Catalytic Degradation of Ortho-Chlorophenol Using Activated Carbon Modified by Different Methods
Abstract
:1. Introduction
2. Results and Discussion
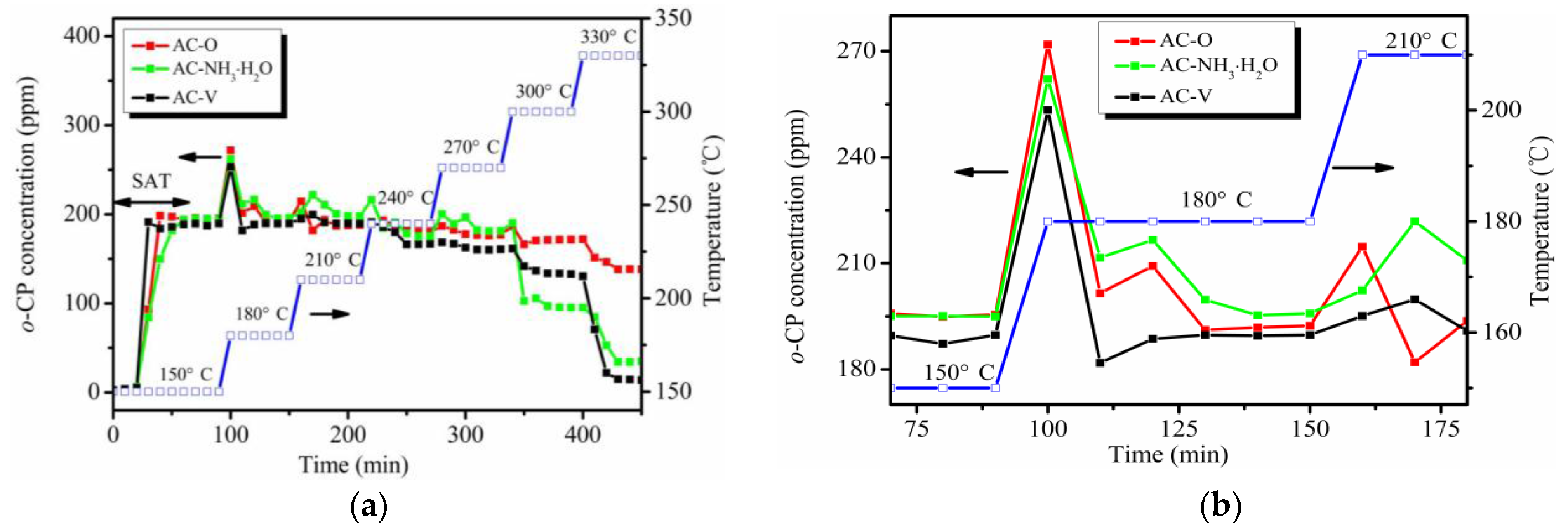
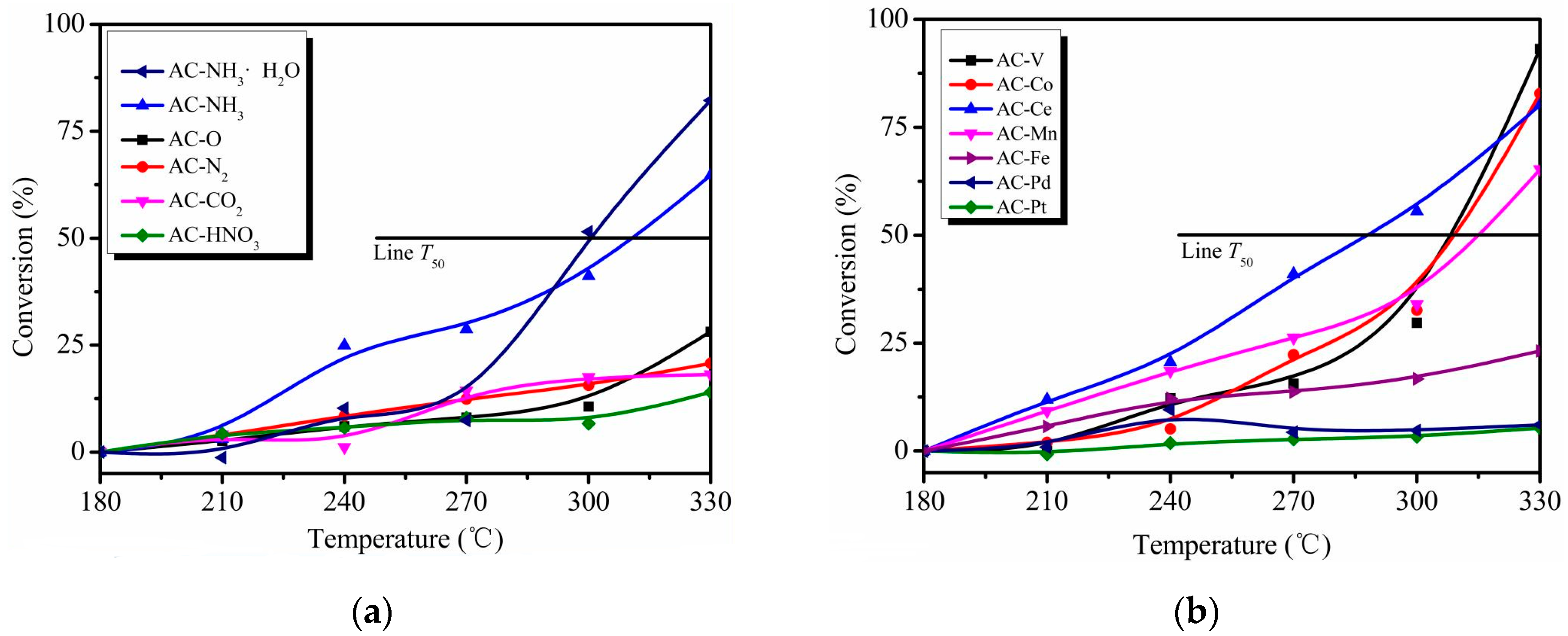
2.1. Catalytic Performance of Different Catalysts for o-CP Oxidation
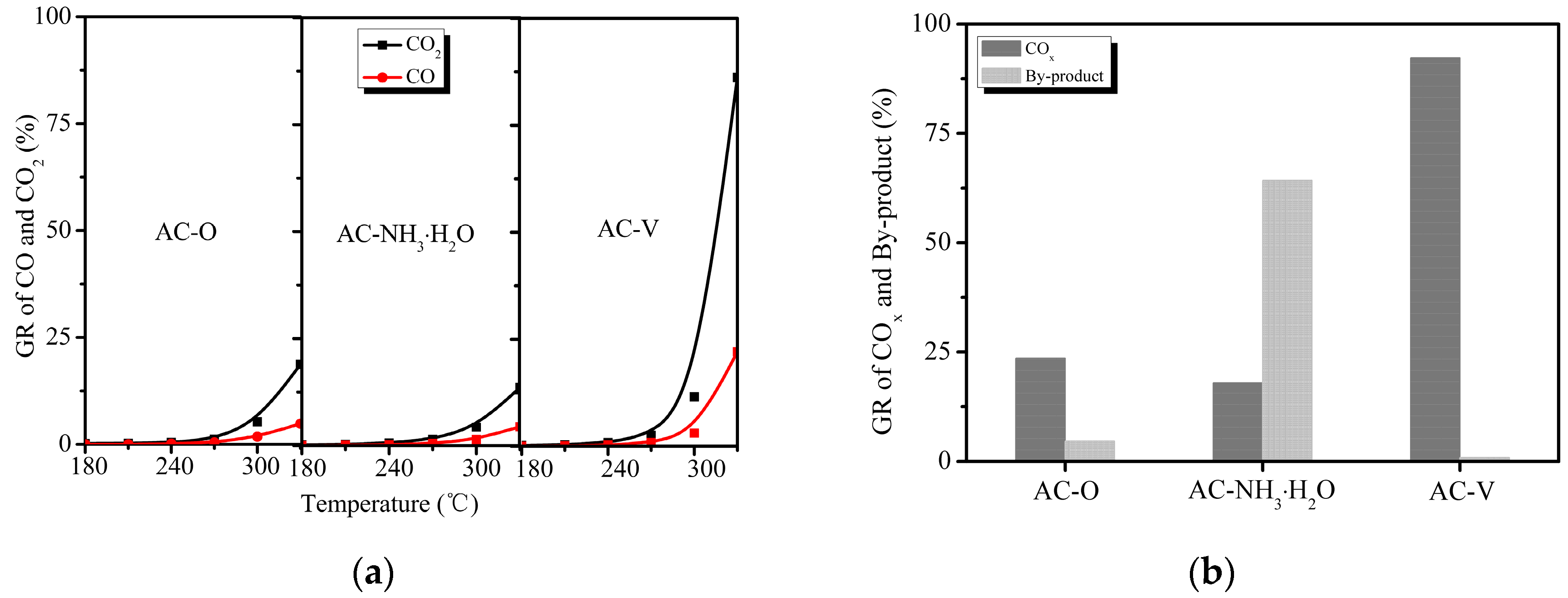
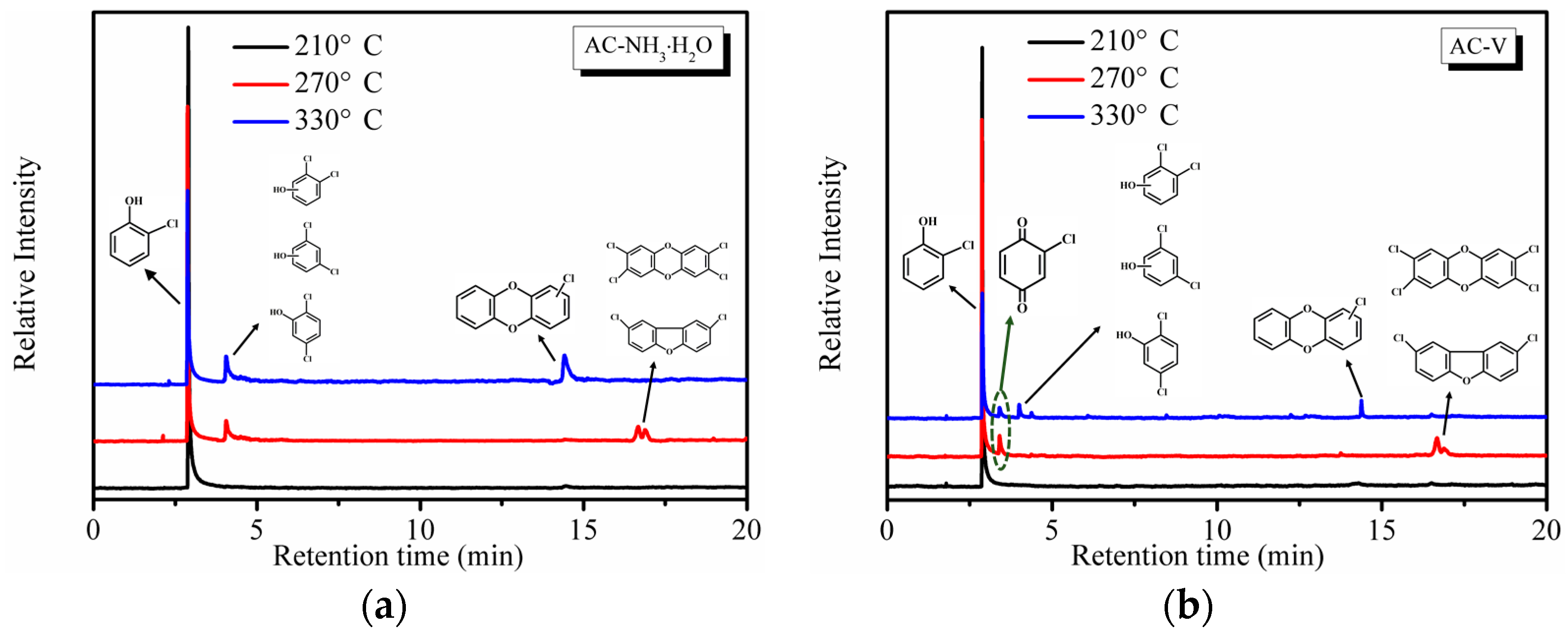
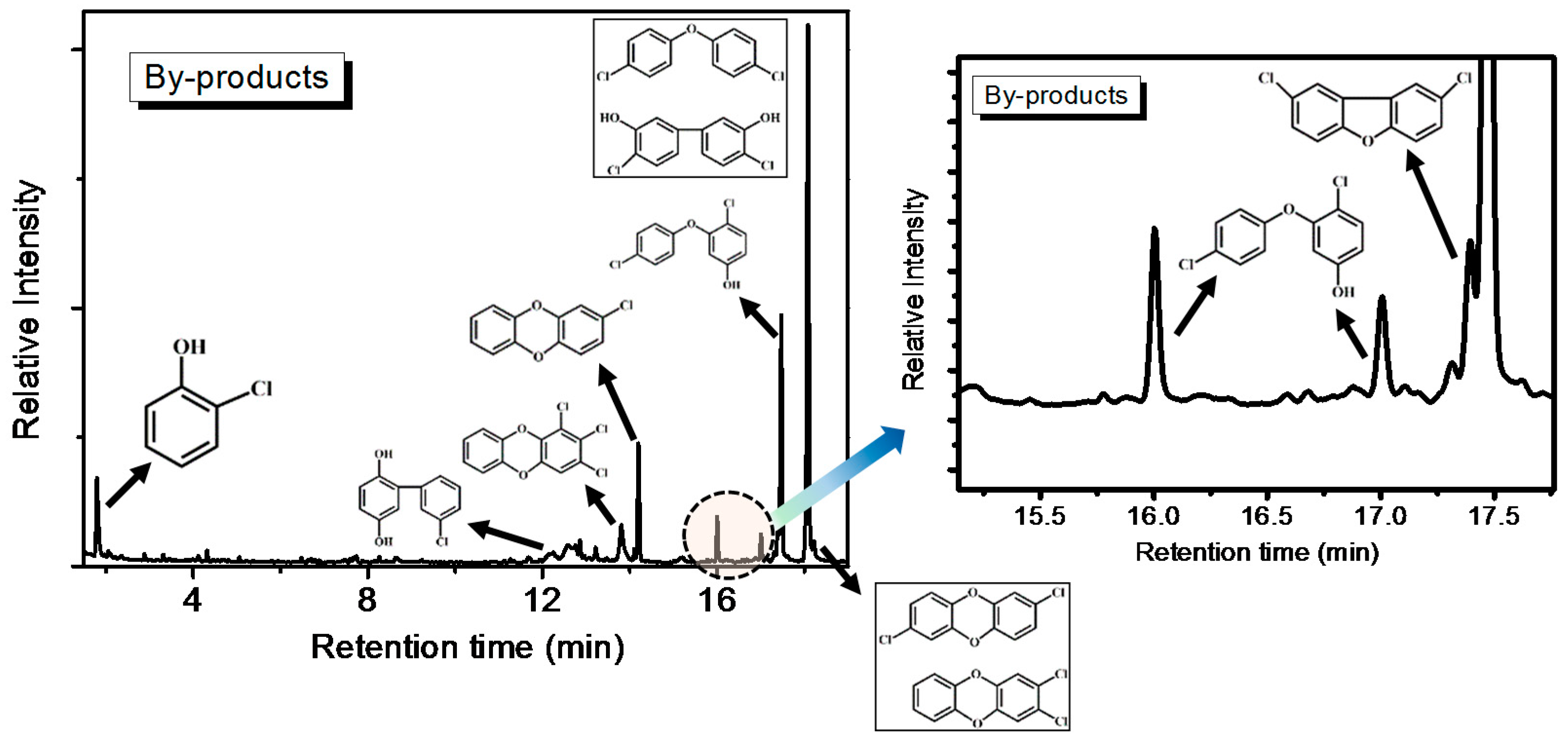
2.2. Catalytic Selectivity and By-Product Analysis
2.2.1. Carbon Mass Balance and Catalytic Selectivity
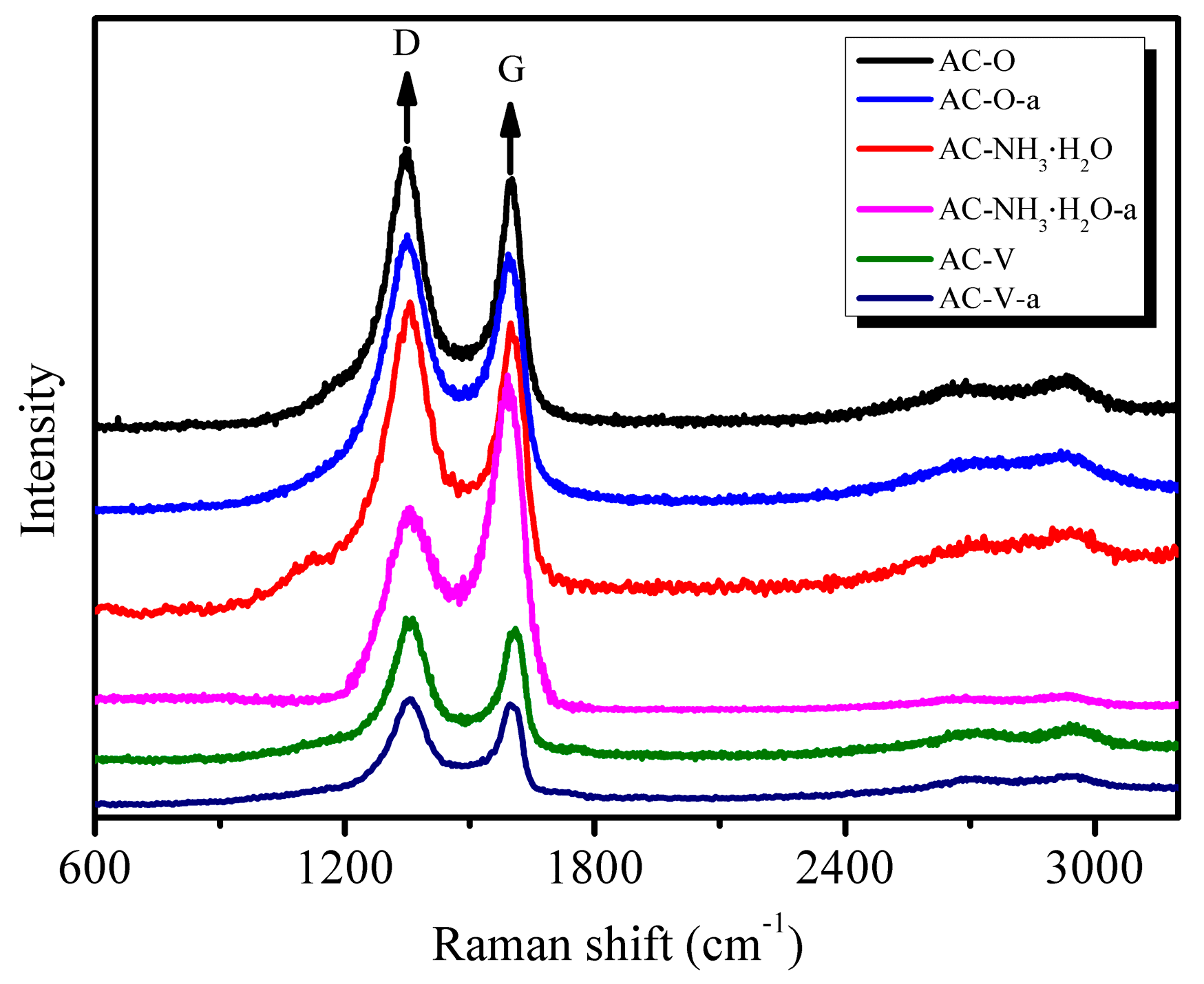
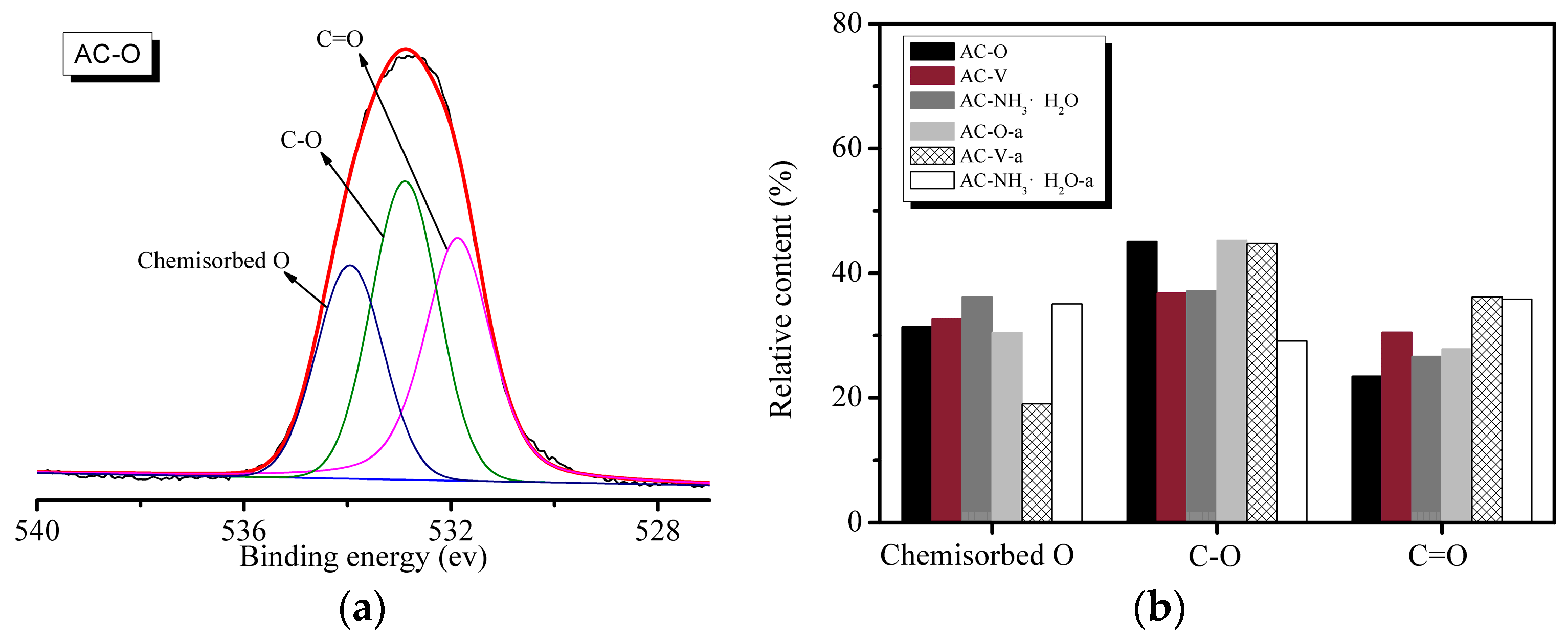
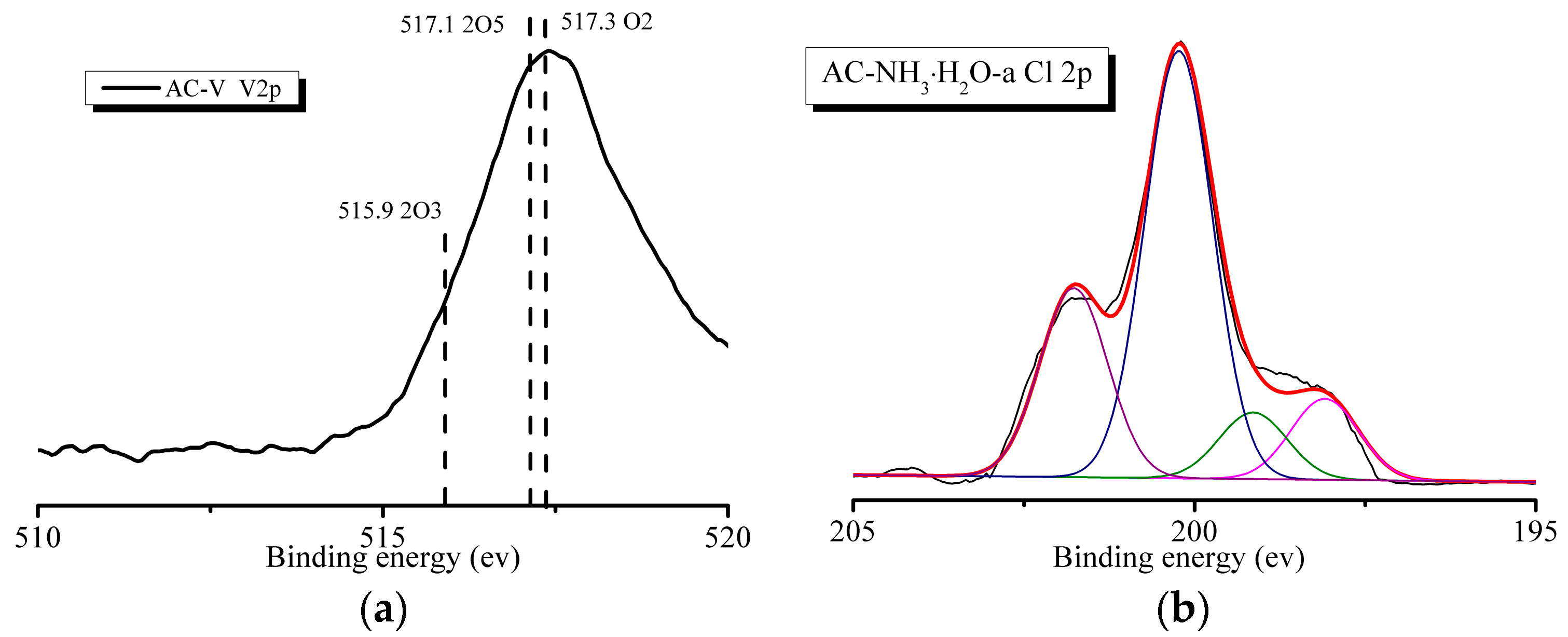
2.2.2. Sample Characterization before and after Reaction
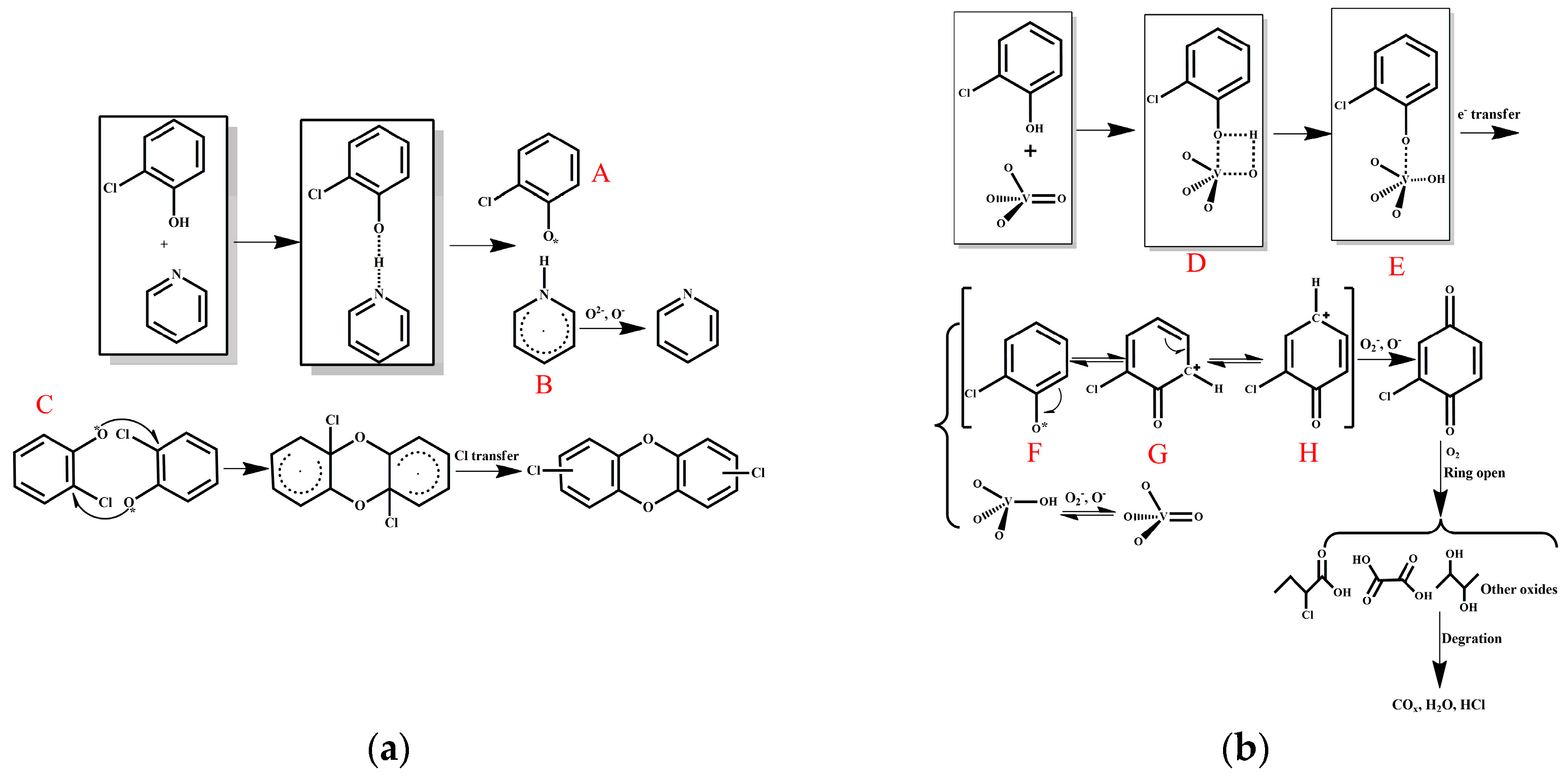
2.3. By-Product Analysis and Catalytic Mechanism
3. Experimental
3.1. Preparation of Modified Activated Carbon
3.1.1. Chemical Treatment
3.1.2. Metal Modification
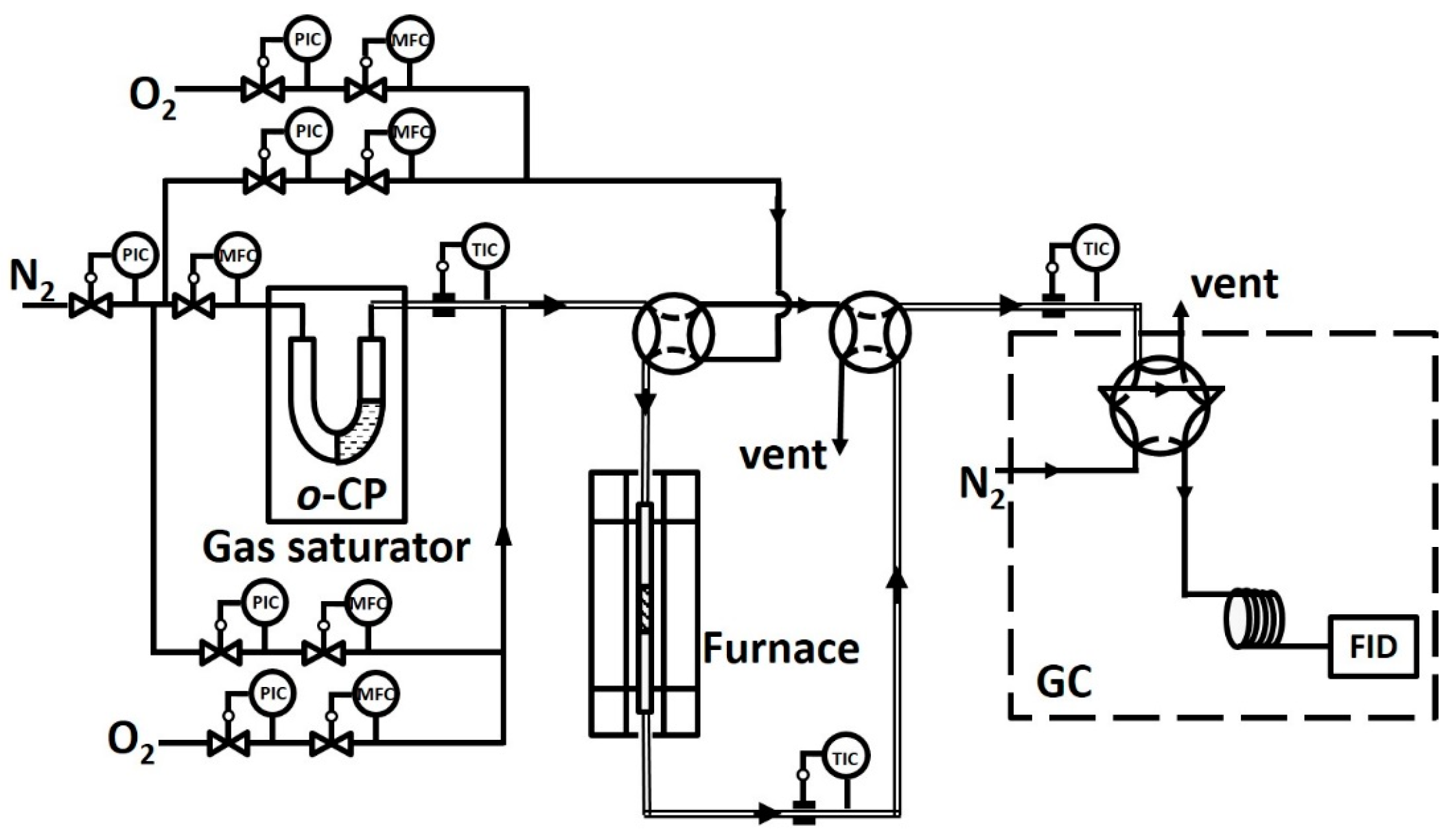
3.2. Catalytic Evaluation and By-Product Measurement
3.3. Sample and Product Characterization
