO2 Specimens Analysis and Assignments. J. Phys. Chem. C 2009, 113, 15110–15123. [Google Scholar] [CrossRef]
- Swaminathan, J.; Ravichandran, S. Insights into the Electrocatalytic Behavior of Defect-Centered Reduced Titania (TiO1.23). J. Phys. Chem. C 2018, 122. [Google Scholar] [CrossRef]
- Chen, J.; Lin, L.B.; Jing, F.Q. Theoretical study of F-type color center in rutile TiO2. J. Phys. Chem. Solids 2001, 62, 1257–1262. [Google Scholar] [CrossRef]
- Kittel, C. Introduction to Solid State Physics, 7th ed.; John Wiley & Sons: New York, NY, USA, 1996; pp. 585–595. ISBN 13: 9780471111818. [Google Scholar]
- Morgan, B.J.; Watson, G.W. Intrinsic n-type Defect Formation in TiO2: A Comparison of Rutile and Anatase from GGA+U Calculations. J. Phys. Chem. C 2010, 114, 2321–2328. [Google Scholar] [CrossRef]
- Na-Phattalung, S.; Smith, M.F.; Kim, K.; Du, M.H.; Wei, S.H.; Zhang, S.B.; Limpijumnong, S. First-principles study of native defects in anatase TiO2. Phys. Rev. B Condens. Matter Mater. Phys. 2006, 73, 1–6. [Google Scholar] [CrossRef]
- Nowotny, M.K.; Sheppard, L.R.; Bak, T.; Nowotny, J. Defect Chemistry of Titanium Dioxide. Application of Defect Engineering in Processing of TiO2 based photocatalysts. J. Phys. Chem. C 2008, 112, 5275–5300. [Google Scholar] [CrossRef]
- Bak, T.; Burg, T.; Kang, S.J.L.; Nowotny, J.; Rekas, M.; Sheppard, L.; Sorrell, C.C.; Vance, E.R.; Yoshida, Y.; Yamawaki, M. Charge transport in polycrystalline titanium dioxide. J. Phys. Chem. Solids 2003, 64, 1089–1095. [Google Scholar] [CrossRef]
- Kofstad, P. High-temperature oxidation of titanium. J. Less Common Met. 1967, 12, 449–464. [Google Scholar] [CrossRef]
- Nowotny, J.; Bak, T.; Burg, T. Electrical properties of polycrystalline TiO2. Ionics 2007, 13, 79–82. [Google Scholar] [CrossRef]
- Tao, J.; Luttrell, T.; Batzill, M. A two-dimensional phase of TiO2 with a reduced bandgap. Nat. Chem. 2011, 3, 296–300. [Google Scholar] [CrossRef]
- Etacheri, V.; Seery, M.K.; Hinder, S.J.; Pillai, S.C. Oxygen rich titania: A dopant free, high temperature stable, and visible-light active anatase photocatalyst. Adv. Funct. Mater. 2011, 21, 3744–3752. [Google Scholar] [CrossRef]
- Bowker, M.; Bennett, R.A. The role of Ti3+ interstitials in TiO2(110) reduction and oxidation. J. Phys. Condens. Matter 2010, 22, 059801. [Google Scholar] [CrossRef]
- Tanaka, T.; Sumiya, A.; Sawada, H.; Kondo, Y.; Takayanagi, K. Direct observation of interstitial titanium ions in TiO2 substrate with gold nanoparticle. Surf. Sci. 2014, 619, 39–43. [Google Scholar] [CrossRef]
- Tanaka, T.; Sano, K.; Ando, M.; Sumiya, A.; Sawada, H.; Hosokawa, F.; Okunishi, E.; Kondo, Y.; Takayanagi, K. Oxygen-rich Ti1−x O2 pillar growth at a gold nanoparticle-TiO2 contact by O2 exposure. Surf. Sci. 2010, 604, L75–L78. [Google Scholar] [CrossRef]
- Ohwada, M.; Kimoto, K.; Mizoguchi, T.; Ebina, Y.; Sasaki, T. Atomic structure of titania nanosheet with vacancies. Sci. Rep. 2013, 3, 2801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nowotny, M.K.; Bak, T.; Nowotny, J.; Sorrell, C.C. Titanium vacancies in nonstoichiometric TiO2 single crystal. Phys. Status Solidi Basic Res. 2005, 242, 88–90. [Google Scholar] [CrossRef]
- Zheng, Q.; Lee, H.-J.; Lee, J.; Choi, W.; Park, N.-B.; Lee, C. Electrochromic titania nanotube arrays for the enhanced photocatalytic degradation of phenol and pharmaceutical compounds. Chem. Eng. J. 2014, 249, 285–292. [Google Scholar] [CrossRef]
- Wang, G.; Wang, H.; Ling, Y.; Tang, Y.; Yang, X.; Fitzmorris, R.C.; Wang, C.; Zhang, J.Z.; Li, Y. Hydrogen-Treated TiO2 Nanowire Arrays for Photoelectrochemical Water Splitting. Nano Lett. 2011, 11, 3026–3033. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, L.; Liu, Z.; Marcus, M.A.; Wang, W.-C.; Oyler, N.A.; Grass, M.E.; Mao, B.; Glans, P.-A.; Yu, P.Y.; et al. Properties of Disorder-Engineered Black Titanium Dioxide Nanoparticles through Hydrogenation. Sci. Rep. 2013, 3, 1510. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Liu, L.; Yu, P.Y.; Mao, S.S. Increasing solar absorption for photocatalysis with black hydrogenated titanium dioxide nanocrystals. Science 2011, 331, 746–750. [Google Scholar] [CrossRef]
