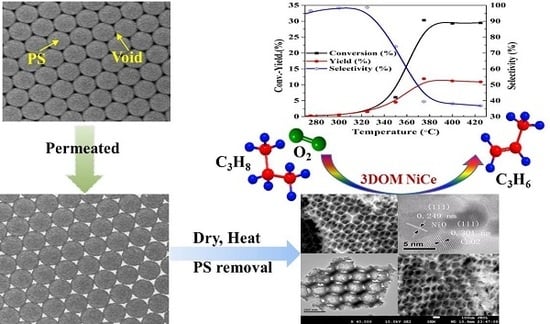
Synthesis of Three-Dimensionally Ordered Macroporous NiCe Catalysts for Oxidative Dehydrogenation of Propane to Propene
Abstract
:1. Introduction
2. Results and Discussion
2.1. Morphology and Structure
2.2. TEM Images
2.3. Powder X-ray Diffraction
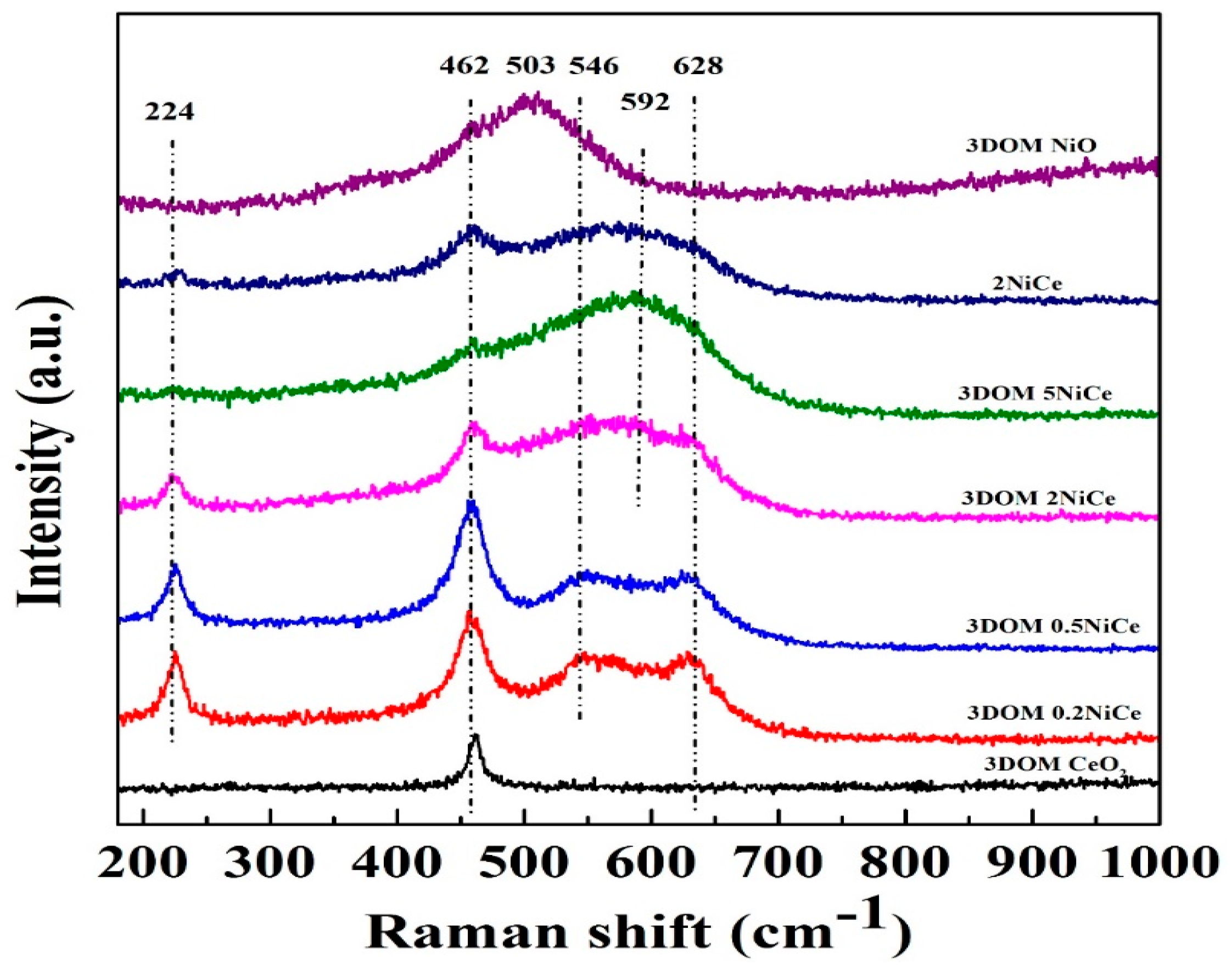
2.4. Raman Spectra
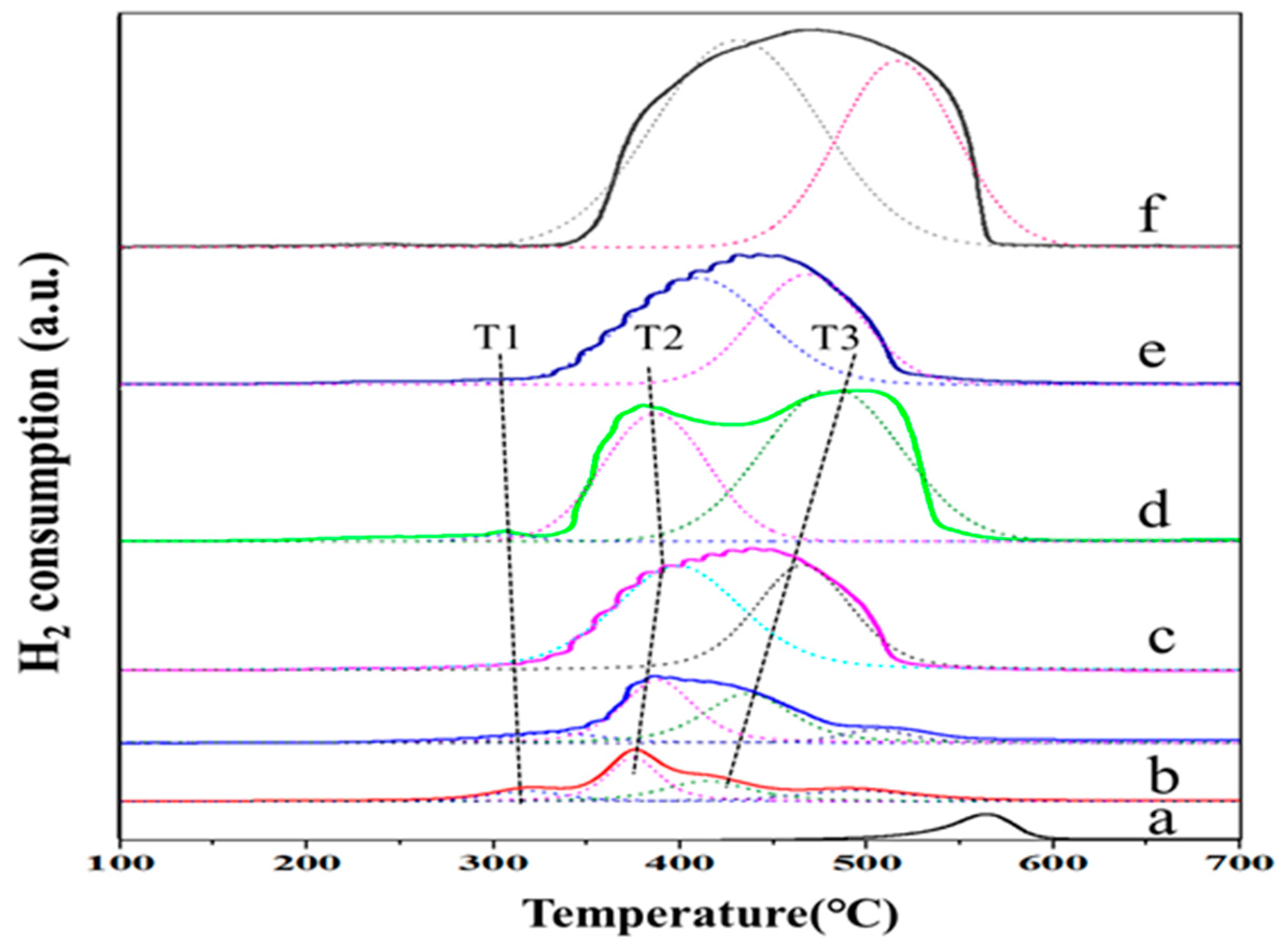
2.5. Temperature-Programmed Reduction
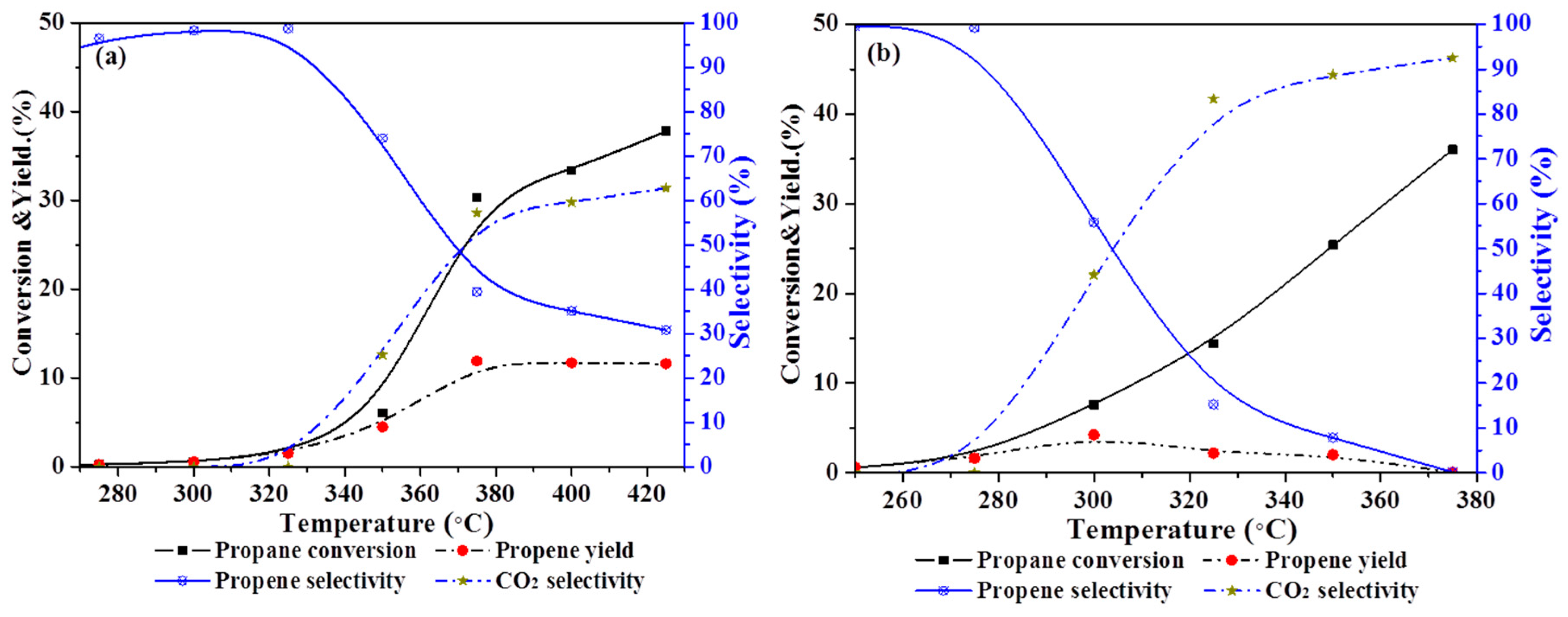
2.6. Catalytic Performance
2.7. Relationship between Catalyst Structure and Catalytic Performance
3. Experimental
3.1. Preparation of the Catalyst
3.2. Catalyst Characterization
3.3. Catalytic Activity Measurements
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chaar, M.A.; Patel, D.; Kung, M.C.; Kung, H.H. Selective oxidative dehydrogenation V-Mg-O of butane over catalysts. J. Catal. 1987, 105, 483–498. [Google Scholar] [CrossRef]
- Siew Hew Sam, D.; Soenen, V.; Volta, J.C. Oxidative dehydrogenation of propane over V-Mg-O catalysts. J. Catal. 1990, 123, 417–435. [Google Scholar] [CrossRef]
- Murgia, V.; Farfán Torres, E.M.; Gottifredi, J.C.; Sham, E.L. Influence of concentration and order of aggregation of the active phases in V–Mo–O catalysts in the oxidative dehydrogenation of propane. Catal. Today 2008, 133–135, 87–91. [Google Scholar] [CrossRef]
