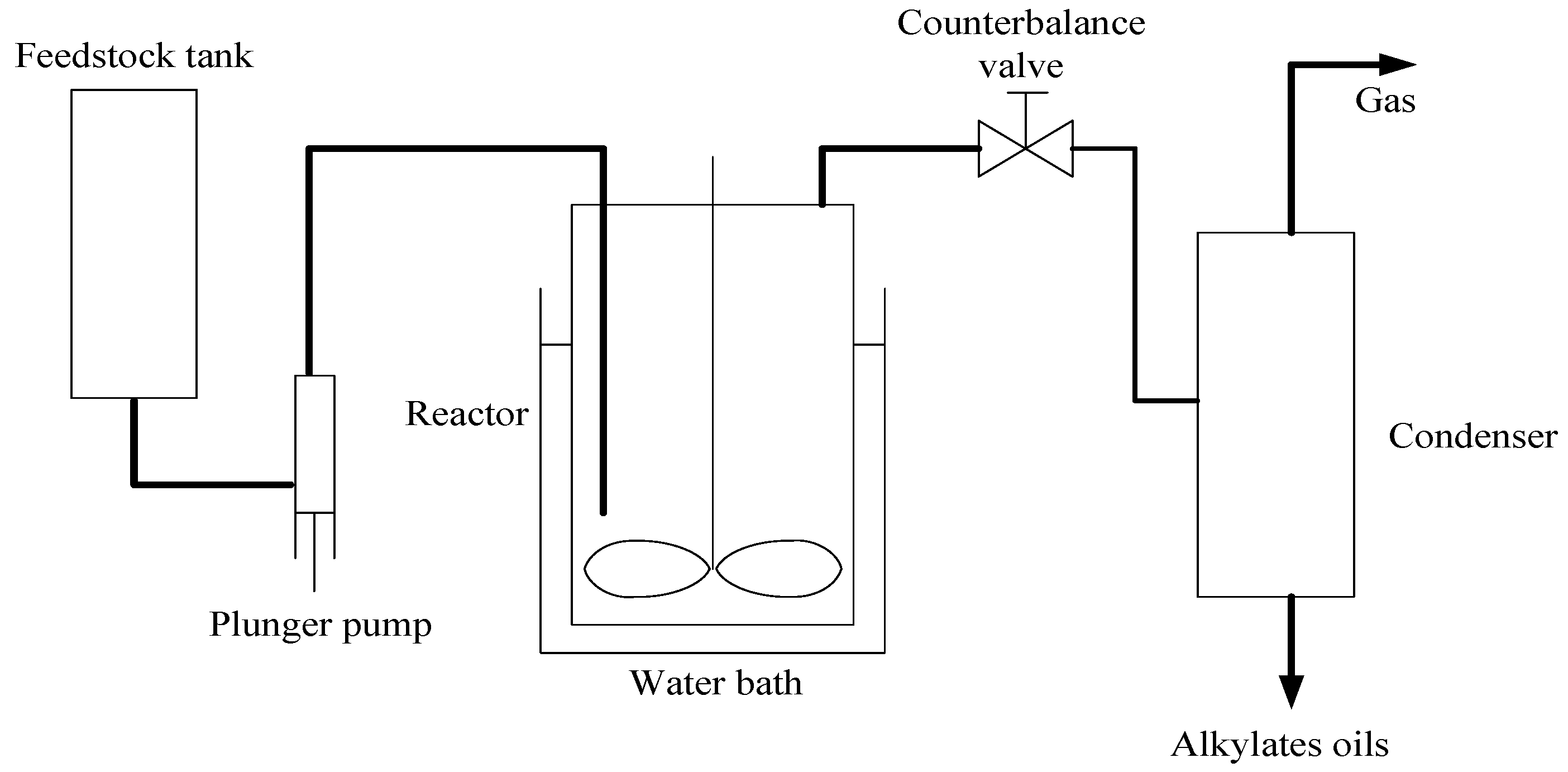
2.1. Effect of Water
The chloroaluminate ionic liquids are very moisture-sensitive. The water content in the alkylation system therefore probably influences the catalytic properties of the ionic liquids. Thus, in this work, the effects of water on the catalytic performances of the ionic liquid, i.e., the lifetime, the activity, and isooctane selectivity, were investigated in continuous alkylation by means of the study on the mass of a feedstock treated with 1 g ionic liquid and the component of the alkylation products. The water content in the feedstock without dehydrating was 1480 ppmw. The water contents in the other feedstocks were reduced to 850 ppmw, 375 ppmw, and 110 ppmw, respectively. The experimental results are reported in
Figure 1,
Figure 2,
Figure 3 and
Figure 4.