- Nakajima, T.; Nakamura, T.; Shinoda, K.; Tsuchiya, T. Rapid formation of black titania photoanodes: Pulsed laser-induced oxygen release and enhanced solar water splitting efficiency. J. Mater. Chem. A 2014, 2, 6762. [Google Scholar] [CrossRef]
- Rahman, M.A.; Bazargan, S.; Srivastava, S.; Wang, X.; Abd-Ellah, M.; Thomas, J.P.; Heinig, N.F.; Pradhan, D.; Leung, K.T. Defect-rich decorated TiO2 nanowires for super-efficient photoelectrochemical water splitting driven by visible light. Energy Environ. Sci. 2015, 8, 3363–3373. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, C.; Lin, T.; Yin, H.; Chen, P.; Wan, D.; Xu, F.; Huang, F.; Lin, J.; Xie, X.; et al. Visible-light photocatalytic, solar thermal and photoelectrochemical properties of aluminium-reduced black titania. Energy Environ. Sci. 2013, 6, 3007. [Google Scholar] [CrossRef]
- Tang, C.; Zhou, D.; Zhang, Q. Synthesis and characterization of Magneli phases: Reduction of TiO2 in a decomposed NH3 atmosphere. Mater. Lett. 2012, 79, 42–44. [Google Scholar] [CrossRef]
- Simon, P.; Pignon, B.; Miao, B.; Coste-Leconte, S.; Leconte, Y.; Marguet, S.; Jegou, P.; Bouchet-Fabre, B.; Reynaud, C.; Herlin-Boime, N. N-doped titanium monoxide nanoparticles with TiO rock-salt structure, low energy band gap, and visible light activity. Chem. Mater. 2010, 22, 3704–3711. [Google Scholar] [CrossRef]
- Takeuchi, T.; Fukushima, J.; Hayashi, Y.; Takizawa, H. Synthesis of Ti4O7 Nanoparticles by Carbothermal Reduction Using Microwave Rapid Heating. Catalysts 2017, 7, 65. [Google Scholar] [CrossRef]
- Tominaka, S.; Tsujimoto, Y.; Matsushita, Y.; Yamaura, K. Synthesis of nanostructured reduced titanium Oxide: Crystal structure transformation maintaining nanomorphology. Angew. Chem. Int. Ed. 2011, 50, 7418–7421. [Google Scholar] [CrossRef]
- Guan, S.; Hao, L.; Lu, Y.; Yoshida, H.; Pan, F.; Asanuma, H. Fabrication of oxygen-deficient TiO2 coatings with nano-fiber morphology for visible-light photocatalysis. Mater. Sci. Semicond. Process. 2016, 41, 358–363. [Google Scholar] [CrossRef]
- Zou, X.; Liu, J.; Su, J.; Zuo, F.; Chen, J.; Feng, P. Facile synthesis of thermal- and photostable titania with paramagnetic oxygen vacancies for visible-light photocatalysis. Chem. A Eur. J. 2013, 19, 2866–2873. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.S.; Lin, Y.H.; Chuang, W.; Shao, P.S.; Chuang, C.H.; Lee, J.F.; Lu, M.L.; Weng, Y.T.; Wu, N.L. Synthesis of High-Performance Titanium Sub-Oxides for Electrochemical Applications Using Combination of Sol-Gel and Vacuum-Carbothermic Processes. ACS Sustain. Chem. Eng. 2018, 6, 3162–3168. [Google Scholar] [CrossRef]
- Sinhamahapatra, A.; Jeon, J.P.; Yu, J.S. A new approach to prepare highly active and stable black titania for visible light-assisted hydrogen production. Energy Environ. Sci. 2015, 8, 3539–3544. [Google Scholar] [CrossRef] [Green Version]
- Grigorov, K.G.; Grigorov, G.I.; Drajeva, L.; Bouchier, D.; Sporken, R.; Caudano, R. Synthesis and characterization of conductive titanium monoxide films. Diffusion of silicon in titanium monoxide films. Vacuum 1998, 51, 153–155. [Google Scholar] [CrossRef]
- Xiong, L.B.; Li, J.L.; Yang, B.; Yu, Y. Ti3+ in the surface of titanium dioxide: Generation, properties and photocatalytic application. J. Nanomater. 2012, 2012. [Google Scholar] [CrossRef]
- Haubrich, J.; Kaxiras, E.; Friend, C.M. The role of surface and subsurface point defects for chemical model studies on TiO2: A first-principles theoretical study of formaldehyde bonding on rutile TiO2(110). Chem. A Eur. J. 2011, 17, 4496–4506. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Li, L.; Zheng, J. Understanding the defect chemistry of oxide nanoparticles for creating new functionalities: A critical review. Sci. China Chem. 2011, 54, 876–886. [Google Scholar] [CrossRef]
- Janotti, A.; Varley, J.B.; Rinke, P.; Umezawa, N.; Kresse, G.; Van de Walle, C.G. Hybrid functional studies of the oxygen vacancy in TiO2. Phys. Rev. B 2010, 81, 085212. [Google Scholar] [CrossRef]