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lomnicki, S.; Dellinger, B. A detailed mechanism of the surface-mediated formation of PCDD/F from the oxidation of 2-Chlorophenol on a CuO/Silica surface. J. Phys. Chem. A 2003, 107, 4387–4395. [Google Scholar] [CrossRef]
- Deng, W.; Dai, Q.; Lao, Y.; Shi, B.; Wang, X. Low temperature catalytic combustion of 1,2-dichlorobenzene over CeO2–TiO2 mixed oxide catalysts. Appl. Catal. B Environ. 2016, 181, 848–861. [Google Scholar] [CrossRef]
- Bjurlid, F.; Kärrman, A.; Ricklund, N.; Hagberg, J. Occurrence of brominated dioxins in a study using various firefighting methods. Sci. Total Environ. 2017, 599, 1213–1221. [Google Scholar] [CrossRef] [PubMed]
- Long, R.Q.; Yang, R.T. Carbon nanotubes as superior sorbent for dioxin removal. J. Am. Chem. Soc. 2001, 123, 2058–2059. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ye, M.; Wang, X.; Liu, W.; Zhu, T. Gas-phase and particle-phase PCDD/F congener distributions in the flue gas from an iron ore sintering plant. J. Environ. Sci. 2017, 54, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Lin, X.; Yu, M.; Mubeen, I.; Buekens, A.; Li, X. Experimental study on PCDD/Fs adsorption onto Nano-graphite. Aerosol Air Qual. Res. 2016, 16, 3281–3289. [Google Scholar] [CrossRef]