- Chen, M.; Wu, J.-L.; Liu, Y.-M.; Cao, Y.; Guo, L.; He, H.-Y.; Fan, K.-N. A practical grinding-assisted dry synthesis of nanocrystalline NiMoO4 polymorphs for oxidative dehydrogenation of propane. J. Solid State Chem. 2011, 184, 3357–3363. [Google Scholar] [CrossRef]
- Cadus, L.E.; Ferretti, O. Characterization of Mo-MnO catalyst for propane oxidative dehydrogenation. Appl. Catal. A Gen. 2002, 233, 239–253. [Google Scholar] [CrossRef]
- Liu, Q.; Li, J.; Zhao, Z.; Gao, M.; Kong, L.; Liu, J.; Wei, Y. Design, synthesis and catalytic performance of vanadium-incorporated mesoporous silica KIT-6 catalysts for the oxidative dehydrogenation of propane to propylene. Catal. Sci. Technol. 2016, 6, 5927–5941. [Google Scholar] [CrossRef]
- Chen, S.; Ma, F.; Xu, A.X.; Wang, L.N.; Chen, F.; Lu, W.M. Study on the structure, acidic properties of V–Zr nanocrystal catalysts in oxidative dehydrogenation of propane. Appl. Surf. Sci. 2014, 289, 316–325. [Google Scholar] [CrossRef]
- Solsona, B.; Blasco, T.; López Nieto, J.M.; Peña, M.L.; Rey, F.; Vidal-Moya, A. Vanadium oxide supported on mesoporous MCM-41 as selective catalysts in the oxidative dehydrogenation of alkanes. J. Catal. 2001, 203, 443–452. [Google Scholar] [CrossRef]
