Over-Expression of the Thermobifida fusca β-Glucosidase in a Yarrowia lipolytica Transformant to Degrade Soybean Isoflavones
Abstract
:1. Introduction
2. Results
2.1. Amplification and Construction of the bgl Gene in a Y. lipolytica Expression System
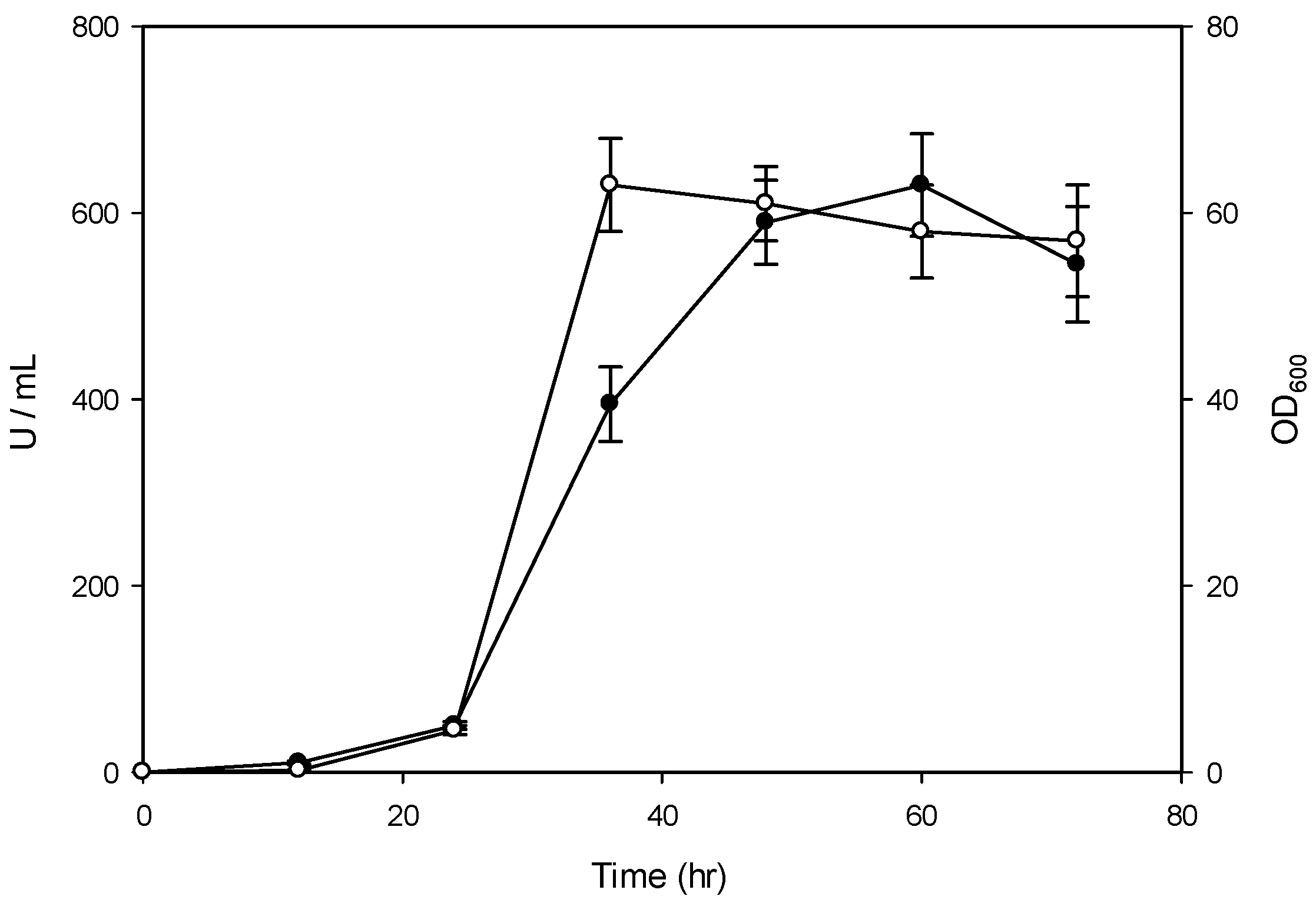
2.2. Constitutive Expression of the bgl Gene in a Y. lipolytica Transformant
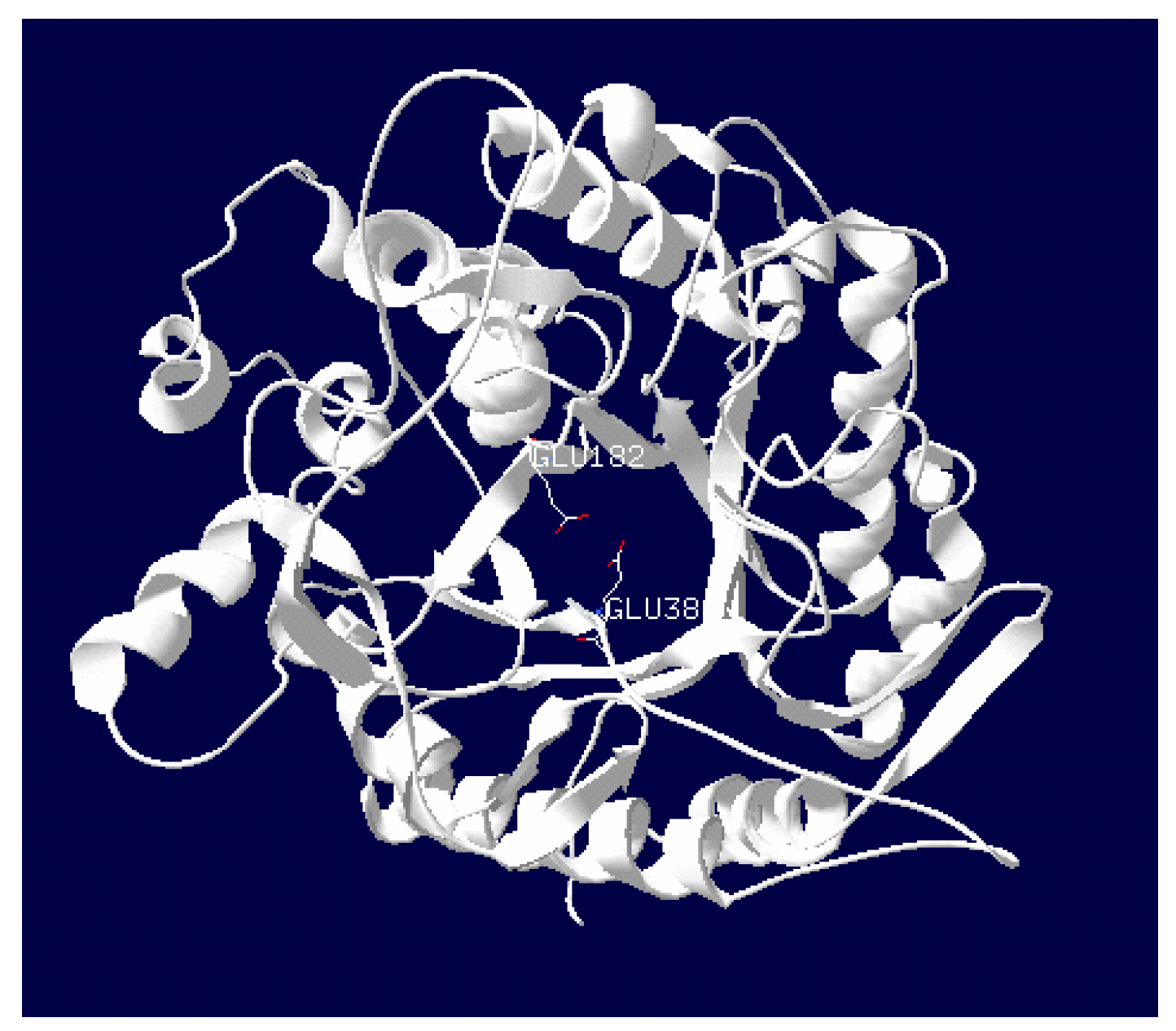
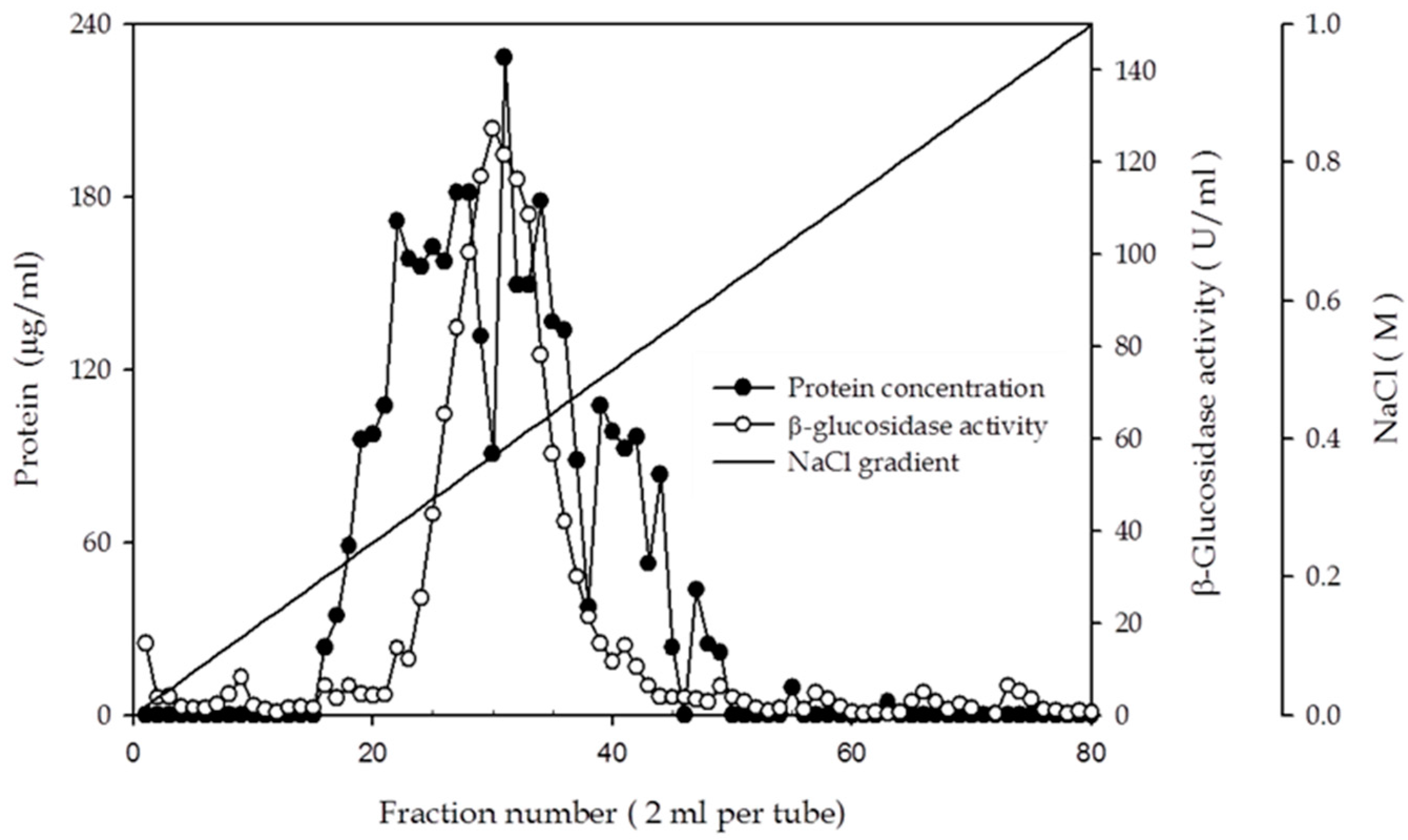
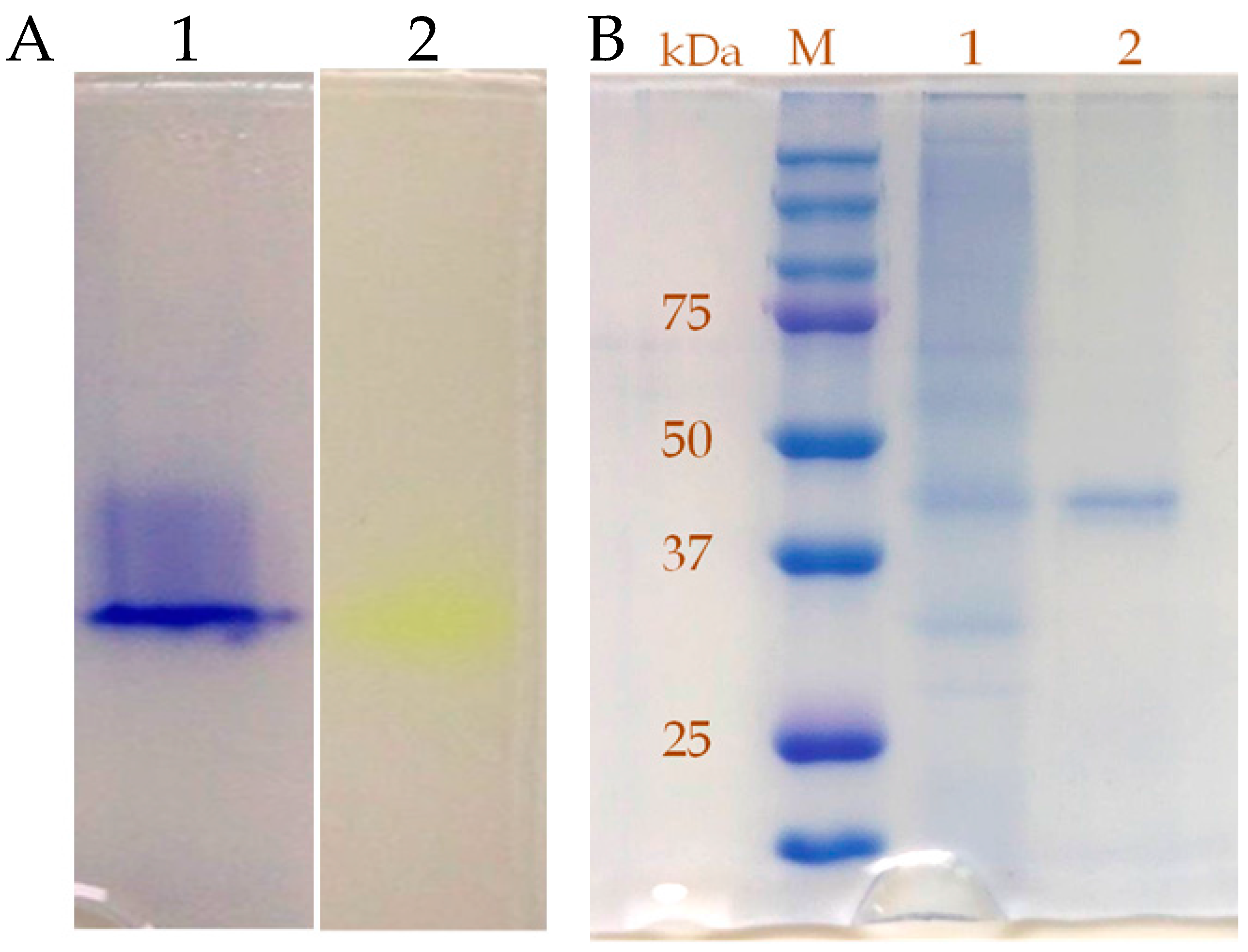
2.3. Purification and Characterization of β-Glucosidase from the Y. lipolytica Transformant (pYLSC1-bgl)
2.4. Substrate-Specific Characterization of the β-Glucosidase from the Y. lipolytica Transformant (pYLSC1-bgl)
3. Discussion
4. Materials and Methods
4.1. Microorganisms and Vectors
4.2. Construction of the β-Glucosidase Expression Plasmid
4.3. Transformation and Screening of Y. lipolytica Transformant
4.4. β-Glucosidase Activity Assay
4.5. Expression of β-Glucosidase in Hinton Flask
4.6. Enzyme Purification
4.7. Hydrolysis of Flavonoid
4.8. Detection of Flavonoid with HPLC
4.9. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hsieh, M.C.; Graham, T.L. Partial purification and characterization of a soybean β-glucosidase with high specific activity towards isoflavone conjugates. Phytochemistry 2001, 58, 995–1005. [Google Scholar] [CrossRef]
