Kinetic and Mechanistic Study on Catalytic Decomposition of Hydrogen Peroxide on Carbon-Nanodots/Graphitic Carbon Nitride Composite
Abstract
:1. Introduction
2. Results and Discussion
2.1. Morphology of the Catalyst
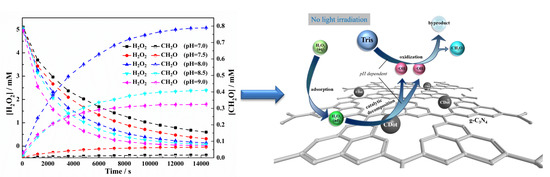
2.2. Kinetic Study
2.3. The Effect of pH
3. Experimental Section
3.1. Instrumentation
3.2. Reagents and Experiments
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- He, J.; Yang, X.F.; Men, B.; Wang, D.S. Interfacial mechanisms of heterogeneous Fenton reactions catalyzed by iron-based materials: A review. J. Environ. Sci. 2016, 39, 97–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nidheesh, P.V.; Gandhimathi, R.; Ramesh, S.T. Degradation of dyes from aqueous solution by Fenton processes: A review. Environ. Sci. Pollut. Res. 2013, 20, 2099–2132. [Google Scholar] [CrossRef] [PubMed]
- Aval, A.E.; Hasani, A.H.; Omrani, G.A.; Karbassi, A. Removal of Landfill Leachate’s Organic load by modified Electro-Fenton process. Int. J. Electrochem. Sci. 2017, 12, 9348–9363. [Google Scholar] [CrossRef]
- Huang, J.; Shi, Q.; Feng, J.; Chen, M.; Li, W.; Li, L. Facile pyrolysis preparation of rosin-derived biochar for supporting silver nanoparticles with antibacterial activity. Compos. Sci. Technol. 2017, 145, 89–95. [Google Scholar] [CrossRef]
- Freyria, F.S.; Armandi, M.; Compagnoni, M.; Ramis, G.; Rossetti, I.; Bonelli, B. Catalytic and Photocatalytic Processes for the Abatement of N-Containing Pollutants from Wastewater. Part 2: Organic Pollutants. J. Nanosci. Nanotechnol. 2017, 17, 3654–3672. [Google Scholar] [CrossRef]
- Chen, C.Y.; Tang, C.; Wang, H.F.; Chen, C.M.; Zhang, X.Y.; Huang, X.; Zhang, Q. Oxygen Reduction Reaction on Graphene in an Electro-Fenton System: In Situ Generation of H2O2 for the Oxidation of Organic Compounds. Chemsuschem 2016, 9, 1194–1199. [Google Scholar] [CrossRef] [PubMed]
- Santos, L.V.D.; Meireles, A.M.; Lange, L.C. Degradation of antibiotics norfloxacin by Fenton, UV and UV/H2O2. J. Environ. Manag. 2015, 154, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.G.; Hsieh, W.P.; Mahmudov, R.; Wei, X.M.; Huang, C.P. Combined ultrasound and Fenton (US-Fenton) process for the treatment of ammunition wastewater. J. Hazard. Mater. 2013, 244, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Nidheesh, P.V. Heterogeneous Fenton catalysts for the abatement of organic pollutants from aqueous solution: A review. RSC Adv. 2015, 5, 40552–40577. [Google Scholar] [CrossRef]
- Oller, I.; Malato, S.; Sanchez-Perez, J.A. Combination of Advanced Oxidation Processes and biological treatments for wastewater decontamination-A review. Sci. Total Environ. 2011, 409, 4141–4166. [Google Scholar] [CrossRef] [PubMed]
- Bokare, A.D.; Choi, W. Review of iron-free Fenton-like systems for activating H2O2 in advanced oxidation processes. J. Hazard. Mater. 2014, 275, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Xie, T.; Duan, X.; Yu, F.B.; Wang, X.; Tang, B. A New Highly Selective and Sensitive Assay for Fluorescence Imaging of ·OH in Living Cells: Effectively Avoiding the Interference of Peroxynitrite. Chem. Eur. J. 2010, 16, 1834–1840. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.Q.; Sui, D.D.; Zhou, W.J.; Lu, C. Highly selective chemiluminescence detection of hydroxyl radical via increased pi-electron densities of rhodamine B on montmorillonite matrix. Sens. Actuators B Chem. 2016, 225, 600–606. [Google Scholar] [CrossRef]
