Rh/CeO2 Thin Catalytic Layer Deposition on Alumina Foams: Catalytic Performance and Controlling Regimes in Biogas Reforming Processes
Abstract
:1. Introduction
2. Results
2.1. Characterization of Samples
2.1.1. Geometrical Properties of OCFs Structures
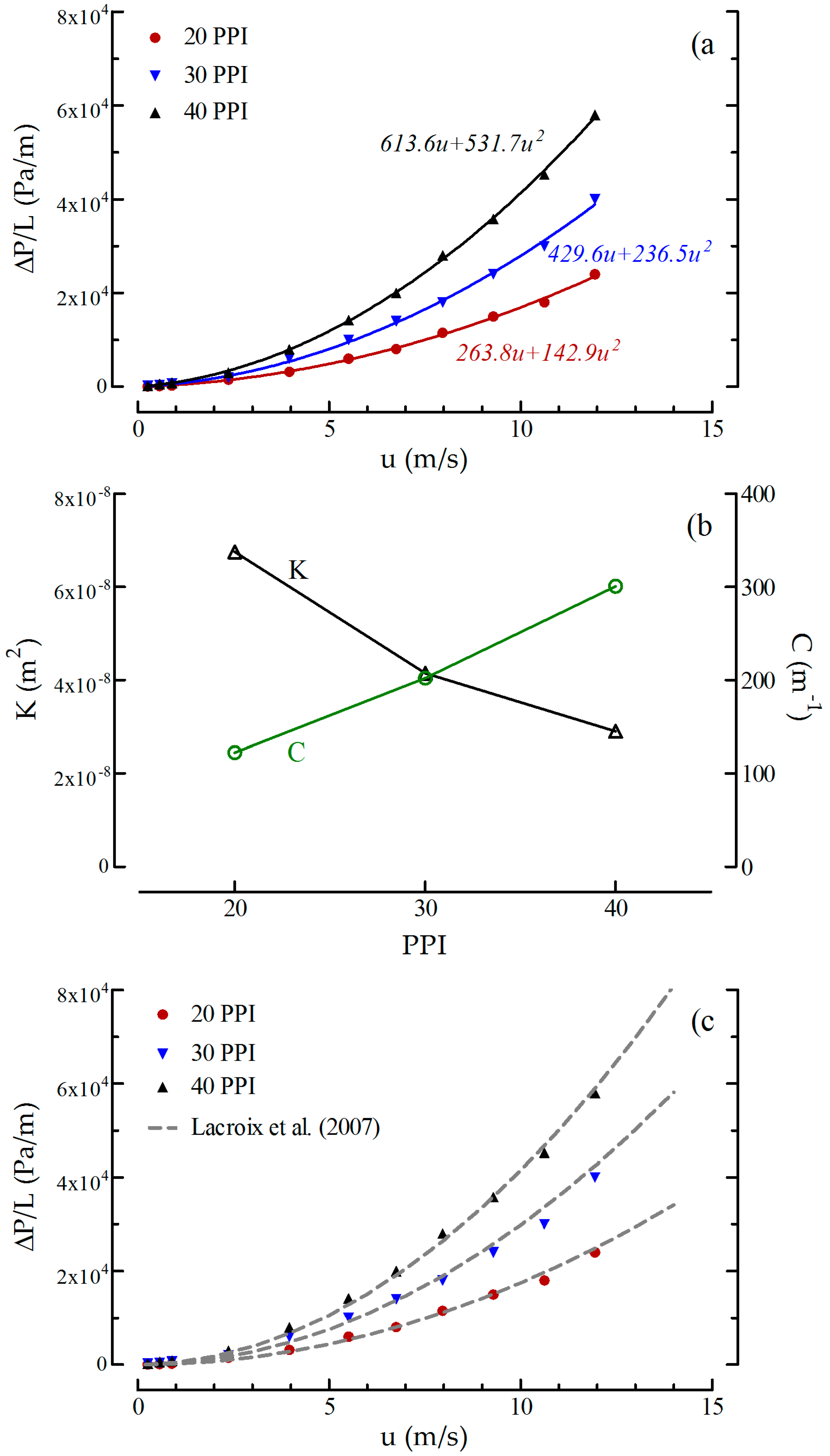
2.1.2. Pressure Drop Measurements
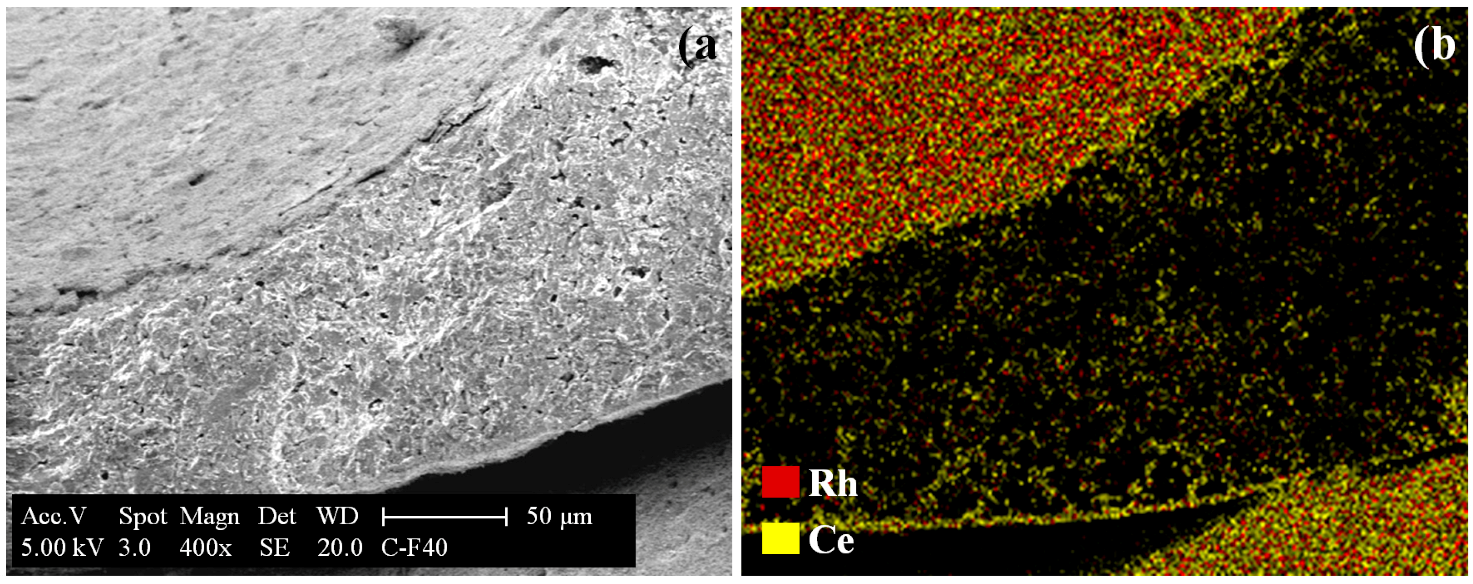
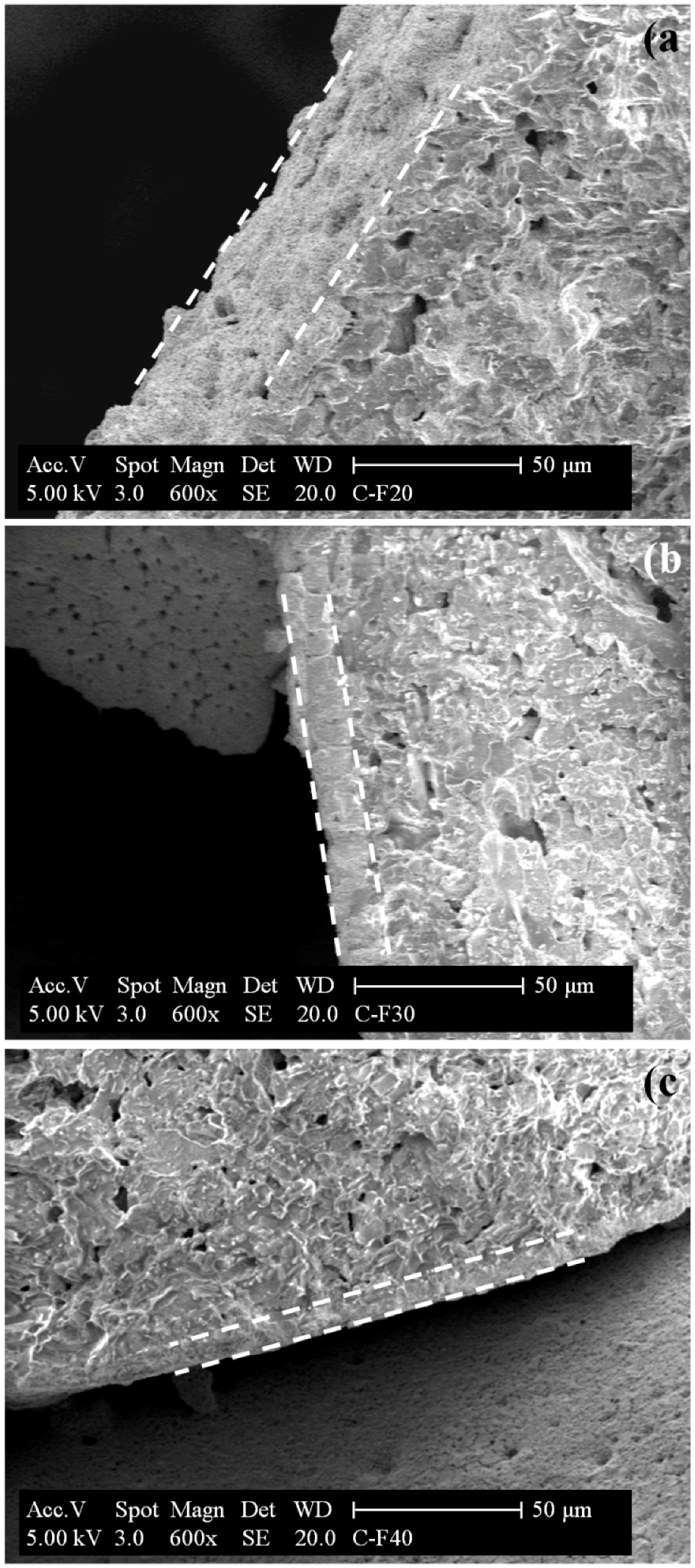
2.1.3. SEM/EDX Measurements
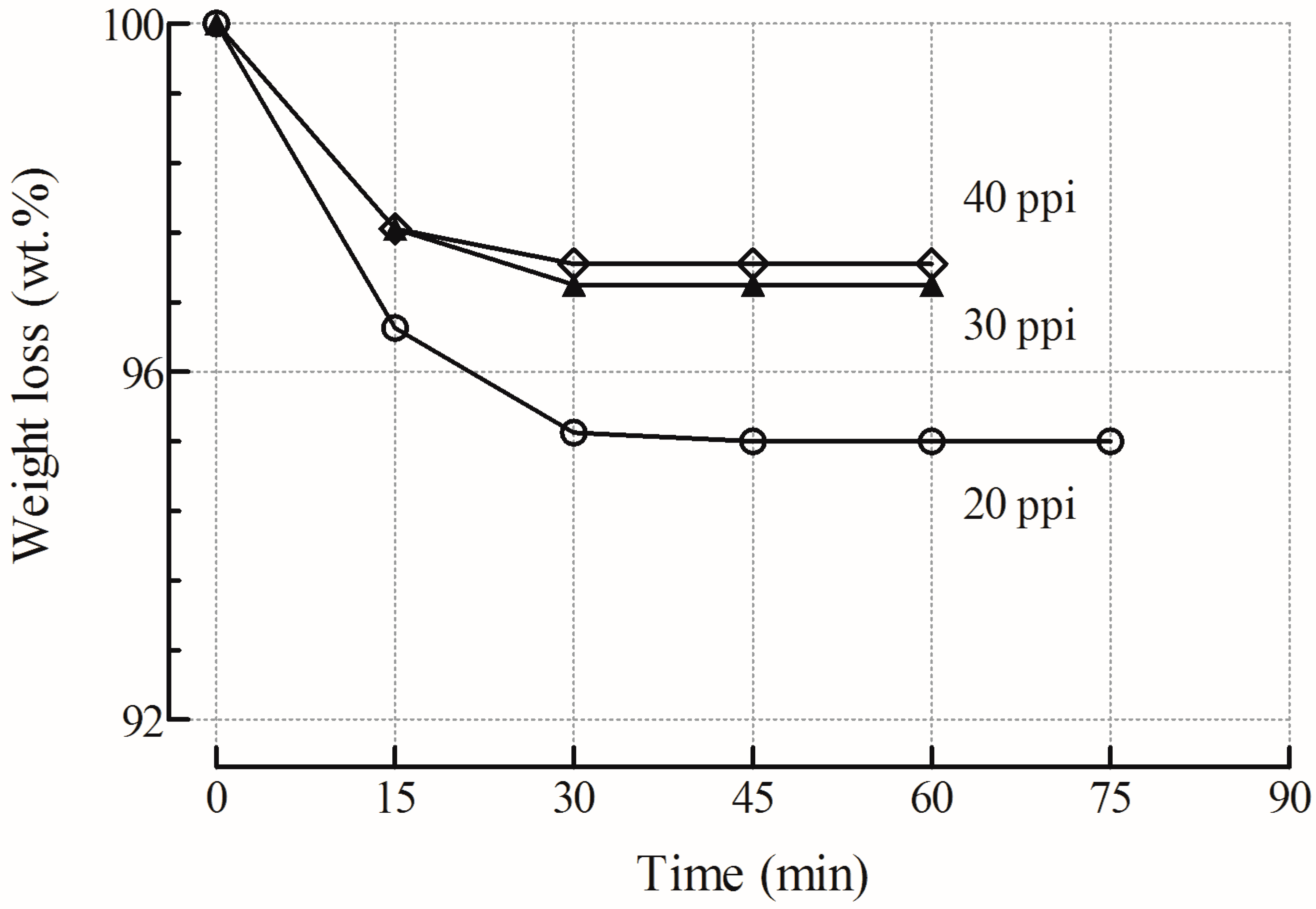
2.1.4. Adhesion Measurements
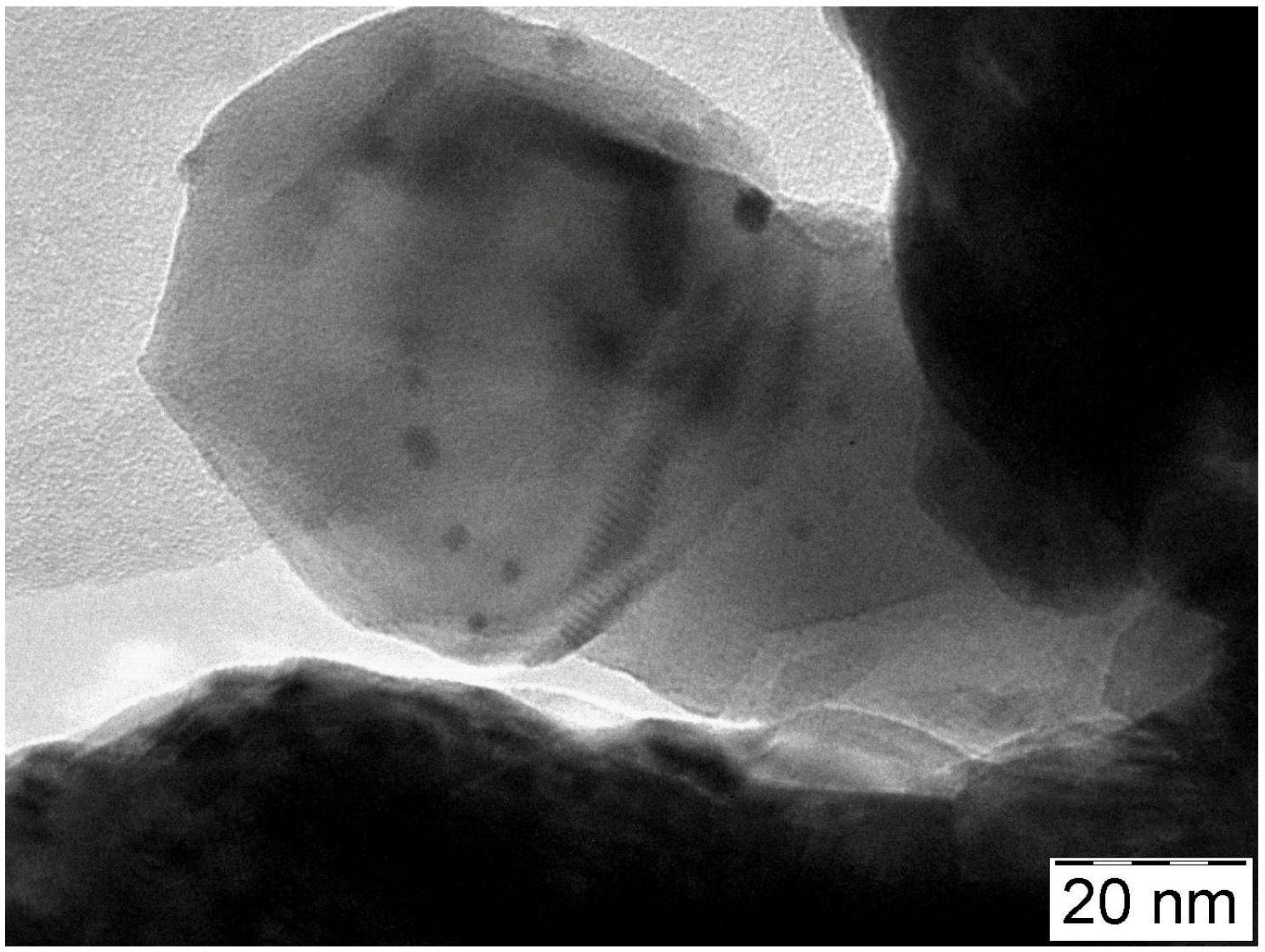
2.1.5. TEM Measurements
2.2. Catalytic Tests towards Biogas Steam and Oxy-Steam Reforming
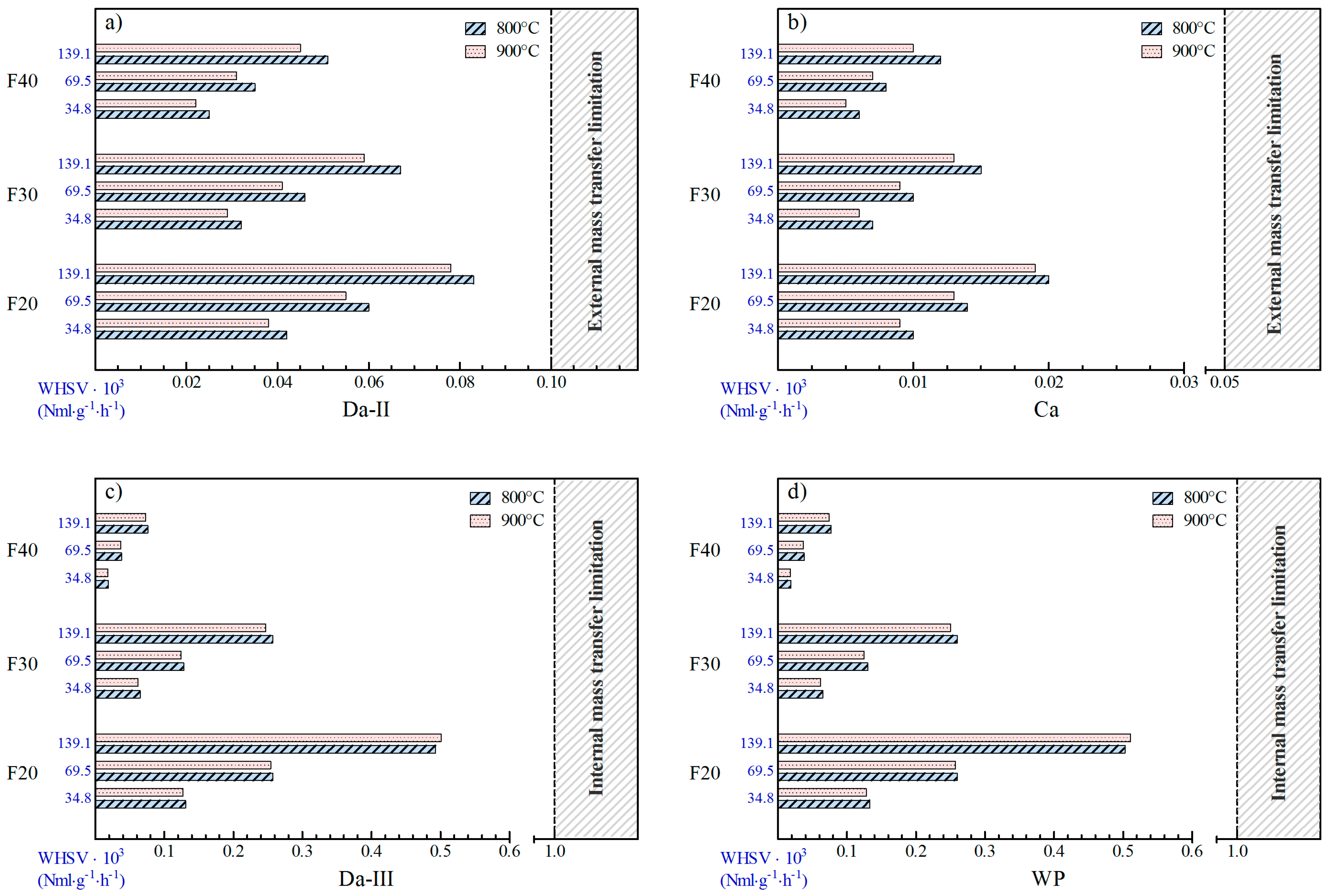
2.2.1. Evaluation of External Interphase (Gas-Solid) and Internal Heat Transfer Limitations
2.2.2. Evaluation of External and Internal Mass Transfer Limitations
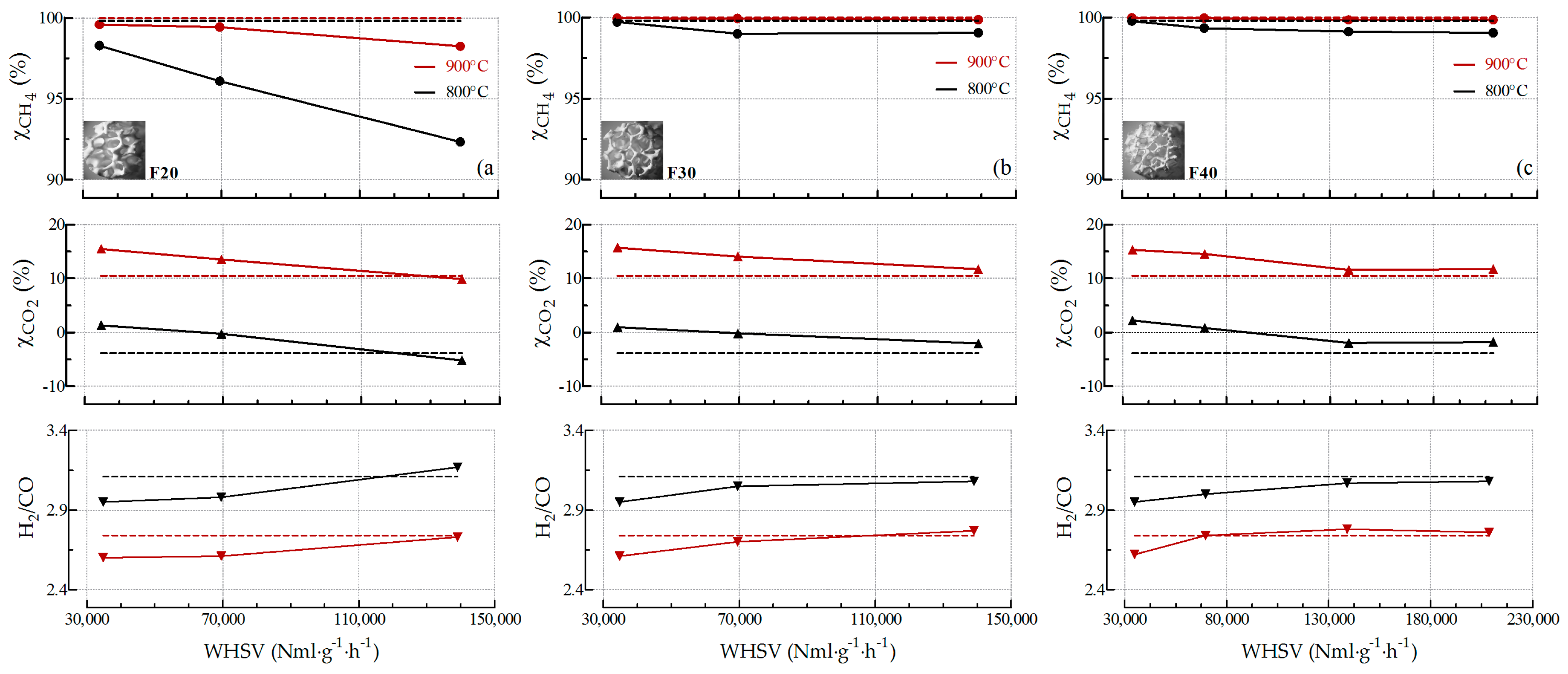
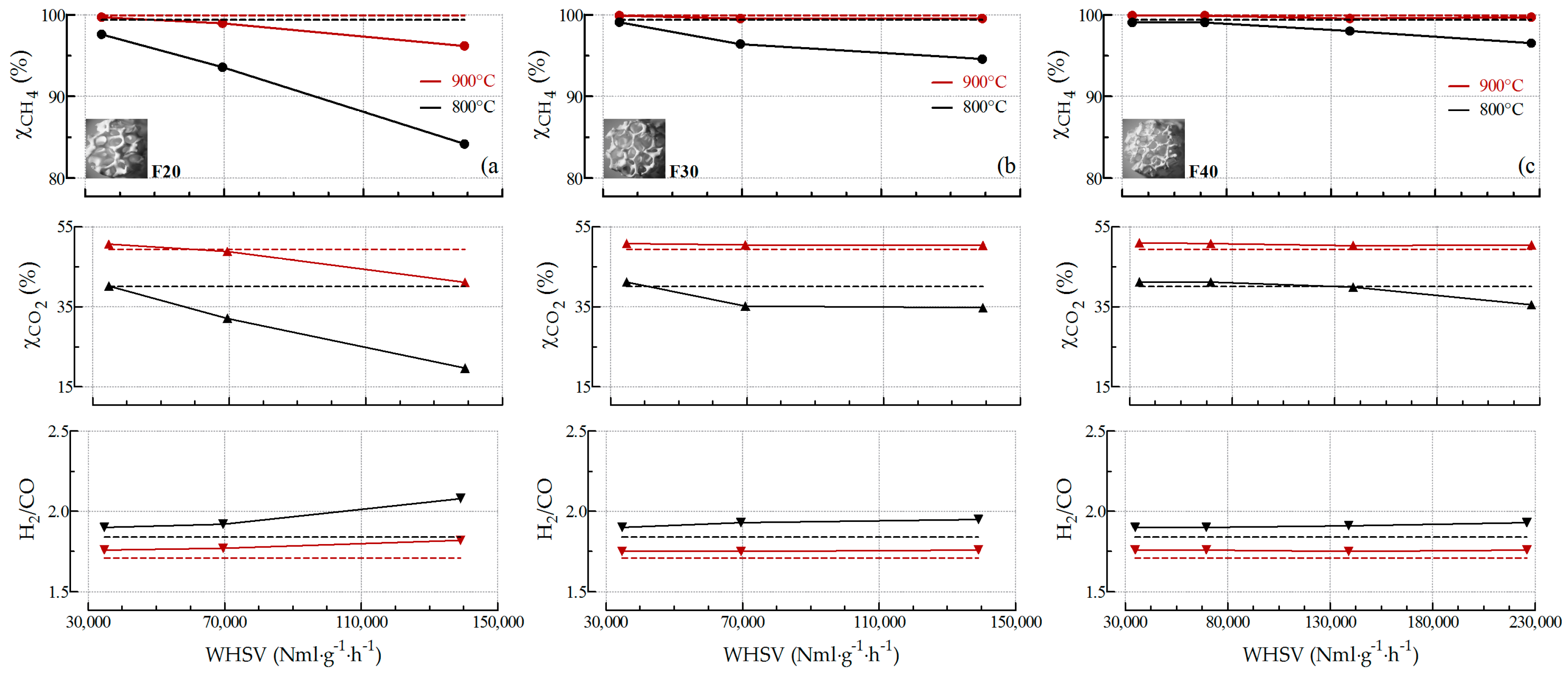
2.2.3. Influence of the Pore Density on Biogas SR and OSR Activity
2.2.4. Stability towards SR and OSR Processes
3. Discussion
4. Experimental Section
4.1. Chemicals and Foams
4.2. Catalysts Preparation
4.3. Physicochemical Characterization
4.4. Catalytic Tests
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| OCFs properties | dp | Average pore diameter (m) |
| df | Average face diameter (m) | |
| GSA | Geometric surface area (m2·m−3) | |
| L | Length (m) | |
| OFA | Open frontal area (m2) | |
| ε | Voidage | |
| ϕ | Diameter (m) | |
| Coated layer properties | dp,c | Average coated pore diameter (m) |
| df,c | Average coated face diameter (m) | |
| rp | Pore radius (m) | |
| δc | Coated layer thickness (m) | |
| εc | Coated layer porosity | |
| λc | Coated layer effective thermal conductivity (kW·m−1·K−1) | |
| ρc | Coated layer density (kg·m−3) | |
| τ | Tortuosity factor | |
| Reaction data | Ea | Apparent activation energy (J·mol−1) |
| Ftot | Total gas flow rate (m3·s−1) | |
| P | Reaction pressure (kPa) | |
| T | Reaction temperature (K) | |
| Tb | Bulk fluid temperature (K) | |
| TIN | Inlet bed temperature (K) | |
| TOUT | Outlet bed temperature (K) | |
| Reaction rate for CH4 (kmol·kg−1·s−1) | ||
| Volumetric reaction rate for CH4 (kmol·m−3·s−1) | ||
| Standard reaction enthalpy (J·mol−1) | ||
| Dimensionless Numbers | Ca | Carberry number |
| E1 | Ergun Constant | |
| E2 | Ergun Constant | |
| Nu | Nusselt number | |
| Re | Reynold number | |
| Sc | Schmidt number | |
| Sh | Sherwood number | |
| WP | Weisz-Prater number | |
| Fluid Properties | Methane concentration in feed mixture (kmol·m−3) | |
| Methane concentration at catalyst surface (kmol·m−3) | ||
| Diffusivity of CH4 in gas phase (m2·s−1) | ||
| Effective diffusivity of CH4 in coated layer (m2·s−1) | ||
| Binary diffusion of CH4 and i gas species (m2·s−1) | ||
| Dk | Knudsen diffusion (m2·s−1) | |
| h | Gas-solid heat transfer coefficient (kW·m−2·K−1) | |
| kG | Mass transfer coefficient of CH4 (m·s−1) | |
| Molecular weight of CH4 (kg·kmol−1) | ||
| Mi | Molecular weight of i compound (kg·kmol−1) | |
| Mj | Molecular weight of j compound (kg·kmol−1) | |
| Mmix | Molecular weight of gas mixture (kg·kmol−1) | |
| Pc,i | Critical pressure of i compound (kPa) | |
| R | Universal gas constant (J·mol−1·K−1) | |
| Tc,i | Critical temperature of i compound (K) | |
| u | Inlet gas velocity (m·s−1) | |
| Mole fraction of i compound | ||
| λi | Thermal conductivity of i compound (kW·m−1·K−1) | |
| λmix | Thermal conductivity of gas mixture (kW·m−1·K−1) | |
| Viscosity of H2 (kg·m−1·s−1) | ||