- Naldoni, A.; Allieta, M.; Santangelo, S.; Marelli, M.; Fabbri, F.; Cappelli, S.; Bianchi, C.L.; Psaro, R.; Dal Santo, V. Effect of nature and location of defects on bandgap narrowing in black TiO2 nanoparticles. J. Am. Chem. Soc. 2012, 134, 7600–7603. [Google Scholar] [CrossRef] [PubMed]
- Parker, J.C.; Siegel, R.W. Raman Microprobe Study of Nanophase TiO2 and Oxidation-Induced Spectral Changes. J. Mater. Res. 1990, 5, 1246–1252. [Google Scholar] [CrossRef]
- Stoyanov, E.; Langenhorst, F.; Steinle-Neumann, G. The effect of valence state and site geometry on Ti L3,2 and O K electron energy-loss spectra of TixOy phases. Am. Mineral. 2007, 92, 577–586. [Google Scholar] [CrossRef]
- Parras, M.; Varela, Á.; Cortés-Gil, R.; Boulahya, K.; Hernando, A.; González-Calbet, J.M. Room-temperature ferromagnetism in reduced rutile TiO2−δ nanoparticles. J. Phys. Chem. Lett. 2013, 4, 2171–2176. [Google Scholar] [CrossRef]
- Walsh, F.C.; Wills, R.G.A. The continuing development of Magnéli phase titanium sub-oxides and Ebonex® electrodes. Electrochim. Acta 2010, 55, 6342–6351. [Google Scholar] [CrossRef]
- Arif, A.F.; Balgis, R.; Ogi, T.; Iskandar, F.; Kinoshita, A.; Nakamura, K.; Okuyama, K. Highly conductive nano-sized Magnéli phases titanium oxide (TiOx). Sci. Rep. 2017, 7, 3646. [Google Scholar] [CrossRef]
- Watanabe, M. Raman spectroscopy of charge-ordered states in Magneli titanium oxides. Phys. Status Solidi 2009, 6, 260–263. [Google Scholar] [CrossRef]
- Åsbrink, S.; Magnéli, A. Crystal structure studies on trititanium pentoxide, Ti3O5. Acta Crystallogr. 1959, 12, 575–581. [Google Scholar] [CrossRef]
- Onoda, M. Phase Transitions of Ti3O5. J. Solid State Chem. 1998, 136, 67–73. [Google Scholar] [CrossRef]
- Ovsyannikov, S.V.; Wu, X.; Shchennikov, V.V.; Karkin, A.E.; Dubrovinskaia, N.; Garbarino, G.; Dubrovinsky, L. Structural stability of a golden semiconducting orthorhombic polymorph of Ti2O3 under high pressures and high temperatures. J. Phys. Condens. Matter 2010, 22. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, Q.; Wu, X.; Qin, S.; Liu, J. High pressure structural study of β-Ti3O5: X-ray diffraction and Raman spectroscopy. J. Solid State Chem. 2012, 192, 356–359. [Google Scholar] [CrossRef]
- Kao, C.H.; Yeh, S.W.; Huang, H.L.; Gan, D.; Shen, P. Study of the TiO to anatase transformation by thermal oxidation of Ti film in air. J. Phys. Chem. C 2011, 115, 5648–5656. [Google Scholar] [CrossRef]
- Kostenko, M.G.; Lukoyanov, A.V.; Rempel, A.A. Electronic structure and stability of nonstoichiometric titanium monoxide TiOy with structural vacancies in one of the sublattices. Phys. Solid State 2013, 55, 2108–2115. [Google Scholar] [CrossRef]
- Bartkowski, S.; Neumann, M. Electronic structure of titanium monoxide. Phys. Rev. B 1997, 56, 10656–10667. [Google Scholar] [CrossRef]
- Kostenko, M.G.; Lukoyanov, A.V.; Zhukov, V.P.; Rempel, A.A. Vacancies in ordered and disordered titanium monoxide: Mechanism of B1 structure stabilization. J. Solid State Chem. 2013, 204, 146–152. [Google Scholar] [CrossRef] [Green Version]
- Barudzija, T.; Gusev, A.; Jugovic, D.; Marinovic-Cincovic, M.; Dramicanin, M.; Zdujic, M.; Jovalekic, C.; Mitric, M. Structural and magnetic properties of mechanochemically synthesized nanocrystalline titanium monoxide. Hem. Ind. 2012, 66, 309–315. [Google Scholar] [CrossRef]
- Guo, C.; Jia, S.; Meng, W.; Zheng, H.; Jin, L.; Liu, Y.; Shi, J.; Wang, J. Orientation domains in vacancy-ordered titanium monoxide. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2013, 69, 589–594. [Google Scholar] [CrossRef]
- Valeeva, A.A.; Rempel, A.A.; Sprengel, W.; Schaefer, H. Identification and study of vacancies in titanium monoxide by means of positron annihilation techniques. Phys. Chem. Chem. Phys. 2003, 5, 2304–2307. [Google Scholar] [CrossRef]
- Fujimura, T.; Iwasaki, H.; Kkegawa, T.; Tsuchida, Y.; Shimomura, O.; Factory, P.; Corporation, K.S.; Iwasaki, H.; Kkegawa, T.; Tsuchida, Y.; et al. Structure changes in vacancy-rich titanium monoxide at high pressures and high temperatures. High Press. Res. 2006, 37–41. [Google Scholar] [CrossRef]
- Denker, S.P. Electronic properties of titanium monoxide. J. Appl. Phys. 1966, 37, 142–149. [Google Scholar] [CrossRef]