- Pei, X.; Yi, Z.; Tang, C.; Wu, Z. Three amino acid changes contribute markedly to the thermostability of β-glucosidase BglC from Thermobifida fusca. Bioresour. Technol. 2011, 102, 3337–3342. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Gautam, A.; Dutt, D. Co-Cultivation of Penicillium sp. AKB-24 and Aspergillus nidulans AKB-25 as a Cost-Effective Method to Produce Cellulases for the Hydrolysis of Pearl Millet Stover. Fermentation 2016, 2, 12. [Google Scholar] [CrossRef]
- Brethauer, S.; Wyman, C.E. Review: Continuous hydrolysis and fermentation for cellulosic ethanol production. Bioresour. Technol. 2010, 101, 4862–4874. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wu, Y.; Liu, Y.; Wu, Z. Complex Enzyme-Assisted Extraction Releases Antioxidative Phenolic Compositions from Guava Leaves. Molecules 2017, 22, 1648. [Google Scholar] [CrossRef] [PubMed]
- Mai, Z.; Su, H.; Zhang, S. Characterization of a Metagenome-Derived β-Glucosidase and Its Application in Conversion of Polydatin to Resveratrol. Catalysts 2016, 6, 35. [Google Scholar] [CrossRef]
- Georgetti, S.R.; Vicentini, F.T.M.C.; Yokoyama, C.Y.; Borin, M.F.; Spadaro, A.C.C.; Fonseca, M.J.V. Enhanced in vitro and in vivo antioxidant activity and mobilization of free phenolic compounds of soybean flour fermented with different β-glucosidase-producing fungi. J. Appl. Microbiol. 2009, 106, 459–466. [Google Scholar] [CrossRef] [PubMed]