In
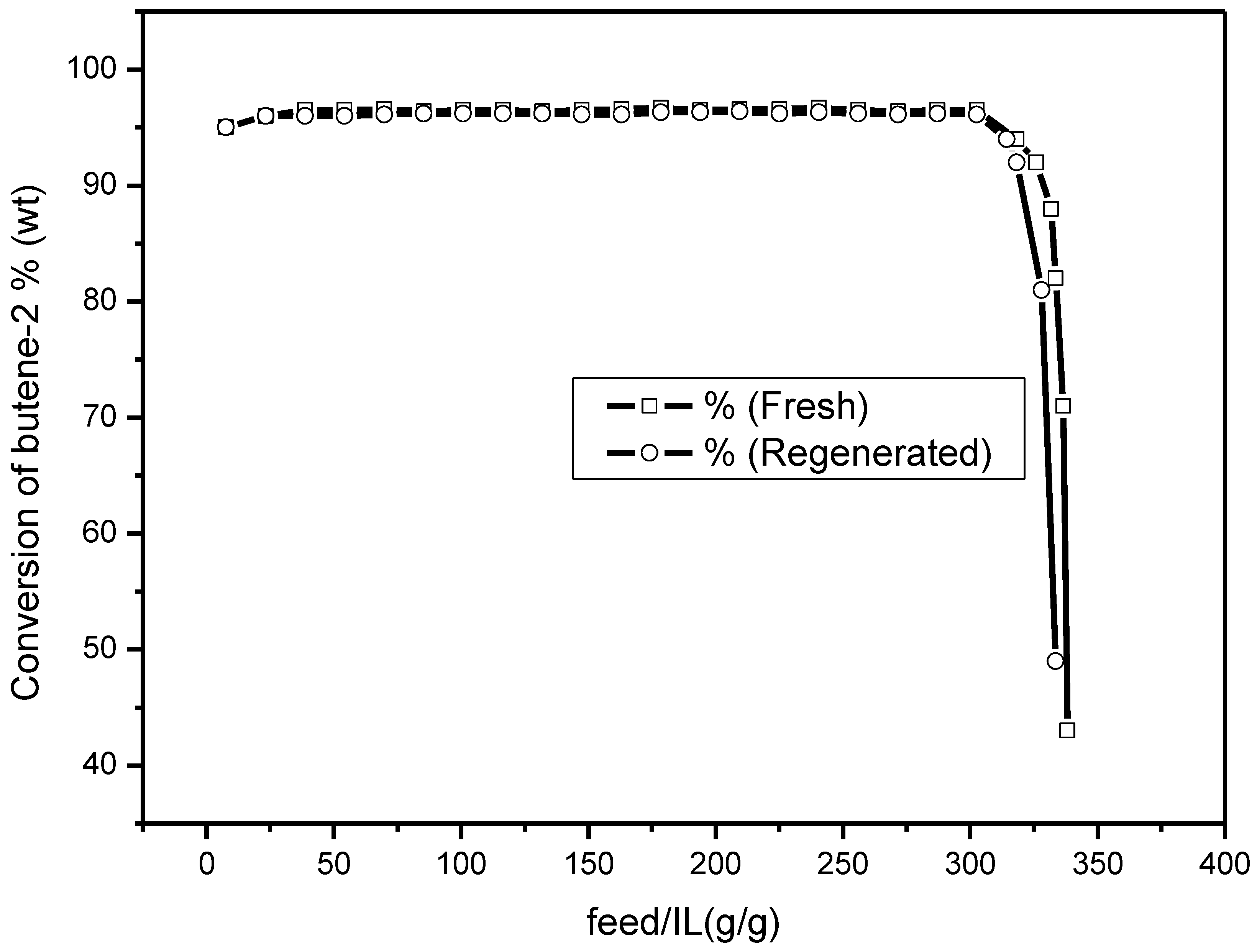
Figure 1, a marked increase of the mass of the feedstock treated with 1 g ionic liquid with the decrease of water content in the feedstock can be seen. When the content of water in the feedstock decreased from 1480 ppmw to 110 ppmw, the mass of feedstock treated with 1 g ionic liquid increased from 29 g to 338 g before deactivation of the ionic liquid (deactivation was considered when the conversion of 2-butene was lower than 50%). It can also be seen that, in the steady period, the higher water content in the feedstock led to higher conversion of 2-butene. This observation indicated that the activity of the chloroaluminate ionic liquid in the high water content system was stronger than that in the low water content system.
Figure 2 shows the effect of water content in feedstocks on the isooctane content in alkylates. It can be seen that there is an induction period of the isooctane content in this ionic liquid catalytic system. In the induction period, the isooctane content in alkylates increased with the increase of the mass of the feedstocks treated by 1 g ionic liquid. After the induction period, a steady period of the isooctane content was observed as expected. In the steady period, the isooctane content remained stable to a certain degree. With the deactivation of the ionic liquid, the isooctane content in the alkylates decreased quickly. The water content in the feedstock also affected the lengths of the induction and the steady periods. High water content in the feedstock resulted in a short induction and steady period. In the case of low water content in the feedstock (110 ppmw and 375 ppmw), the isooctane content in the alkylate was over 80% in the steady period, higher than that of the case of high water content in the feedstock (850 ppmw and 1480 ppmw).