| Viscosity of H2O (kg·m−1·s−1) | ||
| μi | Viscosity of i compound (kg·m−1·s−1) | |
| μj | Viscosity of j compound (kg·m−1·s−1) | |
| μmix | Viscosity of gas mixture (kg·m−1·s−1) | |
| Viscosity of N2 (kg·m−1·s−1) | ||
| ρmix | Density of gas mixture (kg·m−3) | |
| Density of N2 (kg·m−3) | ||
| Molar volume of CH4 (cm3·mol−1) | ||
| νi | Molar volume of i compound (cm3·mol−1) | |
| Characteristic Times | tc | Characteristic contact time (s) |
| text | Characteristic external mass transfer time (s) | |
| tint | Characteristic coated layer diffusion time (s) | |
| tr | Characteristic reaction time (s) |
References
- Lewandowski, I. Bioeconomy. Shaping the Transition to a Sustainable, Biobased Economy; Springer: Berlin, Germany, 2018; pp. 5–74. ISBN 978-3-319-68152-8. [Google Scholar]
- Piemonte, V.; De Falco, M.; Basile, A. Sustainable Development in Chemical Engineering: Innovative Technologies; John Wiley & Sons Ltd.: Chichester, UK, 2013; pp. 95–118. ISBN 978-1-118-62984-0. [Google Scholar]
- Balzarotti, R.; Ciurlia, M.; Cristiani, C.; Paparella, F. Washcoat deposition of Ni- and Co-ZrO2 low surface area powders onto ceramic open-cell foams: Influence of slurry formulation and rheology. Catalysts 2015, 5, 2271–2286. [Google Scholar] [CrossRef] [Green Version]
- Specchia, S.; Ercolino, G.; Karimi, S.; Italiano, C.; Vita, A. Solution combustion synthesis for preparation of structured catalysts: A mini-review on process intensification for energy applications and pollution control. J. Self-Propag. High-Temp. Synth. 2017, 26, 166–186. [Google Scholar] [CrossRef]
- Moulin, J.A.; Stankewicz, A.; Kapteijn, F. The pontential of structured reactors in process intensification. Chem. Sustainable Dev. 2003, 11, 3–9. [Google Scholar]
- Williams, J.L. Monolith structures, materials, properties and uses. Catal. Today 2001, 69, 3–9. [Google Scholar] [CrossRef] [Green Version]
- Ercolino, G.; Karimi, S.; Stelmachowski, P.; Specchia, S. Catalytic combustion of residual methane on alumina monoliths and open cell foams coated with Pd/Co3O4. Chem. Eng. J. 2017, 326, 339–349. [Google Scholar] [CrossRef]
- Cybulski, A.; Moulijn, J.A. Structured Catalysts and Reactors, 2nd ed.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2005; pp. 1–18. ISBN 978-1-4200-2800-3. [Google Scholar]
- Pfau, S.F.; Hagens, J.E.; Dankbaar, B. Biogas between renewable energy and bio-economy policies—Opportunities and constraints resulting from a dual role. Energy. Sustain. Soc. 2017, 7, 1–15. [Google Scholar] [CrossRef]
- Nahar, G.; Mote, D.; Dupont, V. Hydrogen production from reforming of biogas: Review of technological advances and an Indian perspective. Renew. Sust. Energy Rev. 2017, 76, 1032–1052. [Google Scholar] [CrossRef] [Green Version]
- Lo Faro, M.; Vita, A.; Pino, L.; Aricò, A.S. Performance evaluation of a solid oxide fuel cell coupled to an external biogas tri-reforming process. Fuel Process. Technol. 2013, 115, 238–245. [Google Scholar] [CrossRef]
- Farhad, S.; Yoo, Y.; Hamdullahpur, F. Effects of fuel processing methods on industrial scale biogas-fuelled solid oxide fuel cell system for operating in wastewater treatment plants. J. Power Sources 2010, 195, 1446–1453. [Google Scholar] [CrossRef] [Green Version]
- Rasi, S.; Veijanen, A.; Rintala, J. Trace compounds of biogas from different biogas production plants. Energy 2007, 32, 1375–1380. [Google Scholar] [CrossRef]
- Rathod, V.; Bhale, P.V. Experimental investigation on biogas reforming for syngas production over an alumina based nickel catalyst. Energy Procedia 2014, 54, 236–245. [Google Scholar] [CrossRef]