- Jing, Y.; Chaplin, B.P. Mechanistic Study of the Validity of Using Hydroxyl Radical Probes to Characterize Electrochemical Advanced Oxidation Processes. Environ. Sci. Technol. 2017, 51, 2355–2365. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.F.; Guo, X.Q. A novel fluorescence probe for the determination of hydroxyl radicals. Chem. J. Chin. Univ. 2001, 22, 396–398. [Google Scholar]
- Fisher, S.C.; Schoonen, M.A.A.; Brownawell, B.J. Phenylalanine as a hydroxyl radical-specific probe in pyrite slurries. Geochem. Trans. 2012, 13, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Jonsson, M. Evaluation of the O2 and pH Effects on Probes for Surface Bound Hydroxyl Radicals. J. Phys. Chem. C 2014, 118, 7971–7979. [Google Scholar] [CrossRef]
- Lousada, C.M.; Jonsson, M. Kinetics, Mechanism, and Activation Energy of H2O2 Decomposition on the Surface of ZrO2. J. Phys. Chem. C 2010, 114, 11202–11208. [Google Scholar] [CrossRef]
- Nash, T. The colorimetric estimation of formaldehyde by means of the Hantzsch reaction. Biochem. J. 1953, 55, 416–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, P.; Pan, Z.; Zhang, J. Synergistic Enhancement in Catalytic Performance of Superparamagnetic Fe3O4@Bacilus subtilis as Recyclable Fenton-Like Catalyst. Catalysts 2017, 7, 349. [Google Scholar] [CrossRef]
- Yang, H.; Shi, B.; Wang, S. Fe Oxides Loaded on Carbon Cloth by Hydrothermal Process as an Effective and Reusable Heterogenous Fenton Catalyst. Catalysts 2018, 8, 207. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, X.; Grosjean, A.; Soroka, I.; Jonsson, M. Kinetics and Mechanism of the Reaction between H2O2 and Tungsten Powder in Water. J. Phys. Chem. C 2015, 119, 22560–22569. [Google Scholar] [CrossRef]
- Hiroki, A.; LaVerne, J.A. Decomposition of Hydrogen Peroxide at Water−Ceramic Oxide Interfaces. J. Phys. Chem. B 2005, 109, 3364–3370. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, C.M.; Quintanilla, A.; Ocón, P.; Casas, J.A.; Rodriguez, J.J. The use of cyclic voltammetry to assess the activity of carbon materials for hydrogen peroxide decomposition. Carbon 2013, 60, 76–83. [Google Scholar] [CrossRef]
- Lousada, C.M.; Trummer, M.; Jonsson, M. Reactivity of H2O2 towards different UO2-based materials: The relative impact of radiolysis products revisited. J. Nuclear Mater. 2013, 434, 434–439. [Google Scholar] [CrossRef]
- Vilardi, G.; Di Palma, L.; Verdone, N. On the critical use of zero valent iron nanoparticles and Fenton processes for the treatment of tannery wastewater. J. Water Process Eng. 2018, 22, 109–122. [Google Scholar] [CrossRef]
- Björkbacka, Å.; Yang, M.; Gasparrini, C.; Leygraf, C.; Jonsson, M. Kinetics and mechanisms of reactions between H2O2 and copper and copper oxides. Dalton Trans. 2015, 44, 16045–16051. [Google Scholar] [CrossRef] [PubMed]
- Lousada, C.M.; LaVerne, J.A.; Jonsson, M. Enhanced hydrogen formation during the catalytic decomposition of H2O2 on metal oxide surfaces in the presence of HO radical scavengers. Phys. Chem. Chem. Phys. 2013, 15, 12674–12679. [Google Scholar] [CrossRef] [PubMed]
- Pignatello, J.J.; Oliveros, E.; MacKay, A. Advanced oxidation processes for organic contaminant destruction based on the Fenton reaction and related chemistry. Crit. Rev. Environ. Sci. Technol. 2006, 36, 1–84. [Google Scholar] [CrossRef]