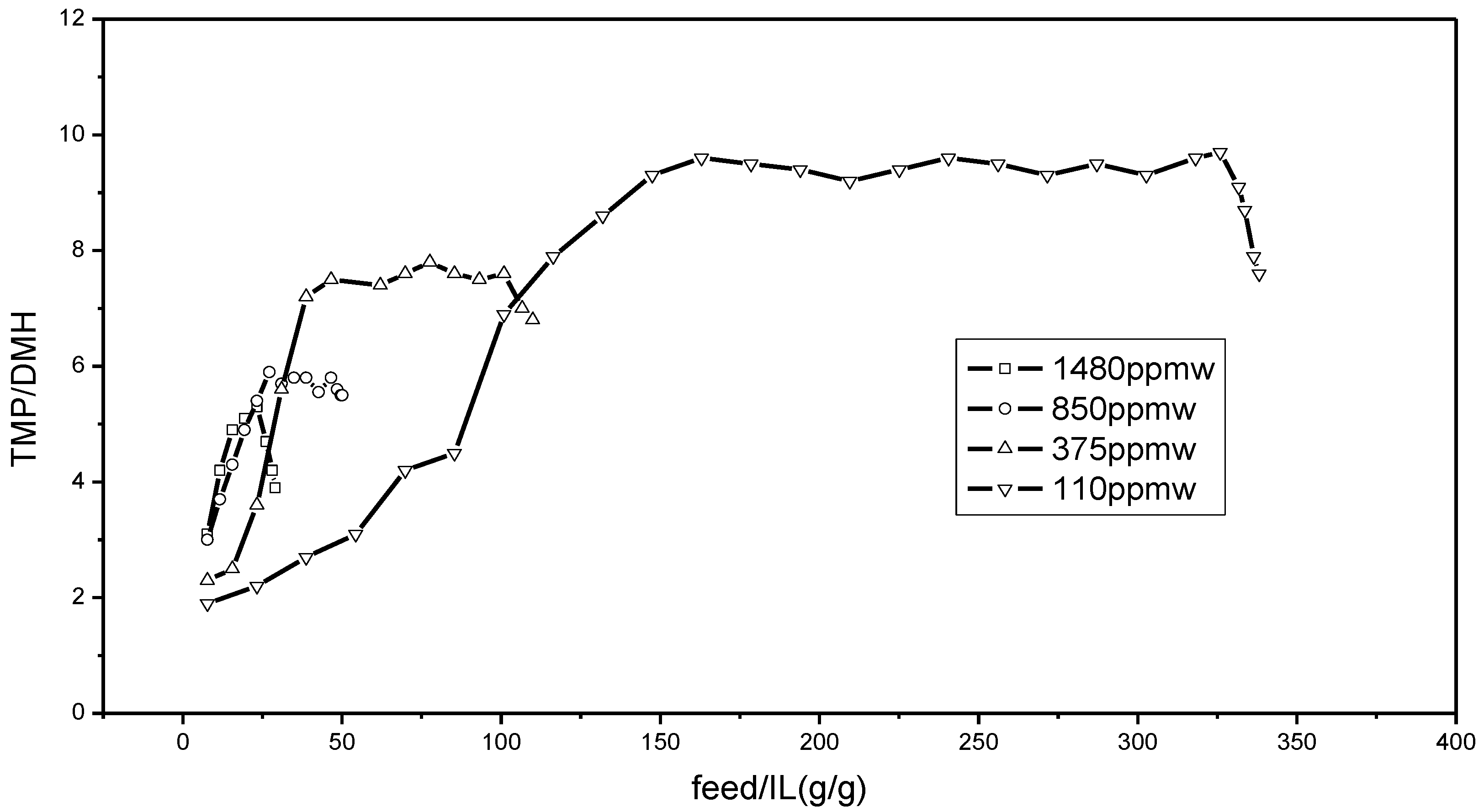
In
Figure 3, the effects of water content in feedstocks on TMP/DMH in alkylates are reported. As with the isooctane content in
Figure 2, a similar trend of TMP/DMH in alkylates is observed. As the amount of the feedstocks treated increased, the ratio of TMP/DMH first increased and then remained stable. Finally, the ratio decreased with the deactivation of the ionic liquid.
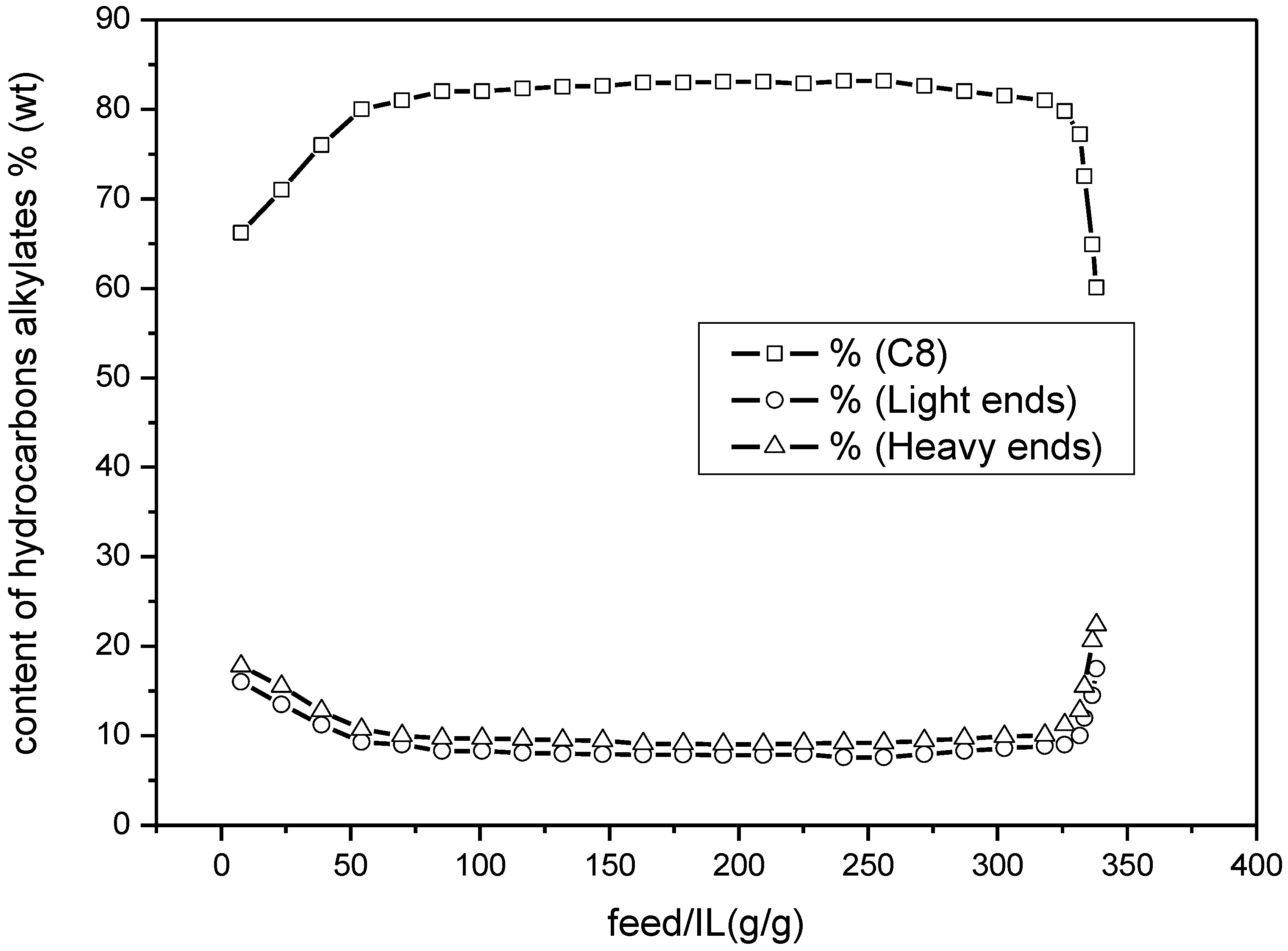
Figure 4 shows the content of different kinds of hydrocarbons in alkylates in the case when the water content in the feedstock was 110 ppmw. The heavy ends, i.e., C9 and C9+, and the light ends, i.e., C5~C7, exhibited similar trend as the increase of the amount of the feedstock treated. In the whole stage of the alkylation, the content of heavy ends was a little higher than that of the light ends.