- Lino, A.V.P.; Assaf, E.M.; Assaf, J.M. Hydrotalcites derived catalysts for syngas production from biogas reforming: Effect of nickel and cerium load. Catal. Today 2017, 289, 78–88. [Google Scholar] [CrossRef]
- Eltejaei, H.; Reza Bozorgzadeh, H.; Towfighi, J.; Reza Omidkhah, M.; Rezaei, M.; Zanganeh, R.; Zamaniyan, A.; Zarrin Ghalam, A. Methane dry reforming on Ni/Ce0.75Zr0.25O2–MgAl2O4 and Ni/Ce0.75Zr0.25O2-γ–alumina: Effects of support composition and water addition. Int. J. Hydrogen Energy 2012, 37, 4107–4118. [Google Scholar] [CrossRef]
- Löfberg, A.; Kane, T.; Guerrero-Caballero, J.; Jalowiecki-Duhamel, L. Chemical looping dry reforming of methane: Toward shale-gas and biogas valorization. Chem. Eng. Process. Process Intensif. 2017, 122, 523–529. [Google Scholar] [CrossRef]
- Lahijani, P.; Zainal, Z.A.; Mohammadi, M.; Mohamed, A.R. Conversion of the greenhouse gas CO2 to the fuel gas CO via the boudouard reaction: A review. Renew. Sustain. Energy Rev. 2015, 41, 615–632. [Google Scholar] [CrossRef]
- Vita, A.; Italiano, C.; Previtali, D.; Fabiano, C.; Palella, A.; Freni, F.; Bozzano, G.; Pino, L.; Manenti, F. Methanol synthesis from biogas: A thermodynamic analysis. Renew. Energy 2018, 118, 673–684. [Google Scholar] [CrossRef]
- Song, C.; Pan, W. Tri-reforming of methane: A novel concept for catalytic production of industrially useful synthesis gas with desired H2/CO ratios. Catal. Today 2004, 98, 463–484. [Google Scholar] [CrossRef]
- Cipitì, F.; Pino, L.; Vita, A.; Laganà, M.; Recupero, V. Model-based investigation of a CO preferential oxidation reactor for polymer electrolyte fuel cell systems. Int. J. Hydrogen Energy 2007, 32, 4040–4051. [Google Scholar] [CrossRef]
- Holladay, J.D.; Hu, J.; King, D.L.; Wang, Y. An overview of hydrogen production technologies. Catal. Today 2009, 139, 244–260. [Google Scholar] [CrossRef]
- Joensen, F.; Rostrup-Nielsen, J.R. Conversion of hydrocarbons and alcohols for fuel cells. J. Power Sources 2002, 105, 195–201. [Google Scholar] [CrossRef]
- Karakaya, M.; Keskin, S.; Avci, A.K. Parametric study of methane steam reforming to syngas in a catalytic microchannel reactor. Appl. Catal. A Gen. 2012, 411–412, 114–122. [Google Scholar] [CrossRef]
- Vita, A.; Italiano, C.; Pino, L.; Frontera, P.; Ferraro, M.; Antonucci, V. Activity and stability of powder and monolith-coated Ni/GDC catalysts for CO2 methanation. Appl. Catal. B Environ. 2018, 226, 384–395. [Google Scholar] [CrossRef]
- Frey, M.; Édouard, D.; Roger, A.-C. Optimization of structured cellular foam-based catalysts for low-temperature carbon dioxide methanation in a platelet milli-reactor. C. R. Chim. 2015, 18, 283–292. [Google Scholar] [CrossRef]
- Ricca, A.; Palma, V.; Martino, M.; Meloni, E. Innovative catalyst design for methane steam reforming intensification. Fuel 2017, 198, 175–182. [Google Scholar] [CrossRef]
- Tomašić, V.; Jović, F. State-of-the-art in the monolithic catalysts/reactors. Appl. Catal. A gen. 2006, 311, 112–121. [Google Scholar] [CrossRef]
- Weng, J.; Lu, X.; Gao, P.-X. Nano-array integrated structured catalysts: A new paradigm upon conventional wash-coated monolithic catalysts? Catalysts 2017, 7, 253. [Google Scholar] [CrossRef]