- Cheng, M.-J.; Chenoweth, K.; Oxgaard, J.; Duin, A.V.; Goddard, W.A., III. Single-site vanadyl activation, functionalization, and reoxidation reaction mechanism for propane oxidative dehydrogenation on the cubic V4O10 cluster. J. Phys. Chem. C 2007, 111, 5115–5127. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, G.; Chen, Z.; Jiang, W.; Zhou, H. In-situ synthesis and characterization of V-MCM-41 for oxidative dehydrogenation of n-butane. Microporous Mesoporous Mater. 2016, 223, 261–267. [Google Scholar] [CrossRef]
- Jalowiecki-Duhamel, L.; Ponchel, A.; Lamonier, C.; D’Huysser, A.; Barbaux, Y. Relationship between structure of CeNixOy mixed oxides and catalytic properties in oxidative dehydrogenation of propane. Langmuir 2001, 17, 1511–1517. [Google Scholar] [CrossRef]
- Boizumault-Moriceau, P.; Pennequin, A.; Grzybowska, B.; Barbaux, Y. Oxidative dehydrogenation of propane on Ni-Ce-O oxide: Effect of the preparation method, effect of potassium addition and physical characterization. Appl. Catal. A Gen. 2003, 245, 55–67. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, L.; Chen, M.; Xu, J.; Cao, Y.; He, H.; Fan, K. Highly selective Ce–Ni–O catalysts for efficient low temperature oxidative dehydrogenation of propane. Catal. Lett. 2009, 130, 350–354. [Google Scholar] [CrossRef]
- Li, T.; Wang, J.; Zhaorigetu, B.; Xu, A. Effect of preparation parameters on the catalytic performance of mesoporous NiO for the oxidative dehydrogenation of propane to propylene. Reac. Kinet. Mech. Cat. 2013, 110, 421–435. [Google Scholar] [CrossRef]