In this section, the trend of the mass of feedstock treated by 1 g ionic liquid affected by the water content in feedstocks was studied. In order to obtain more detailed information about the deactivation of the chloroaluminate ionic liquids, the amount of water accumulated in the ionic liquid when the ionic liquid was deactivated and the amount of the aluminum chloride in the fresh ionic liquid should be compared.
Table 1 shows the amount of accumulated water in 1 g ionic liquid when the ionic liquid was deactivated. There was 4.805 mmol aluminum chloride in the ionic liquid used in this study. It can be clearly seen that, for different water contents in feedstocks, all the molar quantities of accumulated water in 1 g ionic liquid were approximately about 2.4 mmol, i.e., half of the molar quantity of aluminum chloride in 1 g fresh ionic liquid. Aluminum chloride is very moister-sensitive, the reaction between water and aluminum chloride can be described with the Formula (1) below.
According to Formula (1) and the above comparison, it was conjectured that the chloroaluminate ionic liquids would be deactivated in the case of loss of one half of the aluminum chloride in the fresh ionic liquid. Besides, all the molar quantities of the accumulated water were a little less than 2.4 mmol. The gap increased as the water content in the feedstock decreased.
It is well known that the chloroaluminate ionic liquid can have a different acidity by variation of the AlCl
3/Et
3NHCl molar ratio [
16]. In the ionic liquids with AlCl
3/Et
3NHCl molar ratio <1, the dominant anions are Cl
− and AlCl
4−, and the ionic liquids are basic. In the ionic liquids with AlCl
3/Et
3NHCl molar ratio = 1, there is only AlCl
4¯ in the ionic liquid, the ionic liquid is neutral. The basic and neutral ionic liquids have no catalytic activity for the alkylation of isobutane/butene. In the ionic liquids with the AlCl
3/Et
3NHCl molar ratio >1, the dominant anions are AlCl
4− and Al
2Cl
7−, and the ionic liquids are acidic. The Lewis acidity originates from dissociation of AlCl
3 from the dimeric anion, i.e., Al
2Cl
7− [
11]. The Lewis acid site (dissociative AlCl
3) can be transformed to a Brønsted acid site by complexation of aluminum chloride, hydrogen chloride, and a certain proton acceptor such as butene or cuprous chloride [
17]. The complex acts as an effective catalyst to catalyze the alkylation of isobutane and butene. In this case, hydrogen chloride came from the reaction of water in the feedstock and aluminum chloride as shown in Formula (1).
From the discussion above, it can be deduced that the dissociative AlCl
3 would appear only in the case of the AlCl
3/Et
3NHCl molar ratio >1, and the ionic liquid exhibits Lewis acidity and potential catalytic activity for alkylation. In this study, the molar ratio of AlCl
3/Et
3NHCl in the synthesis of ionic liquid is 2:1. Thus, at most half the amount of aluminum chloride can act as the Lewis acid site (co-catalyst) to form the effectively active site. In the acidic ionic liquid, Al
3OCl
8−, Al
3O
2Cl
6−, Al
2OCl
5−, Al
3Cl
9OH
−, and Al
2Cl
6OH
− are the most probable oxychlorlaluminate or hydroxychloroaluminate species, from the reaction between water and aluminum chloride [
18,
19]. The loss of this part of the aluminum chloride would lead to the deactivation of the chloroaluminate ionic liquids. The Formula (1) describes the reaction of the hydrolysis deactivation of active aluminum chloride in the ionic liquids. The other half of the amount of the aluminum chloride only acts as a component of the neutral solvent ([Et
3NH][AlCl
4]) and is catalytically inactive for alkylation [
20].
From the data in
Table 1, it can be seen that the molar quantities of accumulated water are not equal to the amount of aluminum chloride in the ionic liquid. The gap increased with the increased mass of the feedstock treated. This observation suggested that there would be other factors affecting the lifetime of the chloroaluminate ionic liquids. It was without doubt that traces of aluminum chloride dissolved in the hydrocarbons and were carried out of the alkylation system. This part of the aluminum chloride loss was negligible in the case of a low amount of feedstock treated. However, as the amount of feedstock treated increases, it becomes more and more significant. In the case of low butene conversion, the unreacted butene would carry more aluminum chloride because of the chemical interaction between aluminum chloride and butene.
As mention above, the HCl in the catalysis system came from the reaction between water and aluminum chloride. The content of water therefore influences the concentration and the acidity of the effectively catalytic sites. The low water content in the feedstocks leads to proper acidity of the active sites, resulting in relatively high isooctane selectivity and ratio of TMP to DMH. Whereas, the high water content in feedstocks increases the amount of the active sites, leading to high butene conversion relatively.