- Vita, A.; Cristiano, G.; Italiano, C.; Pino, L.; Specchia, S. Syngas production by methane oxy-steam reforming on Me/CeO2 (Me = Rh, Pt, Ni) catalyst lined on cordierite monoliths. Appl. Catal. B Environ. 2015, 162, 551–563. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Sanz, O.; Italiano, C.; Vita, A.; Montes, M.; Specchia, S. Analysis of Ru/La-Al2O3 catalyst loading on alumina monoliths and controlling regimes in methane steam reforming. Chem. Eng. J. 2018, 334, 1792–1807. [Google Scholar] [CrossRef]
- Vita, A.; Cristiano, G.; Italiano, C.; Specchia, S.; Cipitì, F.; Specchia, V. Methane oxy-steam reforming reaction: Performances of Ru/γ-Al2O3 catalysts loaded on structured cordierite monoliths. Int. J. Hydrogen Energy 2014, 39, 18592–18603. [Google Scholar] [CrossRef]
- Roy, P.S.; Song, J.; Kim, K.; Kim, J.-M.; Park, C.S.; Raju, A.S.K. Effects of CeZrO2–Al2O3 support composition of metal-foam-coated Pd–Rh catalysts for the steam-biogas reforming reaction. J. Ind. Eng. Chem. 2018, 62, 120–129. [Google Scholar] [CrossRef]
- Zapf, R.; Thiele, R.; Wichert, M.; O’Connell, M.; Ziogas, A.; Kolb, G. Application of rhodium nanoparticles for steam reforming of propane in microchannels. Catal. Commun. 2013, 41, 140–145. [Google Scholar] [CrossRef]
- Sadykov, V.; Mezentseva, N.; Fedorova, Y.; Lukashevich, A.; Pelipenko, V.; Kuzmin, V.; Simonov, M.; Ishchenko, A.; Vostrikov, Z.; Bobrova, L.; et al. Structured catalysts for steam/autothermal reforming of biofuels on heat-conducting substrates: Design and performance. Catal. Today 2015, 251, 19–27. [Google Scholar] [CrossRef]
- Govender, S.; Friedrich, H.B. Monoliths: A review of the basics, preparation methods and their relevance to oxidation. Catalysts 2017, 7, 62. [Google Scholar] [CrossRef]
- Carty, M.W.; Lednor, P.W. Monolithic ceramics and heterogeneous catalysts: Honeycombs and foams. Curr. Opin. Solid State Mater. Sci. 1996, 1, 88–95. [Google Scholar] [CrossRef]
- Voltolina, S.; Marín, P.; Díez, F.V.; Ordóñez, S. Open-cell foams as beds in multiphase reactors: Residence time distribution and mass transfer. Chem. Eng. J. 2017, 316, 323–331. [Google Scholar] [CrossRef]
- Zhao, G.; Liu, Y.; Lu, Y. Foam/fiber-structured catalysts: Non-dip-coating fabrication strategy and applications in heterogeneous catalysis. Sci. Bull. 2016, 61, 745–748. [Google Scholar] [CrossRef]
- Faure, R.; Rossignol, F.; Chartier, T.; Bonhomme, C.; Maître, A.; Etchegoyen, G.; Del Gallo, P.; Gary, D. Alumina foam catalyst supports for industrial steam reforming processes. J. Eur. Ceram. Soc. 2011, 31, 303–312. [Google Scholar] [CrossRef]
- Faure, R.; Basile, F.; Bersani, I.; Chartier, T.; Cuni, A.; Cornillac, M.; Gallo, P.D.; Etchegoyen, G.; Gary, D.; Rossignol, F.; et al. Foam-supported catalysts tailored for industrial steam reforming processes. In Studies in Surface Science and Catalysis; Gaigneaux, E.M., Devillers, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2010; pp. 241–244. ISBN 978-0-444-53601-3. [Google Scholar]
- Laguna, O.H.; Domínguez, M.I.; Centeno, M.A.; Odriozola, J.A. Catalysts on metallic surfaces: Monoliths and microreactors. In New Materials for Catalytic Applications; Parvulescu, V.I., Kemnitz, E., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 81–120. ISBN 978-0-444-63587-7. [Google Scholar]
- Balzarotti, R.; Italiano, C.; Pino, L.; Cristiani, C.; Vita, A. Ni/CeO2-thin ceramic layer depositions on ceramic monoliths for syngas production by oxy steam reforming of biogas. Fuel Process. Technol. 2016, 149, 40–48. [Google Scholar] [CrossRef]
- Ercolino, G.; Stelmachowski, P.; Specchia, S. Catalytic performance of Pd/Co3O4 on SiC and ZrO2 open cell foams for process intensification of methane combustion in lean conditions. Ind. Eng. Chem. Res. 2017, 56, 6625–6636. [Google Scholar] [CrossRef]