- Hung, P.C.; Lo, W.C.; Chi, K.H.; Chang, S.H.; Chang, M.B. Reduction of dioxin emission by a multi-layer reactor with bead-shaped activated carbon in simulated gas stream and real flue gas of a sinter plant. Chemosphere 2011, 82, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Furubayashi, M.; Hamano, S.; Nagai, K. Regeneration of activated carbon used for removal of dioxins removal from flue gas. Kagaku Kogaku Ronbunshu 2002, 28, 718–725. [Google Scholar] [CrossRef]
- Atkinson, J.D.; Hung, P.C.; Zhang, Z.; Chang, M.B.; Yan, Z.; Rood, M.J. Adsorption and destruction of PCDD/Fs using surface-functionalized activated carbons. Chemosphere 2015, 118, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Everaert, K.; Baeyens, J. Removal of PCDD/F from flue gases in fixed or moving bed adsorbers. Waste Manag. 2004, 24, 37–42. [Google Scholar] [CrossRef]
- Hell, K.; Stieglitz, L.; Altwicker, E.R.; Addink, R.; Will, R. Reactions of 2,4,6-trichlorophenol on model fly ash: Oxidation to CO and CO2, condensation to PCDD/F and conversion into related compounds. Chemosphere 2001, 42, 697–702. [Google Scholar] [CrossRef]