2.2. Effect of Methanol
Methanol is usually present in the feedstock of the industrial alkylation process. A marked exotherm of chloroaluminate ionic liquids was observed after addition of methanol dropwise, indicating a strong chemical interaction. So methanol in the feedstock would also affect the catalytic properties of the chloroaluminate ionic liquids. The effect of methanol therefore was investigated by means of a similar method as in
Section 2.1. The water content in the feedstock was 375 ppmw. Methanol was pumped into the feedstock tank by a plunger pump. The methanol contents in this study were 800 ppmw and 1740 ppmw, respectively.
Figure 5 shows the effect of methanol on the amount of feedstock treated by 1 g ionic liquid. With the introduction of methanol into the catalytic system, a decrease of the lifetime of the ionic liquids was observed. When the content of methanol in the feedstock increased from 0 ppmw to 800 ppmw and 1740 ppmw, the mass of feedstock treated by 1 g ionic liquid decreased from 109 g to 70 g and 49 g before deactivation of the ionic liquid (deactivation was also considered when the conversion of 2-butene was lower than 50%). The addition of methanol sped up the deactivation of the ionic liquid. However, at the steady period of the reaction, the butene conversion was not impacted by methanol, indicating no obvious effect of methanol on the initial activity of the ionic liquid. The deactivation acceleration of ionic liquid may be the result of passivation of a part of the active AlCl
3 by methanol. Besides, no methanol was detected in the liquid products, indicating methanol remained in the ionic liquid through a certain kind of interaction with the ionic liquid.
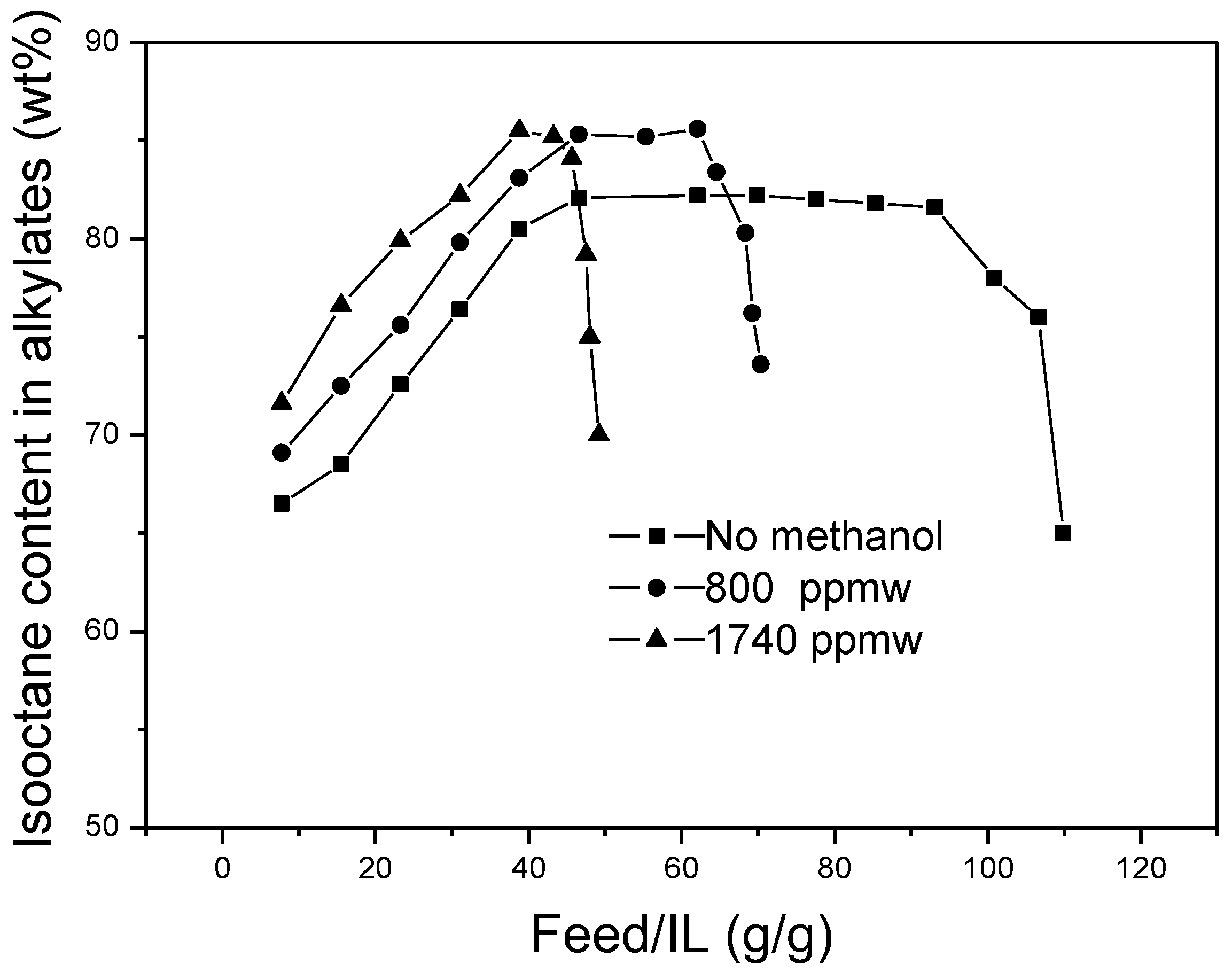
The effects of methanol content in the feedstocks on the isooctane content in alkylates are shown in
Figure 6. In the three curves, the isooctane content in alkylates increased at the onset period and then remained stable to a certain degree, finally decreasing until the deactivation of the ionic liquid. The length of the steady period decreased with the increase of the methanol content in the feedstocks. It also can be seen that the isooctane contents in the products with addition of methanol were generally higher than those without methanol. It was conjectured that addition of methanol was favorable for improvement of isooctane selectivity.