- McCue, P.; Horii, A.; Shetty, K. Mobilization of phenolic antioxidants from defatted soybean powders by Lentinus edodes during solid-state bioprocessing is associated with enhanced production of laccase. Innov. Food Sci. Emerg. Technol. 2004, 5, 385–392. [Google Scholar] [CrossRef]
- Chien, H.L.; Huang, H.Y.; Chou, C.C. Transformation of isoflavone phytoestrogens during the fermentation of soymilk with lactic acid bacteria and bifidobacteria. Food Microbiol. 2006, 23, 772–778. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Wei, Y.T.; Chou, C.C. Enhanced antioxidative activity of soybean koji prepared with various filamentous fungi. Food Microbiol. 2006, 23, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.H.; Yang, C.H. The isolation and identification of a lignocellulolytic and thermophilic actinomycete. Food Sci. Agric. Chem. 2002, 4, 89–94. [Google Scholar]
- Spiridonov, N.A.; Wilson, D.B. Cloning and biochemical characterization of BglC, a β-glucosidase from the cellulolytic actinomycete Thermobifida fusca. Curr. Microbiol. 2001, 42, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Zamost, B.L.; Nielsen, H.K.; Starnes, R.L. Thermostable enzymes for industrial application. J. Ind. Microbiol. 1991, 8, 71–82. [Google Scholar] [CrossRef]
- Madzak, C.; Treton, B.; Blanchin-Roland, S. Strong hybrid promoters and integrative expression/secretion vectors for quasi-constitutive expression of heterologous proteins in the yeast Yarrowia lipolytica. J. Mol. Microbiol. Biotechnol. 2000, 2, 207–216. [Google Scholar] [PubMed]