- Alslaibi, T.M.; Abustan, I.; Ahmad, M.A.; Foul, A.A. A review: Production of activated carbon from agricultural byproducts via conventional and microwave heating. J. Chem. Technol. Biotechnol. 2013, 88, 1183–1190. [Google Scholar] [CrossRef]
- Paulis, M.; Gandía, L.M.; Gil, A.; Sambeth, J.; Odriozola, J.A.; Montes, M. Influence of the surface adsorption–desorption processes on the ignition curves of volatile organic compounds (VOCs) complete oxidation over supported catalysts. Appl. Catal. B Environ. 2000, 26, 37–46. [Google Scholar] [CrossRef]
- Leclercq, J.; Giraud, F.; Bianchi, D.; Fiaty, K.; Gaillard, F. Novel inductively-heated catalytic system for fast VOCs abatement, application to IPA in air. Appl. Catal. B Environ. 2014, 146, 131–137. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Mouscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Wesełucha-Birczyńska, A.; Babeł, K.; Jurewicz, K. Carbonaceous materials for hydrogen storage investigated by 2D Raman correlation spectroscopy. Vib. Spectrosc. 2012, 60, 206–211. [Google Scholar] [CrossRef]
- Savchenko, D.; Vorliček, V.; Kalabukhova, E.; Sitnikov, A.; Vasin, A.; Kysil, D.; Sevostianov, S.; Tertykh, V.; Nazarov, A. Infrared, Raman and magnetic resonance spectroscopic study of SiO2:C Nanopowders. Nanoscale Res. Lett. 2017, 12, 292. [Google Scholar] [CrossRef] [PubMed]
- Alsawat, M.; Altalhi, T.; Santos, A.; Losic, D. Facile and controllable route for nitrogen doping of carbon nanotubes composite membranes by catalyst-free Chemical Vapour Deposition. Carbon 2016, 106, 295–305. [Google Scholar] [CrossRef]
- Hetrick, C.E.; Lichtenberger, J.; Amiridis, M.D. Catalytic oxidation of chlorophenol over V2O5/TiO2 catalysts. Appl. Catal. B Environ. 2008, 77, 255–263. [Google Scholar] [CrossRef]
- Dai, Q.; Wang, W.; Wang, X.; Lu, G. Sandwich-structured CeO2@ZSM-5 hybrid composites for catalytic oxidation of 1, 2-dichloroethane: An integrated solution to coking and chlorine poisoning deactivation. Appl. Catal. B Environ. 2017, 203, 31–42. [Google Scholar] [CrossRef]