- Diya’uddeen, B.H.; Aziz, A.R.A.; Daud, W.M.A.W. On the Limitation of Fenton Oxidation Operational Parameters: A Review. Int. J. Chem. React. Eng. 2012, 10, 1498–1502. [Google Scholar] [CrossRef]
- Garcia-Segura, S.; Bellotindos, L.M.; Huang, Y.H.; Brillas, E.; Lu, M.C. Fluidized-bed Fenton process as alternative wastewater treatment technology-A review. J. Taiwan Inst. Chem. Eng. 2016, 67, 211–225. [Google Scholar] [CrossRef]
- Pouran, S.R.; Raman, A.A.A.; Daud, W.M.A.W. Review on the application of modified iron oxides as heterogeneous catalysts in Fenton reactions. J. Clean. Prod. 2014, 64, 24–35. [Google Scholar] [CrossRef]
- Yao, Y.J.; Chen, H.; Lian, C.; Wei, F.Y.; Zhang, D.W.; Wu, G.D.; Chen, B.J.; Wang, S.B. Fe, Co, Ni nanocrystals encapsulated in nitrogen-doped carbon nanotubes as Fenton-like catalysts for organic pollutant removal. J. Hazard. Mater. 2016, 314, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.Y.; Lu, C.; Yao, Y.Y.; Sun, L.J.; Gong, F.; Li, D.W.; Pei, K.M.; Lu, W.Y.; Chen, W.X. Activated carbon fibers as an effective metal-free catalyst for peracetic acid activation: Implications for the removal of organic pollutants. Chem. Eng. J. 2015, 281, 953–960. [Google Scholar] [CrossRef]
- Zhu, Z.D.; Pan, H.H.; Murugananthan, M.; Gong, J.Y.; Zhang, Y.R. Visible light-driven photocatalytically active g-C3N4 material for enhanced generation of H2O2. Appl. Catal. B Environ. 2018, 232, 19–25. [Google Scholar] [CrossRef]
- Jiang, G.D.; Yang, X.X.; Wu, Y.; Li, Z.W.; Han, Y.H.; Shen, X.D. A study of spherical TiO2/g-C3N4 photocatalyst: Morphology, chemical composition and photocatalytic performance in visible light. Mol. Catal. 2017, 432, 232–241. [Google Scholar] [CrossRef]
- Yan, J.; Fan, Y.M.; Lian, J.B.; Zhao, Y.; Xu, Y.G.; Gu, J.M.; Song, Y.H.; Xu, H.; Li, H.M. Kinetics and mechanism of enhanced photocatalytic activity employing ZnS nanospheres/graphene-like C3N4. Mol. Catal. 2017, 438, 103–112. [Google Scholar] [CrossRef]
- Bicalho, H.A.; Lopez, J.L.; Binatti, I.; Batista, P.F.R.; Ardisson, J.D.; Resende, R.R.; Lorencon, E. Facile synthesis of highly dispersed Fe(II)-doped g-C3N4 and its application in Fenton-like catalysis. Mol. Catal. 2017, 435, 156–165. [Google Scholar] [CrossRef]
- Zhang, M.L.; Yao, Q.F.; Guan, W.J.; Lu, C.; Lin, J.M. Layered Double Hydroxide-Supported Carbon Dots as an Efficient Heterogeneous Fenton-Like Catalyst for Generation of Hydroxyl Radicals. J. Phys. Chem. C 2014, 118, 10441–10447. [Google Scholar] [CrossRef]
- Wang, K.; Li, Q.; Liu, B.S.; Cheng, B.; Ho, W.K.; Yu, J.G. Sulfur-doped g-C3N4 with enhanced photocatalytic CO2-reduction performance. Appl. Catal. B Environ. 2015, 176, 44–52. [Google Scholar] [CrossRef]
- Zhao, D.M.; Chen, J.; Dong, C.L.; Zhou, W.; Huang, Y.C.; Mao, S.S.; Guo, L.J.; Shen, S.H. Interlayer interaction in ultrathin nanosheets of graphitic carbon nitride for efficient photocatalytic hydrogen evolution. J. Catal. 2017, 352, 491–497. [Google Scholar] [CrossRef]