- Rivero-Mendoza, D.E.; Stanley, J.N.G.; Scott, J.; Aguey-Zinsou, K.-F. An alumina-supported Ni-La-based catalyst for producing synthetic natural gas. Catalysts 2016, 6, 170–184. [Google Scholar] [CrossRef]
- Italiano, C.; Vita, A.; Fabiano, C.; Laganà, M.; Pino, L. Bio-hydrogen production by oxidative steam reforming of biogas over nanocrystalline Ni/CeO2 catalysts. Int. J. Hydrogen Energy 2015, 40, 11823–11830. [Google Scholar] [CrossRef]
- Vita, A.; Italiano, C.; Fabiano, C.; Laganà, M.; Pino, L. Influence of Ce-precursor and fuel on structure and catalytic activity of combustion synthesized Ni/CeO2 catalysts for biogas oxidative steam reforming. Mater. Chem. Phys. 2015, 163, 337–347. [Google Scholar] [CrossRef]
- Patcas, F.C.; Garrido, G.I.; Kraushaar-Czarnetzki, B. CO oxidation over structured carriers: A comparison of ceramic foams, honeycombs and beads. Chem. Eng. Sci. 2007, 62, 3984–3990. [Google Scholar] [CrossRef]
- Gibson, L.J.; Ashby, M.F. Cellular Solids Structure and Properties, 2nd ed.; Cambridge University Press: Cambridge, UK, 1999; pp. 15–51. ISBN 0521499119. [Google Scholar]
- Buciuman, F.C.; Kraushaar-Czarnetzki, B. Ceramic foam monoliths as catalyst carriers. 1. Adjustment and Description of the Morphology. Ind. Eng. Chem. Res. 2003, 42, 1863–1869. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Calmidi, V.V.; Mahajan, R.L. Thermophysical properties of high porosity metal foams. Int. J. Heat. Mass Transf. 2002, 45, 1017–1031. [Google Scholar] [CrossRef]
- Boomsma, K.; Poulikakos, D.; Ventikos, Y. Simulations of flow through open cell metal foams using an idealized periodic cell structure. Int. J. Heat Fluid Flow 2003, 24, 825–834. [Google Scholar] [CrossRef]
- Hamadouche, A.; Nebbali, R.; Benahmed, H.; Kouidri, A.; Bousri, A. Experimental investigation of convective heat transfer in an open-cell aluminum foams. Exp. Therm. Fluid Sci. 2016, 71, 86–94. [Google Scholar] [CrossRef]
- Wang, H.; Guo, L. Experimental investigation on pressure drop and heat transfer in metal foam filled tubes under convective boundary condition. Chem. Eng. Sci. 2016, 155, 438–448. [Google Scholar] [CrossRef]
- Richardson, J.; Remue, D.; Hung, J.-K. Properties of ceramic foam catalyst supports: Mass and heat transfer. Appl. Catal. A Gen. 2003, 250, 319–329. [Google Scholar] [CrossRef]
- Richardson, J.; Peng, Y.; Remue, D. Properties of ceramic foam catalyst supports: Pressure drop. Appl. Catal. A Gen. 2000, 204, 19–32. [Google Scholar] [CrossRef]
- Nie, Z.; Lin, Y.; Tong, Q. Numerical investigation of pressure drop and heat transfer through open cell foams with 3D Laguerre-Voronoi model. Int. J. Heat Mass Transfer 2017, 113, 819–839. [Google Scholar] [CrossRef]
- Ergun, S.; Orning, A.A. Fluid flow through randomly packed columns and fluidized beds. Ind. Eng. Chem. 1949, 41, 1179–1184. [Google Scholar] [CrossRef]
- Edouard, D.; Lacroix, M.; Huu, C.P.; Luck, F. Pressure drop modeling on SOLID foam: State-of-the art correlation. Chem. Eng. J. 2008, 144, 299–311. [Google Scholar] [CrossRef]
- Lacroix, M.; Nguyen, P.; Schweich, D.; Huu, C.P.; Savin-Poncet, S.; Edouard, D. Pressure drop measurements and modeling on SiC foams. Chem. Eng. Sci. 2007, 62, 3259–3267. [Google Scholar] [CrossRef]
- Sollier, B.M.; Gómez, L.E.; Boix, A.V.; Miró, E.E. Oxidative coupling of methane on Sr/La2O3 catalysts: Improving the catalytic performance using cordierite monoliths and ceramic foams as structured substrates. Appl. Catal. A Gen. 2017, 532, 65–76. [Google Scholar] [CrossRef]