- Evans, C.S.; Dellinger, B. Mechanisms of dioxin formation from the high-temperature oxidation of 2-chlorophenol. Environ. Sci. Technol. 2005, 39, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Muñiz, J.; Marbán, G.; Fuertes, A.B. Low temperature selective catalytic reduction of NO over modified activated carbon fibres. Appl. Catal. B Environ. 2000, 27, 27–36. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y.; Long, D.; Mochida, I.; Qiao, W.; Zhan, L.; Liu, X.; Yoon, S.; Ling, L. Kinetics and Mechanism study of low-temperature selective catalytic reduction of NO with urea supported on pitch-based spherical activated carbon. Ind. Eng. Chem. Res. 2011, 50, 6017–6027. [Google Scholar] [CrossRef]
- Sun, P.; Wang, W.; Dai, X.; Weng, X.; Wu, Z. Mechanism study on catalytic oxidation of chlorobenzene over MnxCe1−xO2/H-ZSM5 catalysts under dry and humid conditions. Appl. Catal. B Environ. 2016, 198, 389–397. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, Q.; Liu, K.R. Dual degradation of gaseous 1,2-dichlorobenzene and PCDD/Fs using Ce doped VxOy/TiO2 immobilized on cordierite. Chemosphere 2016, 154, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, X.; Liu, X.L.; Zeng, J.L.; Guo, Y.Y.; Zhu, T.Y. Kinetics and mechanism study on catalytic oxidation of chlorobenzene over V2O5/TiO2 catalysts. J. Mol. Catal. A Chem. 2015, 402, 1–9. [Google Scholar] [CrossRef]