- Zhang, Z.; Han, L.; Chai, R.; Zhang, Q.; Li, Y.; Zhao, G.; Liu, Y.; Lu, Y. Microstructured CeO2-NiO-Al2O3/Ni-foam catalyst for oxidative dehydrogenation of ethane to ethylene. Catal. Commun. 2017, 88, 90–93. [Google Scholar] [CrossRef]
- Xie, S.; Deng, J.; Zang, S.; Yang, H.; Guo, G.; Arandiyan, H.; Dai, H. Au–Pd/3DOM Co3O4: Highly active and stable nanocatalysts for toluene oxidation. J. Catal. 2015, 322, 38–48. [Google Scholar] [CrossRef]
- Ji, K.; Dai, H.; Deng, J.; Zang, H.; Arandiyan, H.; Xie, S.; Yang, H. 3DOM BiVO4 supported silver bromide and noble metals: High-performance photocatalysts for the visible-light-driven degradation of 4-chlorophenol. Appl. Catal. B Environ. 2015, 168–169, 274–282. [Google Scholar] [CrossRef]
- Xu, J.; Liu, J.; Zhao, Z.; Xu, C.; Zheng, J.; Duan, A.; Jiang, G. Easy synthesis of three-dimensionally ordered macroporous La1−xKxCoO3 catalysts and their high activities for the catalytic combustion of soot. J. Catal. 2011, 282, 1–12. [Google Scholar] [CrossRef]
- Liu, Y.; Dai, H.; Du, Y.; Deng, J.; Zhang, L.; Zhao, Z.; Au, C.T. Controlled preparation and high catalytic performance of three-dimensionally ordered macroporous LaMnO3 with nanovoid skeletons for the combustion of toluene. J. Catal. 2012, 287, 149–160. [Google Scholar] [CrossRef]