- Madzak, C.; Gaillardin, C.; Beckerich, J.M. Heterologous protein expression and secretion in the non-conventional yeast Yarrowia lipolytica. J. Biotechnol. 2004, 109, 63–81. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.H.; Huang, Y.C.; Chen, C.Y.; Wen, C.Y. Heterologous expression of Thermobifida fusca thermostable alpha-amylase in Yarrowia lipolytica and its application in boiling stable resistant sago starch preparation. J. Ind. Microbiol. Biotechnol. 2010, 37, 953–960. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.C.; Chen, Y.F.; Chen, C.Y.; Chen, W.L.; Ciou, Y.P.; Liu, W.H.; Yang, C.H. Production of ferulic acid from lignocellulolytic agricultural biomass by Thermobifida fusca thermostable esterase produced in Yarrowia lipolytica transformant. Bioresour. Technol. 2011, 102, 8117–8122. [Google Scholar] [CrossRef] [PubMed]
- Coulon, S.; Chemardin, P.; Gueguen, Y.; Arnaud, A.; Galzy, P. Purification and characterization of an intracellular β-glucosidase from Lactobacillus casein ATCC 393. Appl. Biochem. Biotechnol. 1998, 74, 105–114. [Google Scholar] [CrossRef]
- Li, K.B.; Chan, K.Y. Production and properties of β-Glucosidase from Lactobacillus acidophilus. Appl. Environ. Microbiol. 1983, 46, 1380–1387. [Google Scholar] [PubMed]
- Riou, C.; Salmon, J.; Vallier, M.; Gunata, Z.; Barre, P. Purification, characterization, and substrate specificity of a novel highly glucose-tolerant β-glucosidase from Aspergillus oryzae. Appl. Environ. Microbiol. 1998, 64, 3607–3614. [Google Scholar] [PubMed]