- Gallastegi-Villa, M.; Aranzabal, A.; González-Marcos, J.A.; González-Velasco, J.R. Metal-loaded ZSM5 zeolites for catalytic purification of dioxin/furans and NOx containing exhaust gases from MWI plants: Effect of different metal cations. Appl. Catal. B Environ. 2016, 184, 238–245. [Google Scholar] [CrossRef]
- Yu, M.; Li, W.; Li, X.; Lin, X.; Chen, T.; Yan, J. Development of new transition metal oxide catalysts for the destruction of PCDD/Fs. Chemosphere 2016, 156, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Debecker, D.P.; Bertinchamps, F.; Blangenois, N.; Eloy, P.; Gaigneaux, E.M. On the impact of the choice of model VOC in the evaluation of V-based catalysts for the total oxidation of dioxins: Furan vs. chlorobenzene. Appl. Catal. B Environ. 2007, 74, 223–232. [Google Scholar] [CrossRef]

| Catalysts | SBET/m2·g−1 | Vtotal/cm3·g−1 | Vmi/cm3·g−1 | Pore Size/nm |
|---|---|---|---|---|
| AC–O | 553.1 | 0.3157 | 0.2783 | 0.3692 |
| AC–NH3·H2O | 600.9 | 0.3494 | 0.2984 | 0.3729 |
| AC–V | 599.3 | 0.3440 | 0.2981 | 0.3762 |