- Gunda, N.S.H.; Puchala, B.; Van der Ven, A. Resolving phase stability in the Ti-O binary with first-principles statistical mechanics methods. Phys. Rev. Mater. 2018, 2, 033604. [Google Scholar] [CrossRef]
- Jostsons, A.; McDougall, P.G. Fault Structures in Ti2O. Phys. Status Solidi 1968, 29, 873–889. [Google Scholar] [CrossRef]
- Wang, Q.; Fan, J.; Zhang, S.; Yun, Y.; Zhang, J.; Zhang, P.; Hu, J.; Wang, L.; Shao, G. In situ coupling of Ti2O with rutile TiO2 as a core-shell structure and its photocatalysis performance. RSC Adv. 2017, 7, 54662–54667. [Google Scholar] [CrossRef]
- Burton, B.P.; Van De Walle, A. First principles phase diagram calculations for the octahedral-interstitial system αTiOX, 0 ≤ X ≤ 1/2. Calphad 2012, 39, 97–103. [Google Scholar] [CrossRef]
- Hamdy, M.S.; Amrollahi, R.; Mul, G. Surface Ti3+-containing (blue) titania: A unique photocatalyst with high activity and selectivity in visible light-stimulated selective oxidation. ACS Catal. 2012, 2, 2641–2647. [Google Scholar] [CrossRef]
- Liu, G.; Yin, L.C.; Wang, J.; Niu, P.; Zhen, C.; Xie, Y.; Cheng, H.M. A red anatase TiO2 photocatalyst for solar energy conversion. Energy Environ. Sci. 2012, 5, 9603–9610. [Google Scholar] [CrossRef]
- Liu, G.; Yang, H.G.; Wang, X.; Cheng, L.; Lu, H.; Wang, L.; Lu, G.Q.; Cheng, H.M. Enhanced photoactivity of oxygen-deficient anatase TiO2 sheets with dominant {001} facets. J. Phys. Chem. C 2009, 113, 21784–21788. [Google Scholar] [CrossRef]
- Zhu, Q.; Peng, Y.; Lin, L.; Fan, C.M.; Gao, G.Q.; Wang, R.X.; Xu, A.W. Stable blue TiO2−x nanoparticles for efficient visible light photocatalysts. J. Mater. Chem. A 2014, 2, 4429–4437. [Google Scholar] [CrossRef]
- Mu, J.; Spiecker, E.; Schmuki, P. Black TiO2 Nanotubes: Cocatalyst-Free Open-Circuit Hydrogen Generation. Nano Lett. 2014, 14, 3309–3313. [Google Scholar] [CrossRef]
- Kuznetsov, V.N.; Emeline, A.V.; Rudakova, A.V.; Aleksandrov, M.S.; Glazkova, N.I.; Lovtcius, V.A.; Kataeva, G.V.; Mikhaylov, R.V.; Ryabchuk, V.K.; Serpone, N. Visible-NIR light absorption of titania thermochemically fabricated from titanium and its alloys; UV-and visible-light-induced photochromism of yellow titania. J. Phys. Chem. C 2013, 117, 25852–25864. [Google Scholar] [CrossRef]
- Wang, H.; Lin, T.; Zhu, G.; Yin, H.; Lü, X.; Li, Y.; Huang, F. Colored titania nanocrystals and excellent photocatalysis for water cleaning. Catal. Commun. 2015, 60, 55–59. [Google Scholar] [CrossRef]
- Kako, T.; Umezawa, N.; Xie, K.; Ye, J. Undoped visible-light-sensitive titania photocatalyst. J. Mater. Sci. 2013, 48, 108–114. [Google Scholar] [CrossRef]
- Regonini, D.; Adamaki, V.; Bowen, C.R.; Pennock, S.R.; Taylor, J.; Dent, A.C.E. AC electrical properties of TiO2 and Magnéli phases, TinO2n−1. Solid State Ion. 2012, 229, 38–44. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, M.; Fu, Q.; Lei, B.; Lin, W.; Guo, H.; Wu, M.; Lei, Y. Observation of defect state in highly ordered titanium dioxide nanotube arrays. Nanotechnology 2014, 25, 275603. [Google Scholar] [CrossRef]
- Nowotny, M.K.; Bak, T.; Nowotny, J. Electrical Properties and Defect Chemistry of TiO2 Single Crystal. IV. Prolonged Oxidation Kinetics and Chemical Diffusion. J. Phys. Chem. B 2006, 110, 16270–16282. [Google Scholar] [CrossRef]
- Santara, B.; Giri, P.K.; Imakita, K.; Fujii, M. Evidence for Ti Interstitial Induced Extended Visible Absorption and Near Infrared Photoluminescence from Undoped TiO2 Nanoribbons: An In Situ Photoluminescence Study. J. Phys. Chem. C 2013, 117, 23402–23411. [Google Scholar] [CrossRef]
- Kevane, C.J. Oxygen Vaccancies and Electrical conduction in Metal Oxides. Phys. Rev. 1964, 133, A1431. [Google Scholar] [CrossRef]
- Hossain, F.M.; Murch, G.E.; Sheppard, L.; Nowotny, J. The Effect of Defect Disorder on the Electronic Structure of Rutile TiO2−x. Defect Diffus. Forum 2006, 251–252, 1–12. [Google Scholar] [CrossRef]
- Guo, Q.; Zhou, C.; Ma, Z.; Ren, Z.; Fan, H. Fundamental Processes in Surface Photocatalysis on TiO2. In Heterogeneous Photocatalysis—From Fundamentals to Green Applications; Springer: London, UK; pp. 361–370. ISBN 978-3-662-48717-4.361-370.