- Zhou, M.; Yang, P.J.; Yuan, R.S.; Asiri, A.M.; Wakeel, M.; Wang, X.C. Modulating Crystallinity of Graphitic Carbon Nitride for Photocatalytic Oxidation of Alcohols. Chemsuschem 2017, 10, 4451–4456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, K.Y.A.; Lin, J.T. Ferrocene-functionalized graphitic carbon nitride as an enhanced heterogeneous catalyst of Fenton reaction for degradation of Rhodamine B under visible light irradiation. Chemosphere 2017, 182, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, Y.; Liu, N.Y.; Han, Y.Z.; Zhang, X.; Huang, H.; Lifshitz, Y.; Lee, S.T.; Zhong, J.; Kang, Z.H. Metal-free efficient photocatalyst for stable visible water splitting via a two-electron pathway. Science 2015, 347, 970–974. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Chen, T.; Guo, Y.; Zhang, Z.; Fang, X. Ultrathin g-C3N4 nanosheets coupled with carbon nanodots as 2D/0D composites for efficient photocatalytic H2 evolution. Appl. Catal. B Environ. 2016, 193, 248–258. [Google Scholar] [CrossRef]
- Oh, J.; Lee, J.M.; Yoo, Y.; Kim, J.; Hwang, S.J.; Park, S. New insight of the photocatalytic behaviors of graphitic carbon nitrides for hydrogen evolution and their associations with grain size, porosity, and photophysical properties. Appl. Catal. B Environ. 2017, 218, 349–358. [Google Scholar] [CrossRef]
- Liu, J.H.; Zhang, Y.W.; Lu, L.H.; Wu, G.; Chen, W. Self-regenerated solar-driven photocatalytic water-splitting by urea derived graphitic carbon nitride with platinum nanoparticles. Chem. Commun. 2012, 48, 8826–8828. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.H.; Chen, P.; Wang, F.L.; Zhang, Q.X.; Chen, T.S.; Wang, Y.F.; Yao, K.; Lv, W.Y.; Liu, G.G. Decoration of TiO2/g-C3N4 Z-scheme by carbon dots as a novel photocatalyst with improved visible-light photocatalytic performance for the degradation of enrofloxacin. RSC Adv. 2017, 7, 34096–34103. [Google Scholar] [CrossRef]
- Fang, S.; Xia, Y.; Lv, K.L.; Li, Q.; Sun, J.; Li, M. Effect of carbon-dots modification on the structure and photocatalytic activity of g-C3N4. Appl. Catal. B Environ. 2016, 185, 225–232. [Google Scholar] [CrossRef]
- Wang, X.F.; Cheng, J.J.; Yu, H.G.; Yu, J.G. A facile hydrothermal synthesis of carbon dots modified g-C3N4 for enhanced photocatalytic H2-evolution performance. Dalton Trans. 2017, 46, 6417–6424. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.L.; Chen, P.; Feng, Y.P.; Xie, Z.J.; Liu, Y.; Su, Y.H.; Zhang, Q.X.; Wang, Y.F.; Yao, K.; Lv, W.Y.; et al. Facile synthesis of N-doped carbon dots/g-C3N4 photocatalyst with enhanced visible-light photocatalytic activity for the degradation of indomethacin. Appl. Catal. B Environ. 2017, 207, 103–113. [Google Scholar] [CrossRef]
- Dadigala, R.; Bandi, R.; Gangapuram, B.R.; Guttena, V. Carbon dots and Ag nanoparticles decorated g-C3N4 nanosheets for enhanced organic pollutants degradation under sunlight irradiation. J. Photochem. Photobiol. A 2017, 342, 42–52. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, L.X.; Geng, F.L.; Guo, L.H.; Wan, B.; Yang, Y. Carbon dots decorated graphitic carbon nitride as an efficient metal-free photocatalyst for phenol degradation. Appl. Catal. B Environ. 2016, 180, 656–662. [Google Scholar] [CrossRef] [Green Version]
- Lousada, C.M.; Yang, M.; Nilsson, K.; Jonsson, M. Catalytic decomposition of hydrogen peroxide on transition metal and lanthanide oxides. J. Mol. Catal. A-Chem. 2013, 379, 178–184. [Google Scholar] [CrossRef]
- Yang, M.; Jonsson, M. Surface reactivity of hydroxyl radicals formed upon catalytic decomposition of H2O2 on ZrO2. J. Mol. Catal. A Chem. 2015, 400, 49–55. [Google Scholar] [CrossRef]