- Palma, V.; Pisano, D.; Martino, M. The influence of the textural properties of aluminum foams as catalyst carriers for water gas shift process. Int. J. Hydrogen Energy 2017, 42, 23517–23525. [Google Scholar] [CrossRef]
- Chai, R.; Zhang, Z.; Chen, P.; Zhao, G.; Liu, Y.; Lu, Y. Ni-foam-structured NiO-MOx-Al2O3 (M = Ce or Mg) nanocomposite catalyst for high throughput catalytic partial oxidation of methane to syngas. Microporous Mesoporous Mater. 2017, 253, 123–128. [Google Scholar] [CrossRef]
- Méndez, F.J.; Sanz, O.; Montes, M.; Guerra, J.; Olivera-Fuentes, C.; Curbelo, S.; Brito, J.L. Selective hydrogenation of 1,3-butadiene in the presence of 1-butene under liquid phase conditions using structured catalysts. Catal. Today 2016, 151–161. [Google Scholar] [CrossRef]
- Sanz, O.; Javier Echave, F.; Sánchez, M.; Monzón, A.; Montes, M. Aluminium foams as structured supports for volatile organic compounds (VOCs) oxidation. Appl. Catal. A Gen. 2008, 340, 125–132. [Google Scholar] [CrossRef]
- Ertl, G.; Knözinger, H.; Schüth, F.; Weitkamp, J. Handbook of Heterogeneous Catalysis, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2008; ISBN 978-3-527-31241-2. [Google Scholar]
- Mears, D.E. Diagnostic criteria for heat transport limitations in fixed bed reactors. J. Catal. 1971, 20, 127–131. [Google Scholar] [CrossRef]
- Anderson, J.B. A criterion for isothermal behaviour of a catalyst pellet. Chem. Eng. Sci. 1963, 18, 147–148. [Google Scholar]
- Daele, T.V.; del Pozo, D.F.; Hauwermeiren, D.V.; Gernaey, K.V.; Wohlgemuth, R.; Nopens, I. A generic model-based methodology for quantification of mass transfer limitations in microreactors. Chem. Eng. J. 2016, 300, 193–208. [Google Scholar] [CrossRef] [Green Version]
- Wijaya, W.Y.; Kawasaki, S.; Watanabe, H.; Okazaki, K. Damköhler number as a descriptive parameter in methanol steam reforming and its integration with absorption heat pump system. Appl. Energy 2012, 94, 141–147. [Google Scholar] [CrossRef]
- Liao, C.; Erickson, P.A. Characteristic time as a descriptive parameter in steam reformation hydrogen production processes. Int. J. Hydrogen Energy 2008, 33, 1652–1660. [Google Scholar] [CrossRef]
- Talebian-Kiakalaieh, A.; Amin, N.A.S. Theoretical and experimental evaluation of mass transfer limitation in gas phase dehydration of glycerol to acrolein over supported hsiw catalyst. J. Taiwan Inst. Chem. Eng. 2016, 59, 11–17. [Google Scholar] [CrossRef]
- Bandopadhyay, A.; Borgne, T.L.; Méheust, Y.; Dentz, M. Enhanced reaction kinetics and reactive mixing scale dynamics in mixing fronts under shear flow for arbitrary Damköhler numbers. Adv. Water Resour. 2017, 100, 78–95. [Google Scholar] [CrossRef] [Green Version]
- Metkar, P.S.; Salazar, N.; Muncrief, R.; Balakotaiah, V.; Harold, M.P. Selective catalytic reduction of NO with NH3 on iron zeolite monolithic catalysts: Steady-state and transient kinetics. Appl. Catal. B Environ. 2011, 104, 110–126. [Google Scholar] [CrossRef]
- Groppi, G.; Tronconi, E. Design of novel monolith catalyst supports for gas/solid reactions with heat exchange. Chem. Eng. Sci. 2000, 55, 2161–2171. [Google Scholar] [CrossRef]
- Boldrini, D.E.; Sánchez, M.J.F.; Tonetto, G.M.; Damiani, D.E. Monolithic stirrer reactor: Performance in the partial hydrogenation of sunflower oil. Ind. Eng. Chem. Res. 2012, 51, 12222–12232. [Google Scholar] [CrossRef]