- Suzuki, H.; Takahashi, S.; Watanabe, R.; Fukushima, Y.; Fujita, N.; Noguchi, A.; Yokoyama, R.; Nishitani, K.; Nishino, T.; Nakayama, T. An isoflavone conjugate-hydrolyzing β-glucosidase from the roots of soybean (Glycine max) seedlings: Purification, gene cloning, phylogenetics, and cellular localization. J. Biol. Chem. 2006, 281, 30251–30259. [Google Scholar] [CrossRef] [PubMed]
- Berrin, J.G.; Czjzek, M.; Kroon, P.A.; Mclauchlan, W.R.; Puigserver, A.; Williamson, G.; Juge, N. Substrate (aglycone) specificity of human cytosolic β-glucosidase. Biochem. J. 2003, 373, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Wolosowska, S.; Synowiecki, J. Thermostable β-glucosidase with a broad substrate specifity suitable for processing of lactose-containing products. Food Chem. 2004, 85, 181–187. [Google Scholar] [CrossRef]
- Wang, H.; Murphy, P.A. Isoflavone content in commercial soybean foods. J. Agric. Food Chem. 2009, 42, 1666–1673. [Google Scholar] [CrossRef]
- Otieno, D.O.; Ashton, J.F.; Shah, N.P. Evaluation of enzymic for biotransformation of isoflavone phytoestrogen in soymilk by Bifidobacterium animalis, Lactobacillus acidophilus and Lactobacillus casei. Food Res. Int. 2006, 39, 394–407. [Google Scholar] [CrossRef]