| AC–O-a | 155.5 | 0.1027 | 0.0740 | 0.4086 |
| AC–NH3·H2O-a | 91.2 | 0.0676 | 0.0417 | 0.4253 |
| AC–V-a | 172.8 | 0.1270 | 0.0795 | 0.3911 |
| Samples | AC–O | AC–NH3·H2O | AC–V | AC–O-a | AC–NH3·H2O-a | AC–V-a |
|---|---|---|---|---|---|---|
| N/C | 0.68 | 0.54 | 0.58 | 0.76 | 0.43 | 0.79 |
| O/C | 19.37 | 19.56 | 21.31 | 20.84 | 21.39 | 22.79 |
| Cl/C | 0.05 | 0.04 | 0.04 | 0.84 | 0.40 | 0.49 |
| V/C | - | - | 0.60 | - | - | 0.55 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Y.; Guo, Y.; Luo, L.; Zhu, T. Catalytic Degradation of Ortho-Chlorophenol Using Activated Carbon Modified by Different Methods. Catalysts 2018, 8, 37. https://doi.org/10.3390/catal8010037
Zheng Y, Guo Y, Luo L, Zhu T. Catalytic Degradation of Ortho-Chlorophenol Using Activated Carbon Modified by Different Methods. Catalysts. 2018; 8(1):37. https://doi.org/10.3390/catal8010037
Chicago/Turabian StyleZheng, Yang, Yangyang Guo, Lei Luo, and Tingyu Zhu. 2018. "Catalytic Degradation of Ortho-Chlorophenol Using Activated Carbon Modified by Different Methods" Catalysts 8, no. 1: 37. https://doi.org/10.3390/catal8010037