In
Figure 7, the effects of methanol content in feedstocks on TMP/DMH in alkylates were reported. As with the isooctane content in
Figure 7, a similar trend of TMP/DMH in alkylates was observed. As the amount of the feedstocks treated increased, the ratio of TMP/DMH first increased and then remained stable. Finally, the ratio decreased with the deactivation of the ionic liquid. The ratios of TMP/DMH in the products with addition of methanol were also higher than those without methanol.
The reason for the rapid deactivation of the ionic liquid by addition of methanol was studied by means of comparison of the amount of active AlCl
3 in the fresh ionic liquid and the amount of water and methanol accumulated. The results are listed in
Table 2.
Table 2 shows the amount of accumulated methanol and water in 1 g ionic liquid when the ionic liquid was deactivated. As mentioned above, there was 2.402 mmol active aluminum chloride in 1 g of fresh ionic liquid used in this study. It can be clearly seen that, the molar quantities of the accumulated methanol were approximately twice as much as the molar quantities of active aluminum chloride except the aluminum chloride was deactivated by water. This result revealed that one mole of active aluminum chloride would be deactivated by two moles of methanol by a certain kind of chemical interaction. In a previous study on isobutane/butene alkylation catalyzed by solid aluminum chloride, the complex of AlCl
3•CH
3OH was a good catalyst and the catalytic performance was better than with AlCl
3 itself. However, the product of the complexation of aluminum chloride with two molecular proportions of methanol, i.e., AlCl
3•2CH
3OH, was not catalytically active for alkylation [
21,
22]. This conclusion was consistent with the result in this work. An improvement of catalytic performances such as isooctane selectivity and TMP/DMH was also observed in the continuous alkylation catalyzed by ionic liquids. Furthermore, it can be conjectured that the mechanism of alkylation catalyzed by the chloroaluminate ionic liquid would be analogous to that of alkylation catalyzed by solid aluminum chloride.