- Williamson, G.; Plumb, G.W.; Uda, Y.; Price, K.R.; Rhodes, M.J.C. Dietary quercetin glycosides: Antioxidant activity and induction of the anticarcinogenic phase II marker enzyme quinone reductase in Hepalclc7 cells. Carcinogenesis 1996, 17, 2385–2387. [Google Scholar] [CrossRef] [PubMed]
- Day, A.J.; DuPont, M.S.; Ridley, S.; Rhodes, M.; Rhodes, M.J.C.; Morgan, M.R.A.; Williamson, G. Deglycosylation of favonoid and isofavonoid glycosides by human small intestine and liver β-glucosidase activity. FEBS Lett. 1998, 436, 71–75. [Google Scholar] [CrossRef]
- Schmidt, S.; Rainieri, S.; Witte, S.; Matern, U.; Martens, S. Identification of a Saccharomyces cerevisiae glucosidase that hydrolyzes flavonoid glucosides. Appl. Environ. Microbiol. 2011, 77, 1751–1757. [Google Scholar] [CrossRef] [PubMed]


| Total Protein (mg) | Total Activity (U) | Specific Activity (U/mg) | Purification (fold) | Yield (%) | |
|---|---|---|---|---|---|
| Culture Filtration | 40,176 | 2730 | 0.068 | 1.0 | 100 |
| Pellicon ultrafiltration | 16,797 | 2402 | 0.143 | 2.1 | 88 |
| DEAE-sepharose FF | 1840 | 1141 | 0.62 | 9.2 | 41.8 |
| Metal Salt/Chemical Reagent a | Relative Activity (%) b |
|---|---|
| Control | 100 |
| CoCl2 | 105 |
| MnCl2 | 105 |
| FeCl3 | 100 |
| CaCl2 | 93 |
| ZnCl2 | 90 |
| MgCl2 | 87 |
| AgNO3 | 22 |
| BaCl2 | 17 |
| CuCl2 | 14 |
| HgCl2 | 0 |
| 2-Mercaptoethanol | 96 |
| EDTA | 93 |
| DTT c | 85 |
| PMSF c | 84 |
| Iodoacetate | 7 |
| PCMB c | 0 |
| Substrate | Specific Activity a |
|---|---|
| Cellobiose b | 22.03 |
| Lactose | 2.70 |