- Sekiya, T.; Yagisawa, T.; Kamiya, N.; Mulmi, D.D.; Kurita, S.; Murakami, Y.; Kodaira, T. Defects in anatase TiO2 single crystal controlled by heat treatments. J. Phys. Soc. Jpn. 2004, 73, 703–710. [Google Scholar] [CrossRef]
- Kitada, A.; Hasegawa, G.; Kobayashi, Y.; Kanamori, K.; Nakanishi, K.; Kageyama, H. Selective preparation of macroporous monoliths of conductive titanium oxides TinO2n−1 (n = 2, 3, 4, 6). J. Am. Chem. Soc. 2012, 134, 10894–10898. [Google Scholar] [CrossRef] [PubMed]
- Eder, D.; Kramer, R. Stoichiometry of titanium suboxide Part 2 Electric properties. Phys. Chem. Chem. Phys. 2003, 5, 1314–1319. [Google Scholar] [CrossRef]
- Kieslich, G.; Burkhardt, U.; Birkel, C.S.; Veremchuk, I.; Douglas, J.E.; Gaultois, M.W.; Lieberwirth, I.; Seshadri, R.; Stucky, G.D.; Grin, Y.; et al. Enhanced thermoelectric properties of the n-type Magneli phase WO2.90: Reduced thermal conductivity through microstructure engineering. J. Mater. Chem. A 2014, 2, 13492–13497. [Google Scholar] [CrossRef]
- Nowotny, J.; Radecka, M.; Rekas, M. Semiconducting properties of undoped TiO2. J. Phys. Chem Solids 1997, 58, 927–937. [Google Scholar] [CrossRef]
- Shibuya, T.; Yasuoka, K.; Mirbt, S.; Sanyal, B. A systematic study of polarons due to oxygen vacancy formation at the rutile TiO(110) surface by GGA+U and HSE06 methods. J. Phys. Condens. Matter 2012, 24. [Google Scholar] [CrossRef] [PubMed]
- Gono, P.; Wiktor, J.; Ambrosio, F.; Pasquarello, A. Surface Polarons Reducing Overpotentials in the Oxygen Evolution Reaction. ACS Catal. 2018, 8, 5847–5851. [Google Scholar] [CrossRef]
- Gravelle, P.C.; Juillet, F.; Meriaudeau, P.; Teichner, S.J. Surface reactivity of reduced titanium dioxide. Discuss. Faraday Soc. 1971, 52, 140–148. [Google Scholar] [CrossRef]
- Amakawa, K.; Sun, L.; Guo, C.; Hävecker, M.; Kube, P.; Wachs, I.E.; Lwin, S.; Frenkel, A.I.; Patlolla, A.; Hermann, K.; et al. How Strain Affects the Reactivity of Surface Metal Oxide Catalysts. Angew. Chem. Int. Ed. 2013, 52, 13553–13557. [Google Scholar] [CrossRef]
- Horikoshi, S.; Minatodani, Y.; Tsutsumi, H.; Uchida, H.; Abe, M.; Serpone, N. Influence of lattice distortion and oxygen vacancies on the uv-driven/microwave-assisted TiO2 photocatalysis. J. Photochem. Photobiol. A Chem. 2013, 265, 20–28. [Google Scholar] [CrossRef]
- Aschauer, U.; He, Y.; Cheng, H.; Li, S.C.; Diebold, U.; Selloni, A. Influence of subsurface defects on the surface reactivity of TiO2: Water on anatase (101). J. Phys. Chem. C 2010, 114, 1278–1284. [Google Scholar] [CrossRef]
- Schaub, R.; Thostrup, P.; Lopez, N.; Lægsgaard, E.; Stensgaard, I.; Nørskov, J.K.; Besenbacher, F. Oxygen vacancies as active sites for water dissociation on rutile TiO2(110). Phys. Rev. Lett. 2001, 87. [Google Scholar] [CrossRef] [PubMed]
- Jensen, S.C.; Friend, C.M. The dynamic roles of interstitial and surface defects on oxidation and reduction reactions on titania. Top. Catal. 2013, 56, 1377–1388. [Google Scholar] [CrossRef]
- Tilocca, A.; Selloni, A. Reaction pathway and free energy barrier for defect-induced water dissociation on the (101) surface of TiO2-anatase. J. Chem. Phys. 2003, 119, 7445–7450. [Google Scholar] [CrossRef]
- Petrik, N.G.; Kimmel, G.A. Reaction Kinetics of Water Molecules with Oxygen Vacancies on Rutile TiO2(110). J. Phys. Chem. C 2015, 119, 23059–23067. [Google Scholar] [CrossRef]
- Kolbrecka, K.; Przyluski, J. Sub-stoichiometric titanium oxides as ceramic electrodes for oxygen evolution-structural aspects of the voltammetric behaviour of TinO2n−1. Electrochim. Acta 1994, 39, 1591–1595. [Google Scholar] [CrossRef]
- Shan, Z.; Archana, P.S.; Shen, G.; Gupta, A.; Bakker, M.G.; Pan, S. NanoCOT: Low-Cost Nanostructured Electrode Containing Carbon, Oxygen, and Titanium for Efficient Oxygen Evolution Reaction. J. Am. Chem. Soc. 2015, 137, 11996–12005. [Google Scholar] [CrossRef]
- Zhang, Z.; Cao, K.; Yates, J.T. Defect-electron spreading on the TiO2(110) semiconductor surface by water adsorption. J. Phys. Chem. Lett. 2013, 4, 674–679. [Google Scholar] [CrossRef]
- Nosaka, Y.; Nosaka, A.Y. Generation and Detection of Reactive Oxygen Species in Photocatalysis. Chem. Rev. 2017, 117, 11302–11336. [Google Scholar] [CrossRef]
- Imanishi, A.; Okamura, T.; Ohashi, N.; Nakamura, R.; Nakato, Y. Mechanism of water photooxidation, at n-TiO2 (rutile) (110) and (100) surfaces: Dependence on solution pH. J. Am. Chem. Soc. 2007, 2, 11569–11578. [Google Scholar] [CrossRef] [PubMed]
- Komaguchi, K.; Maruoka, T.; Nakano, H.; Imae, I.; Ooyama, Y.; Harima, Y. Electron-transfer reaction of oxygen species on TiO2 nanoparticles induced by Sub-band-gap illumination. J. Phys. Chem. C 2010, 114, 1240–1245. [Google Scholar] [CrossRef]