- Fidalgo, A.B.; Dahlgren, B.; Brinck, T.; Jonsson, M. Surface Reactions of H2O2, H2, and O2 in Aqueous Systems Containing ZrO2. J. Phys. Chem. C 2016, 120, 1609–1614. [Google Scholar] [CrossRef]
- Pirsaheb, M.; Moradi, S.; Shahlaei, M.; Farhadian, N. Application of carbon dots as efficient catalyst for the green oxidation of phenol: Kinetic study of the degradation and optimization using response surface methodology. J. Hazard. Mater. 2018, 353, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Lousada, C.M.; Johansson, A.J.; Brinck, T.; Jonsson, M. Mechanism of H2O2 Decomposition on Transition Metal Oxide Surfaces. J. Phys. Chem. C 2012, 116, 9533–9543. [Google Scholar] [CrossRef]
- Ming, H.; Ma, Z.; Liu, Y.; Pan, K.; Yu, H.; Wang, F.; Kang, Z. Large scale electrochemical synthesis of high quality carbon nanodots and their photocatalytic property. Dalton Trans. 2012, 41, 9526–9531. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.Y.; Yan, X.Q.; Kang, Z.; Zhang, Z.; Cao, S.Y.; Liu, Y.C.; Zhang, Y. Carbon Quantum Dots Decorated C3N4/TiO2 Heterostructure Nanorod Arrays for Enhanced Photoelectrochemical Performance. J. Electrochem. Soc. 2017, 164, H515–H520. [Google Scholar] [CrossRef]
- Guo, F.; Shi, W.L.; Guan, W.S.; Huang, H.; Liu, Y. Carbon dots/g-C3N4/ZnO nanocomposite as efficient visible-light driven photocatalyst for tetracycline total degradation. Sep. Purif. Technol. 2017, 173, 295–303. [Google Scholar] [CrossRef]
- Hochanadel, C.J. Effects of Cobalt γ-Radiation on Water and Aqueous Solutions. J. Phys. Chem. 1952, 21, 587–594. [Google Scholar] [CrossRef]
- Ghormley, J.A.; Stewart, A.C. Effects of γ-Radiation on Ice1. J. Am. Chem. Soc. 1956, 78, 2934–2939. [Google Scholar] [CrossRef]









| Sample | Description |
|---|---|
| 1 | 13.4 mg/L CDots |
| 2 | 4 × 105 m−1 g-C3N4 |
| 3 | physical mixture of Sample 1 and Sample 2 |
| 4 | 133 mg/L CDots |
| 5 | 4 × 105 m−1 CDots/g-C3N4 composite |
| SA/V (105 m−1) | k1 (10−4 s−1) | Standard Deviation (10−4 s−1) | R2 (%) |
|---|---|---|---|
| 3.2 | 2.61 | 0.078 | 99.38 |
| 4.0 | 3.52 | 0.068 | 99.74 |
| 4.8 | 4.53 | 0.084 | 99.76 |
| 5.6 | 6.05 | 1.063 | 99.49 |
| 6.4 | 7.16 | 0.094 | 99.88 |
| T(K) | k1 (10−4 s−1) | Standard Deviation (10−4 s−1) | R2 (%) |
|---|---|---|---|
| 293 | 3.02 | 0.071 | 99.61 |
| 298 | 3.52 | 0.068 | 99.74 |
| 303 | 4.50 | 0.088 | 99.74 |
| 308 | 5.37 | 0.090 | 99.80 |
| 313 | 6.27 | 0.104 | 99.81 |
| 318 | 7.44 | 0.129 | 99.80 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Shen, Q.; Zhou, C.; Fang, L.; Yang, M.; Xia, T. Kinetic and Mechanistic Study on Catalytic Decomposition of Hydrogen Peroxide on Carbon-Nanodots/Graphitic Carbon Nitride Composite. Catalysts 2018, 8, 445. https://doi.org/10.3390/catal8100445
Liu Z, Shen Q, Zhou C, Fang L, Yang M, Xia T. Kinetic and Mechanistic Study on Catalytic Decomposition of Hydrogen Peroxide on Carbon-Nanodots/Graphitic Carbon Nitride Composite. Catalysts. 2018; 8(10):445. https://doi.org/10.3390/catal8100445
Chicago/Turabian StyleLiu, Zhongda, Qiumiao Shen, Chunsun Zhou, Lijuan Fang, Miao Yang, and Tao Xia. 2018. "Kinetic and Mechanistic Study on Catalytic Decomposition of Hydrogen Peroxide on Carbon-Nanodots/Graphitic Carbon Nitride Composite" Catalysts 8, no. 10: 445. https://doi.org/10.3390/catal8100445