2.3. Effect of Diethyl Ether
Ether is another kind of impurity in the feedstock of the industrial alkylation process. A strong chemical interaction also occurred when ethers made contact with chloroaluminate ionic liquid. Therefore, the effect of ether on the catalytic performance of the ionic liquid was also investigated by means of a similar method as in
Section 2.2 and
Section 2.3. Diethyl ether was used as a representative in this study. The water content in the feedstock was 375 ppmw. Diethyl ether was added into the feedstock tank by the same method as in
Section 3.3. The diethyl ether contents in this study were 680 ppmw and 1525 ppmw, respectively.
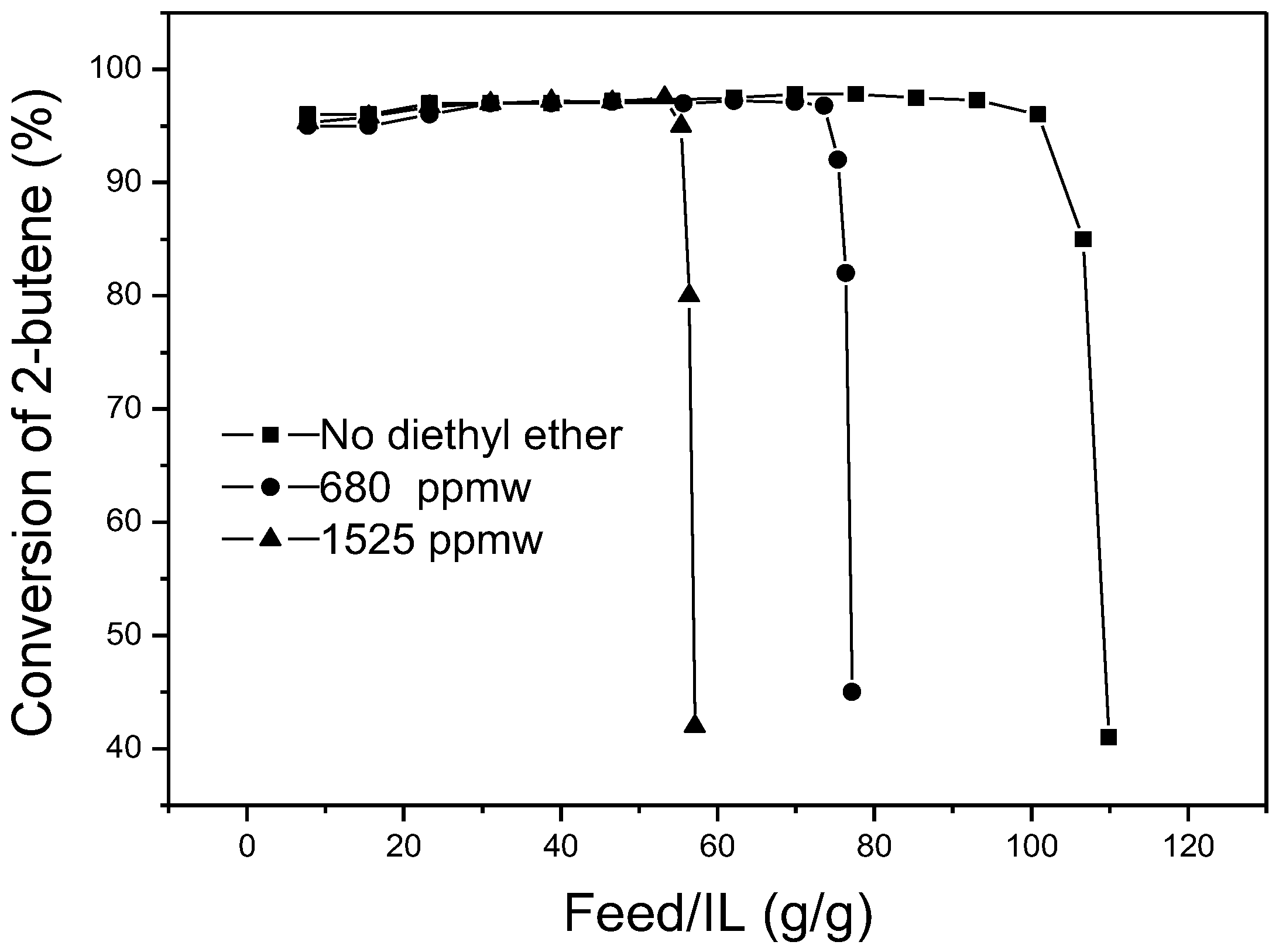
Figure 8 shows the effect of diethyl ether on the amount of feedstock treated by 1 g ionic liquid. As with the effect of methanol, with the addition of diethyl ether into the catalytic system, the lifetime of the ionic liquids diminished. When the content of diethyl ether in the feedstock increased from 0 ppmw to 680 ppmw and 1525 ppmw, the mass of feedstock treated by 1 g ionic liquid decreased from 109 g to 77 g and 57 g before deactivation of the ionic liquid. The addition of diethyl ether also sped up the deactivation of the ionic liquid. Similarly, in the steady period of the reaction, the butene conversion was not impacted by diethyl ether and no diethyl ether was detected in the liquid products. It can be deduced that the deactivation acceleration of ionic liquid may also be the result of passivation of a part of the active AlCl
3 by diethyl ether through complexation, as methanol and diethyl ether with a pair of unshared electrons are both basic.
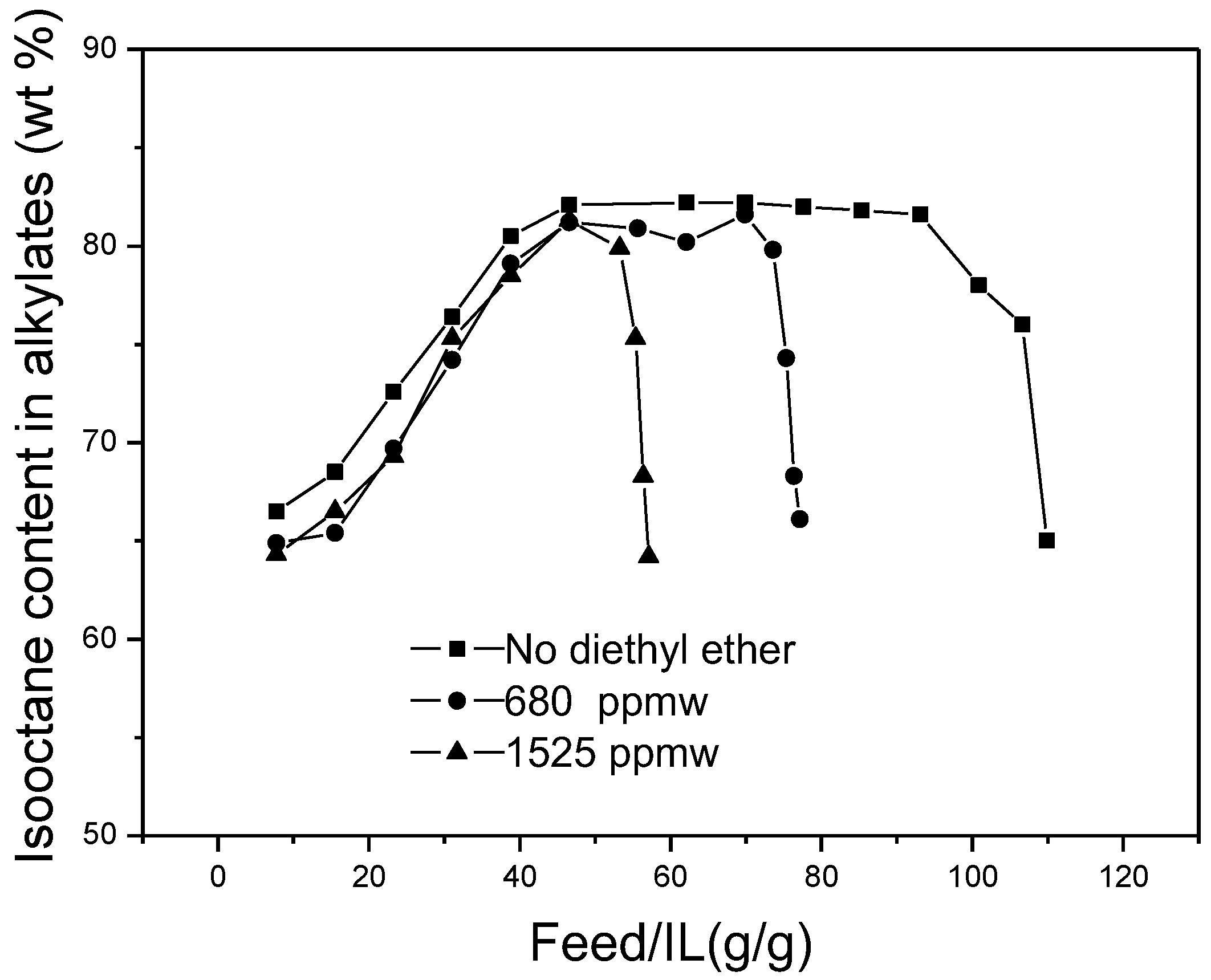
The effects of diethyl ether content in the feedstocks on the isooctane content in alkylates are shown in
Figure 9. Under different conditions, the isooctane content in the alkylates exhibited a similar trend. No improvement of isooctane selectivity was observed.