- Pan, L.; Wang, S.; Zou, J.J.; Huang, Z.F.; Wang, L.; Zhang, X. Ti3+-defected and V-doped TiO2 quantum dots loaded on MCM-41. Chem. Commun. 2014, 50, 988–990. [Google Scholar] [CrossRef]
- Fujishima, A.; Zhang, X.; Tryk, D.A. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Di Paola, A.; Bellardita, M.; Palmisano, L.; Barbieriková, Z.; Brezová, V. Influence of crystallinity and OH surface density on the photocatalytic activity of TiO2 powders. J. Photochem. Photobiol. A Chem. 2014, 273, 59–67. [Google Scholar] [CrossRef]
- Radecka, M.; Trenczek-Zajac, A.; Zakrzewska, K.; Rekas, M. Effect of oxygen nonstoichiometry on photo-electrochemical properties of TiO2−x. J. Power Sources 2007, 173, 816–821. [Google Scholar] [CrossRef]
- Wheeler, D.A.; Ling, Y.; Dillon, R.J.; Fitzmorris, R.C.; Dudzik, C.G.; Zavodivker, L.; Rajh, T.; Dimitrijevic, N.M.; Millhauser, G.; Bardeen, C.; et al. Probing the nature of bandgap states in hydrogen-treated TiO2 nanowires. J. Phys. Chem. C 2013, 117, 26821–26830. [Google Scholar] [CrossRef]
- Lo, H.H.; Gopal, N.O.; Ke, S.C. Origin of photoactivity of oxygen-deficient TiO2 under visible light. Appl. Phys. Lett. 2009, 95, 2007–2010. [Google Scholar] [CrossRef]
- Zhuang, J.; Weng, S.; Dai, W.; Liu, P.; Liu, Q. Effects of interface defects on charge transfer and photoinduced properties of TiO2 bilayer films. J. Phys. Chem. C 2012, 116, 25354–25361. [Google Scholar] [CrossRef]
- Ma, S.; Huang, S.D.; Fang, Y.H.; Liu, Z.P. TiH Hydride Formed on Amorphous Black Titania: Unprecedented Active Species for Photocatalytic Hydrogen Evolution. ACS Catal. 2018, 8, 9711–9721. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, H.; Tan, S.; Feng, H.; Cheng, Z.; Zhao, J.; Zhao, A.; Wang, B.; Luo, Y.; Yang, J.; et al. Role of point defects on the reactivity of reconstructed anatase titanium dioxide (001) surface. Nat. Commun. 2013, 4, 1–8. [Google Scholar] [CrossRef]
- Wan, Z.; Huang, G.F.; Huang, W.Q.; Jiao, C.; Yan, X.G.; Yang, Z.M.; Zhang, Q. The enhanced photocatalytic activity of Ti3+ self-doped TiO2 by a reduction method. Mater. Lett. 2014, 122, 33–36. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, N.; Schmuki, P. Photocatalysis with TiO2 Nanotubes: “Colorful” Reactivity and Designing Site-Specific Photocatalytic Centers into TiO2 Nanotubes. ACS Catal. 2017, 7, 3210–3235. [Google Scholar] [CrossRef]
- Choudhury, B.; Choudhury, A. Oxygen defect dependent variation of band gap, Urbach energy and luminescence property of anatase, anatase-rutile mixed phase and of rutile phases of TiO2 nanoparticles. Phys. E Low Dimens. Syst. Nanostruct. 2014, 56, 364–371. [Google Scholar] [CrossRef]
- Das, T.K.; Ilaiyaraja, P.; Mocherla, P.S.V.; Bhalerao, G.M.; Sudakar, C. Influence of surface disorder, oxygen defects and bandgap in TiO2 nanostructures on the photovoltaic properties of dye sensitized solar cells. Sol. Energy Mater. Sol. Cells 2016, 144, 194–209. [Google Scholar] [CrossRef]
- Pan, S.; Liu, X.; Guo, M.; Yu, S.F.; Huang, H.; Fan, H.; Li, G. Engineering the intermediate band states in amorphous Ti3+-doped TiO2 for hybrid dye-sensitized solar cell applications. J. Mater. Chem. A 2015, 3, 11437–11443. [Google Scholar] [CrossRef]
- Bharti, B.; Kumar, S.; Lee, H.-N.; Kumar, R. Formation of oxygen vacancies and Ti3+ state in TiO2 thin film and enhanced optical properties by air plasma treatment. Sci. Rep. 2016, 6, 32355. [Google Scholar] [CrossRef] [PubMed]
- Saputera, W.H.; Mul, G.; Hamdy, M.S. Ti3+-containing titania: Synthesis tactics and photocatalytic performance. Catal. Today 2015, 246, 60–66. [Google Scholar] [CrossRef]
- Zhu, G.; Shan, Y.; Lin, T.; Zhao, W.; Xu, J.; Tian, Z.; Zhang, H.; Zheng, C.; Huang, F. Hydrogenated blue titania with high solar absorption and greatly improved photocatalysis. Nanoscale 2016, 8, 4705–4712. [Google Scholar] [CrossRef]
- Wei, W.; Yaru, N.; Chunhua, L.; Zhongzi, X. Hydrogenation of TiO2 nanosheets with exposed {001} facets for enhanced photocatalytc activity. RSC Adv. 2012, 2, 8286–8288. [Google Scholar] [CrossRef]
- Ali, A.; Ruzybayev, I.; Yassitepe, E.; Karim, A.; Shah, S.I.; Bhatti, A.S. Phase transformations in the pulsed laser deposition grown TiO2 thin films as a consequence of O2 partial pressure and Nd doping. J. Phys. Chem. C 2015, 119, 11578–11587. [Google Scholar] [CrossRef]
- Lepcha, A.; Maccato, C.; Mettenbörger, A.; Andreu, T.; Mayrhofer, L.; Walter, M.; Olthof, S.; Ruoko, T.P.; Klein, A.; Moseler, M.; et al. Electrospun Black Titania Nanofibers: Influence of Hydrogen Plasma-Induced Disorder on the Electronic Structure and Photoelectrochemical Performance. J. Phys. Chem. C 2015, 119, 18835–18842. [Google Scholar] [CrossRef]