- Dekker, F.H.M.; Bliek, A.; Kapteijn, F.; Moulijn, J.A. Analysis of mass and heat transfer in transient experiments over heterogeneous catalysts. Chem. Eng. Sci. 1995, 50, 3573–3580. [Google Scholar] [CrossRef] [Green Version]
- Hayes, R.E.; Kolaczkowskib, S.T.; Li, P.K.C.; Awdry, S. Evaluating the effective diffusivity of methane in the washcoat of a honeycomb monolith. Appl. Catal. B Environ. 2000, 25, 93–104. [Google Scholar] [CrossRef]
- Joshi, S.Y.; Harold, M.P.; Balakotaiah, V. Overall mass transfer coefficients and controlling regimes in catalytic monoliths. Chem. Eng. Sci. 2010, 65, 1729–1747. [Google Scholar] [CrossRef]
- Roy, P.S.; Raju, A.S.K.; Kim, K. Influence of S/C ratio and temperature on steam reforming of model biogas over a metal-foam-coated Pd–Rh/(CeZrO2–Al2O3) catalyst. Fuel 2015, 139, 314–320. [Google Scholar] [CrossRef]
- Ashrafi, M.; Pröll, T.; Pfeifer, C.; Hofbauer, H. Experimental study of model biogas catalytic steam reforming: 1. Thermodynamic optimization. Energy Fuels 2008, 22, 4182–4189. [Google Scholar] [CrossRef]
- Zeppieri, M.; Villa, P.L.; Verdone, N.; Scarsella, M.; De Filippis, P. Kinetic of methane steam reforming reaction over nickel- and rhodium-based catalysts. Appl. Catal. A Gen. 2010, 387, 147–154. [Google Scholar] [CrossRef]
- Roh, H. Methane-reforming reactions over Ni/Ce-ZrO2/θ-Al2O3 catalysts. Appl. Catal. A Gen. 2003, 251, 275–283. [Google Scholar] [CrossRef]
- Zhang, Z.; Ding, J.; Chai, R.; Zhao, G.; Liu, Y.; Lu, Y. Oxidative dehydrogenation of ethane to ethylene: A promising CeO2-ZrO2-modified NiO-Al2O3/Ni-foam catalyst. Appl. Catal. A Gen. 2018, 550, 151–159. [Google Scholar] [CrossRef]
- Mbodji, M.; Commenge, J.M.; Falk, L.; Di Marco, D.; Rossignol, F.; Prost, L.; Valentin, S.; Joly, R.; Del-Gallo, P. Steam methane reforming reaction process intensification by using a millistructured reactor: Experimental setup and model validation for global kinetic reaction rate estimation. Chem. Eng. J. 2012, 207–208, 871–884. [Google Scholar] [CrossRef]
- Arzamendi, G.; Diéguez, P.M.; Montes, M.; Odriozola, J.A.; Sousa-Aguiar, E.F.; Gandía, L.M. Methane steam reforming in a microchannel reactor for GTL intensification: A computational fluid dynamics simulation study. Chem. Eng. J. 2009, 154, 168–173. [Google Scholar] [CrossRef]
- Zhou, W.; Ke, Y.; Wang, Q.; Wan, S.; Lin, J.; Zhang, J.; Hui, K.S. Development of cylindrical laminated methanol steam reforming microreactor with cascading metal foams as catalyst support. Fuel 2017, 191, 46–53. [Google Scholar] [CrossRef] [Green Version]
- Mbodji, M.; Commenge, J.-M.; Falk, L. Preliminary design and simulation of a microstructured reactor for production of synthesis gas by steam methane reforming. Chem. Eng. Res. Des. 2014, 92, 1728–1739. [Google Scholar] [CrossRef]
- González-Castaño, M.; Ivanova, S.; Laguna, O.H.; Martínez, T.L.M.; Centeno, M.A.; Odriozola, J.A. Structuring Pt/CeO2/Al2O3 WGS catalyst: Introduction of buffer layer. Appl. Catal. B Environ. 2017, 200, 420–427. [Google Scholar]
- Deutschmann, O.; Schwiedemoch, R.; Maier, L.I.; Chatterjee, D. Natural gas conversion in monolithic catalysts: Interaction of chemical reactions and transport phenomena. Stud. Surf. Sci. Catal. 2001, 251–258. [Google Scholar]
- Stutz, M.J.; Poulikakos, D. Optimum washcoat thickness of a monolith reactor for syngas production by partial oxidation of methane. Chem. Eng. Sci. 2008, 63, 1761–1770. [Google Scholar] [CrossRef]
- Montebelli, A.; Visconti, C.G.; Groppi, G.; Tronconi, E.; Kohler, S.; Venvik, H.J.; Myrstad, R. Washcoating and chemical testing of a commercial Cu/ZnO/Al2O3 catalyst for the methanol synthesis over copper open-cell foams. Appl. Catal. A Gen. 2014, 481, 96–103. [Google Scholar] [CrossRef]