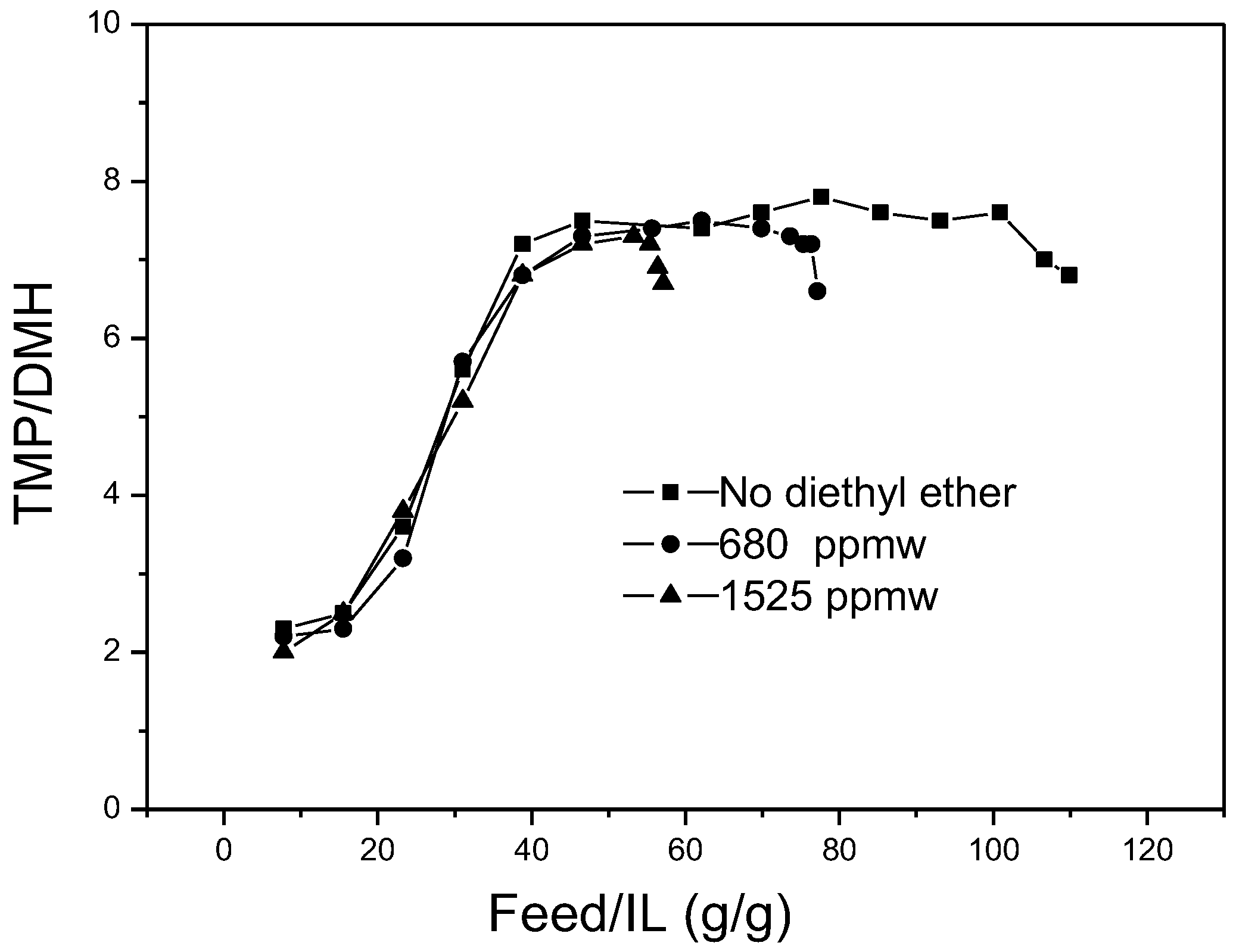
In
Figure 10, the effects of diethyl ether content in feedstocks on TMP/DMH in alkylates are displayed. As with the isooctane content in
Figure 9, a similar trend of TMP/DMH in alkylates was observed. Generally, the ratios of TMP to DMH were not impacted by addition of diethyl ether. No obvious increase or decrease of the ratio was seen with the change of diethyl ether content in the feedstock.
The amount of active AlCl
3 in the fresh ionic liquid and the amount of water and diethyl ether accumulated were also compared in order to study the deactivation of aluminum chloride resulting from addition of diethyl ether. The results are listed in
Table 3.
Table 3 shows the amount of accumulated diethyl ether and water in 1 g ionic liquid when the ionic liquid was deactivated. The molar quantity of active aluminum chloride in 1 g fresh ionic liquid used in this study was 2.402 mmol. It can be clearly seen that the molar quantities of the accumulated diethyl ether were approximately equal to the molar quantities of active aluminum chloride except the aluminum chloride was deactivated by water. This result revealed that the active aluminum chloride would be deactivated by diethyl ether with equimolar proportion through complexation described as AlCl
3•C
2H
5OC
2H
5. In a previous study, the researchers found that, although the equimolar complexes of aluminum chloride with dialkyl ethers had good solvency for the reactants, they were catalytically inactive for hydrocarbon alkylation. These complexes dissolved excess aluminum chloride to form an active alkylation catalyst [
22,
23,
24]. This conclusion was consistent with the result in this work. The availability of the electron pair determined the extent of interaction of methanol or diethyl ether with aluminum chloride. The electron donating ability (i.e., the basicity) of diethyl ether seems stronger than that of methanol, thus it could inactivate aluminum chloride with an equimolar proportion, while the complexation of aluminum chloride with two molecular proportions of methanol was needed to inactivate the aluminum chloride.