| Maltose | 0.41 |
| Sucrose | 0.21 |
| α-Cellulose | 0.20 |
| Carboxymethyl cellulose | 0.23 |
| p-Nitrophenyl-β-d-glucopyranoside c | 28.12 |
| p-Nitrophenyl-β-d-galactopyranoside | 19.29 |
| p-Nitrophenyl-β-d-xylopyranoside | 0.28 |
| p-Nitrophenyl-phosphate | 1.86 |
| Substrate | Conversion Rate (μmol/min/unit) |
|---|---|
| Genistein-7-glucoside | 50.0 |
| Daidzein-7-glucoside | 80.5 |
| Cyanidin-3-glucoside | 24.4 |
| Luteolin-7-glucoside | 0 |
| Quercetin-3-glucoside | 0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.-L.; Yang, Y.-M.; Guo, G.-W.; Chen, C.-Y.; Huang, Y.-C.; Liu, W.-H.; Huang, K.-F.; Yang, C.-H. Over-Expression of the Thermobifida fusca β-Glucosidase in a Yarrowia lipolytica Transformant to Degrade Soybean Isoflavones. Catalysts 2018, 8, 24. https://doi.org/10.3390/catal8010024
Chen W-L, Yang Y-M, Guo G-W, Chen C-Y, Huang Y-C, Liu W-H, Huang K-F, Yang C-H. Over-Expression of the Thermobifida fusca β-Glucosidase in a Yarrowia lipolytica Transformant to Degrade Soybean Isoflavones. Catalysts. 2018; 8(1):24. https://doi.org/10.3390/catal8010024
Chicago/Turabian StyleChen, Wei-Lin, Yo-Ming Yang, Gui-Wen Guo, Cheng-Yu Chen, Yu-Chun Huang, Wen-Hsiung Liu, Keh-Feng Huang, and Chao-Hsun Yang. 2018. "Over-Expression of the Thermobifida fusca β-Glucosidase in a Yarrowia lipolytica Transformant to Degrade Soybean Isoflavones" Catalysts 8, no. 1: 24. https://doi.org/10.3390/catal8010024