- Huang, C.N.; Bow, J.S.; Zheng, Y.; Chen, S.Y.; Ho, N.J.; Shen, P. Nonstoichiometric titanium oxides via pulsed laser ablation in water. Nanoscale Res. Lett. 2010, 5, 972–985. [Google Scholar] [CrossRef] [PubMed]
- Forster, M.; Potter, R.J.; Ling, Y.; Yang, Y.; Klug, D.R.; Cowan, A.J. Oxygen deficient α-Fe2O3 photoelectrodes: A balance between enhanced electrical properties and trap-mediated losses. Chem. Sci. 2015, 6, 4009–4016. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Li, W.; Wang, J.; Qu, Y.; Yang, Y.; Xie, Y.; Zhang, K.; Wang, L.; Fu, H.; Zhao, D. Ordered Mesoporous Black TiO2 as Highly Efficient Hydrogen Evolution Photocatalyst. J. Am. Chem. Soc. 2014, 136, 9280–9283. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, Y. Enhancing the capacitance of TiO2 nanotube arrays by a facile cathodic reduction process. J. Power Sources 2013, 239, 128–131. [Google Scholar] [CrossRef]
- Lin, T. Effective nonmetal incorporation in black titania with enhanced solar energy utilization. Energy Environ. Sci. 2014, 6. [Google Scholar] [CrossRef]
- Cui, H.; Zhao, W.; Yang, C.; Yin, H.; Lin, T.; Shan, Y.; Xie, Y.; Gu, H.; Huang, F. Black TiO2 nanotube arrays for high-efficiency photoelectrochemical water-splitting. J. Mater. Chem. A 2014, 8612–8616. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, C.; Lin, T.; Yin, H.; Chen, P.; Wan, D.; Xu, F.; Huang, F.; Lin, J.; Xie, X.; et al. H-doped black titania with very high solar absorption and excellent photocatalysis enhanced by localized surface plasmon resonance. Adv. Funct. Mater. 2013, 23, 5444–5450. [Google Scholar] [CrossRef]
- Pei, D.-N.; Gong, L.; Zhang, A.-Y.; Zhang, X.; Chen, J.-J.; Mu, Y.; Yu, H.-Q. Defective titanium dioxide single crystals exposed by high-energy {001} facets for efficient oxygen reduction. Nat. Commun. 2015, 6, 8696. [Google Scholar] [CrossRef] [Green Version]
- Bonanni, S.; Aït-Mansour, K.; Harbich, W.; Brune, H. Effect of the TiO2 reduction state on the catalytic CO oxidation on deposited size-selected Pt clusters. J. Am. Chem. Soc. 2012, 134, 3445–3450. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Yang, M.; Fu, X.; Zhang, N.; Xu, Y. Defective TiO2 with oxygen vacancies: Synthesis, properties and photocatalytic applications. Nanoscale 2013, 5, 3601. [Google Scholar] [CrossRef]
- Tan, L.L.; Ong, W.J.; Chai, S.P.; Goh, B.T.; Mohamed, A.R. Visible-light-active oxygen-rich TiO2 decorated 2D graphene oxide with enhanced photocatalytic activity toward carbon dioxide reduction. Appl. Catal. B Environ. 2015, 179, 160–170. [Google Scholar] [CrossRef]
- Ramchiary, A.; Samdarshi, S.K. Hydrogenation based disorder-engineered visible active N-doped mixed phase titania. Sol. Energy Mater. Sol. Cells 2015, 134, 381–388. [Google Scholar] [CrossRef]
- Kumar, V.; Ntwaeaborwa, O.M.; Holsa, J.; Motaung, D.E.; Swart, H.C. The role of oxygen and titanium related defects on the emission of TiO2: Tb3+ nano-phosphor for blue lighting applications. Opt. Mater. 2015, 46, 510–516. [Google Scholar] [CrossRef]
- Kang, Q.; Cao, J.; Zhang, Y.; Liu, L.; Xu, H.; Ye, J. Reduced TiO2 nanotube arrays for photoelectrochemical water splitting. J. Mater. Chem. A 2013, 1, 5766–5774. [Google Scholar] [CrossRef]
- Su, J.; Zou, X.; Zou, Y.; Li, G.; Wang, P.; Chen, J. Porous Titania with Heavily Self-Doped Ti3+ for Specific Sensing of CO at Room Temperature. Inorg. Chem. 2013, 52, 5924–5930. [Google Scholar] [CrossRef] [PubMed]
- Zhao, A.; Masa, J.; Xia, W. Oxygen-deficient titania as alternative support for Pt catalysts for the oxygen reduction reaction. J. Energy Chem. 2014, 23, 701–707. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, Y. Electrochemically Self-Doped TiO2 Nanotube Arrays for Supercapacitors. J. Phys. Chem. C 2014, 118, 5626–5636. [Google Scholar] [CrossRef]
- Li, Y.; Weng, Y.; Zhang, J.; Ding, J.; Zhu, Y.; Wang, Q.; Yang, Y.; Cheng, Y.; Zhang, Q.; Li, P.; et al. Observation of superconductivity in structure-selected Ti2O3 thin films. NPG Asia Mater. 2018, 10, 522–532. [Google Scholar] [CrossRef]
- Wei, X.; Skomski, R.; Balamurugan, B.; Sun, Z.G.; Ducharme, S.; Sellmyer, D.J. Magnetism of TiO and TiO2 nanoclusters. J. Appl. Phys. 2009, 105. [Google Scholar] [CrossRef]
- Han, X.; Huang, J.; Jing, X.; Yang, D.; Lin, H.; Wang, Z.; Li, P.; Chen, Y. Oxygen-Deficient Black Titania for Synergistic/Enhanced Sonodynamic and Photoinduced Cancer Therapy at Near Infrared-II Biowindow. ACS Nano 2018, 12, 4545–4555. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.L.; Ong, W.J.; Chai, S.P.; Mohamed, A.R. Band gap engineered, oxygen-rich TiO2 for visible light induced photocatalytic reduction of CO2. Chem. Commun. 2014, 50, 6923–6926. [Google Scholar] [CrossRef] [PubMed]
- Chong, S.V.; Kadowaki, K.; Xia, J.; Idriss, H. Interesting magnetic behavior from reduced titanium dioxide nanobelts. Appl. Phys. Lett. 2008, 92, 1–4. [Google Scholar] [CrossRef]