- Italiano, C.; Balzarotti, R.; Vita, A.; Latorrata, S.; Fabiano, C.; Pino, L.; Cristiani, C. Preparation of structured catalysts with Ni and Ni–Rh/CeO2 catalytic layers for syngas production by biogas reforming processes. Catal. Today 2016, 273, 3–11. [Google Scholar] [CrossRef]
- Cao, C.; Zhang, N.; Cheng, Y. Numerical analysis on steam methane reforming in a plate microchannel reactor: Effect of washcoat properties. Int. J. Hydrog. Energy 2016, 41, 18921–18941. [Google Scholar] [CrossRef]
- Wójcik, S.; Ercolino, G.; Gajewska, M.; Moncada Quintero, C.M.; Specchia, S.; Kotarba, A. Robust Co3O4|α–Al2O3|cordierite structured catalyst for N2O abatement—Validation of the SCS method for active phase synthesis and deposition. Chem. Eng. J. 2018, in press. [Google Scholar]
- Palma, V.; Pisano, D.; Martino, M.; Ciambelli, P. Structured catalysts with high thermoconductive properties for the intensification of water gas shift process. Chem. Eng. J. 2016, 304, 544–551. [Google Scholar] [CrossRef]
- Vita, A.; Italiano, C.; Pino, L.; Laganà, M.; Recupero, V. Hydrogen-rich gas production by steam reforming of n-dodecane. Part II: Stability, regenerability and sulfur poisoning of low loading Rh-based catalyst. Appl. Catal. B Environ. 2017, 218, 317–326. [Google Scholar] [CrossRef]
- Zhang, M.; Ning, T.; Sun, P.; Yan, Y.; Zhang, D.; Li, Z. Effect of Al2O3-SiO2 substrate on gas-sensing properties of TiO2 based lambda sensor at high temperature. Ceram. Int. 2018, 44, 3000–3004. [Google Scholar] [CrossRef]
- Shi, R.; Shang, Y.; Zhang, Y.; Wang, P.; Zhang, A.; Yang, P. Synthesis of ultrafine α-Al2O3 powder by two-step hydrolysis. Ceram. Int. 2018, 44, 3741–3750. [Google Scholar] [CrossRef]





| Structured Substrate | F20 | F30 | F40 |
|---|---|---|---|
 |  |  | |
| Pore per inch, ppi | 20 | 30 | 40 |
| Diameter, ϕ—Length, L (mm) | 10–15 | 10–15 | 10–15 |
| Hole area, A (mm2) | 2.88 | 1.55 | 0.92 |
| Average pore diameter, dp (mm) | 1.92 | 1.40 | 1.08 |
| Average strut thickness, ts (mm) | 0.51 | 0.41 | 0.33 |
| Face diameter, df (mm) | 2.42 | 1.81 | 1.41 |
| Relative density, ρr | 0.11 | 0.13 | 0.14 |
| Voidage, ε | 0.89 | 0.87 | 0.86 |
| Bed porosity, Vp (%) | 88.3 | 85.1 | 83.6 |
| Geometric surface area, GSA (m2·m−3) | 669 | 967 | 1273 |
| Exposed surface area, SAOCF (mm2) | 788 | 1138 | 1500 |
| Catalyst loading, Cload (mg·cm−2) | 22.2 | 15.2 | 12.0 |
| Catalytic layer thickness, δc (μm) | 25–40 | 15–30 | 5–20 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Italiano, C.; Ashraf, M.A.; Pino, L.; Moncada Quintero, C.W.; Specchia, S.; Vita, A. Rh/CeO2 Thin Catalytic Layer Deposition on Alumina Foams: Catalytic Performance and Controlling Regimes in Biogas Reforming Processes. Catalysts 2018, 8, 448. https://doi.org/10.3390/catal8100448
Italiano C, Ashraf MA, Pino L, Moncada Quintero CW, Specchia S, Vita A. Rh/CeO2 Thin Catalytic Layer Deposition on Alumina Foams: Catalytic Performance and Controlling Regimes in Biogas Reforming Processes. Catalysts. 2018; 8(10):448. https://doi.org/10.3390/catal8100448
Chicago/Turabian StyleItaliano, Cristina, Muhammad Arsalan Ashraf, Lidia Pino, Carmen Williana Moncada Quintero, Stefania Specchia, and Antonio Vita. 2018. "Rh/CeO2 Thin Catalytic Layer Deposition on Alumina Foams: Catalytic Performance and Controlling Regimes in Biogas Reforming Processes" Catalysts 8, no. 10: 448. https://doi.org/10.3390/catal8100448