- Guillemot, F.; Porté, M.C.; Labrugère, C.; Baquey, C. Ti4+ to Ti3+ conversion of TiO2 uppermost layer by low-temperature vacuum annealing: Interest for titanium biomedical applications. J. Colloid Interface Sci. 2002, 255, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.M.; Ruan, J.M.; Zhou, Z.C.; Sang, S.B. Magneli phase titanium sub-oxide conductive ceramic TinO2n−1 as support for electrocatalyst toward oxygen reduction reaction with high activity and stability. J. Cent. South Univ. 2015, 22, 1212–1219. [Google Scholar] [CrossRef]
- Li, X.; Zhu, A.L.; Qu, W.; Wang, H.; Hui, R.; Zhang, L.; Zhang, J. Magneli phase Ti4O7 electrode for oxygen reduction reaction and its implication for zinc-air rechargeable batteries. Electrochim. Acta 2010, 55, 5891–5898. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, T.; Le, L.; Ruan, X.; Fang, P.; Pan, C.; Xiong, R.; Shi, J.; Wei, J. Quick and facile preparation of visible light-driven TiO2 photocatalyst with high absorption and photocatalytic activity. Sci. Rep. 2014, 4, 7045. [Google Scholar] [CrossRef]
- Liu, Y.; Xing, M.; Zhang, J. Ti3+ and carbon co-doped TiO2 with improved visible light photocatalytic activity. Chin. J. Catal. 2014, 35, 1511–1519. [Google Scholar] [CrossRef]
- Shahid, M.; Choi, S.Y.; Liu, J.; Kwon, Y.U. Reduced titania films with ordered nanopores and their application to visible light water splitting. Bull. Korean Chem. Soc. 2013, 34, 2271–2275. [Google Scholar] [CrossRef]
- Ren, Y.; Li, J.; Yu, J. Enhanced electrochemical performance of TiO2 by Ti3+ doping using a facile solvothermal method as anode materials for lithium-ion batteries. Electrochim. Acta 2014, 138, 41–47. [Google Scholar] [CrossRef]
- Xia, T.; Zhang, W.; Wang, Z.; Zhang, Y.; Song, X.; Murowchick, J.; Battaglia, V.; Liu, G.; Chen., X. Amorphous carbon-coated TiO2 nanocrysals for improved lithium-ion battery and photocatalytic performance. Nano Energy 2014, 6, 109–118. [Google Scholar] [CrossRef]
- Liu, H.; Yang, W.; Ma, Y.; Yao, J. Extended visible light response of binary TiO2-Ti2O3 photocatalyst prepared by a photo-assisted sol-gel method. Appl. Catal. A Gen. 2006, 299, 218–223. [Google Scholar] [CrossRef]
- Yin, G.; Huang, X.; Chen, T.; Zhao, W.; Bi, Q.; Xu, J.; Han, Y.; Huang, F. Hydrogenated Blue Titania for Efficient Solar to Chemical Conversions: Preparation, Characterization, and Reaction Mechanism of CO2 Reduction. ACS Catal. 2018, 8, 1009–1017. [Google Scholar] [CrossRef]
- Yeh, Y.C.; Li, S.S.; Wu, C.C.; Shao, T.W.; Kuo, P.C.; Chen, C.W. Stoichiometric dependence of TiOx as a cathode modifier on band alignment of polymer solar cells. Sol. Energy Mater. Sol. Cells 2014, 125, 233–238. [Google Scholar] [CrossRef]
- Pan, S.S.; Lu, W.; Zhao, Y.H.; Tong, W.; Li, M.; Jin, L.M.; Choi, J.Y.; Qi, F.; Chen, S.G.; Fei, L.F.; et al. Self-doped rutile titania with high performance for direct and ultrafast assay of H2O2. ACS Appl. Mater. Interfaces 2013, 5, 12784–12788. [Google Scholar] [CrossRef]










| Bulk | Surface |
|---|---|
| Interstitial atom | Adatom |
| Vacancy | Vacancy |
| Interstitial Cluster | Adatom island |
| Vacancy Cluster | Vacancy island |
| Kick-in/Kick-out | Exhange diffusion |
| Vacancy-interstitial formation | Vacancy-adatom formation |
| Kroger-Vink Notation | Meaning |
|---|---|
| Ti3+ ion in the interstitial site | |
| Ti4+ ion in the interstitial site | |
| Oxygen vacancy | |
| O2− ion in the interstitial site | |
| Titanium vacancy |
| Compound | X in TiOx | Structure |
|---|---|---|
| TiO2 | 2 | Rutile |
| Ti10O19 | 1.9 | Anatase |
| Ti9O17 | 1.89 | Triclinic |
| Ti8O15 | 1.875 | Triclinic |
| Ti7O13 | 1.857 | Triclinic |
| Ti6O11 | 1.833 | Triclinic |
| Ti5O9 | 1.8 | Triclinic |
| Ti4O7 | 1.75 | Triclinic |
| γTi3O5 | 1.67 | Monoclinic |
| Ti2O3 | 1.5 | Tetragonal |
| TiO | 1.0 | Hexagonal Cubic Monoclinic |
| Ti2O | 0.5 | Hexagonal |
| Ti | 0 | Hexagonal |
| m | Predominant Defects | Conductivity Type |
|---|---|---|
| 6 | , e | n |
| 4 | n | |
| 5 | , , e’ | n |
| ∞ | h● | p |
| 5 | , h● | p |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jayashree, S.; Ashokkumar, M. Switchable Intrinsic Defect Chemistry of Titania for Catalytic Applications. Catalysts 2018, 8, 601. https://doi.org/10.3390/catal8120601
Jayashree S, Ashokkumar M. Switchable Intrinsic Defect Chemistry of Titania for Catalytic Applications. Catalysts. 2018; 8(12):601. https://doi.org/10.3390/catal8120601
Chicago/Turabian StyleJayashree, Swaminathan, and Meiyazhagan Ashokkumar. 2018. "Switchable Intrinsic Defect Chemistry of Titania for Catalytic Applications" Catalysts 8, no. 12: 601. https://doi.org/10.3390/catal8120601