- Wei, Y.; Zhao, Z.; Li, T.; Liu, J.; Duan, A.; Jiang, G. The novel catalysts of truncated polyhedron Pt nanoparticles supported on three-dimensionally ordered macroporous oxides (Mn, Fe, Co, Ni, Cu) with nanoporous walls for soot combustion. Appl. Catal. B Environ. 2014, 146, 57–70. [Google Scholar] [CrossRef]
- Lu, Y.; Cao, B.; Yu, F.; Liu, J.; Bao, Z.; Gao, J. High selectivity higher alcohols synthesis from syngas over three-dimensionally ordered macroporous Cu-Fe catalysts. ChemCatChem 2014, 6, 473–478. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, K.; Wang, H.; Wang, Y.; Tian, D.; Wei, Y.; Zhu, X.; Zeng, C.; Luo, Y. Structure dependence and reaction mechanism of CO oxidation: A model study on macroporous CeO2 and CeO2-ZrO2 catalysts. J. Catal. 2016, 344, 365–377. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, K.; Wang, H.; Tian, D.; Wang, Y.; Zhu, X.; Wei, Y.; Zheng, M.; Luo, Y. Designed oxygen carriers from macroporous LaFeO3 supported CeO2 for chemical-looping reforming of methane. Appl. Catal. B Environ. 2017, 202, 51–63. [Google Scholar] [CrossRef]
- Zhang, J.; Jin, Y.; Li, C.; Shen, Y.; Han, L.; Hu, Z.; Di, X.; Liu, Z. Creation of three-dimensionally ordered macroporous Au/CeO2 catalysts with controlled pore sizes and their enhanced catalytic performance for formaldehyde oxidation. Appl. Catal. B Environ. 2009, 91, 11–20. [Google Scholar] [CrossRef]
- Liu, B.; Liu, Y.; Li, C.; Hu, W.; Jing, P.; Wang, Q.; Zhang, J. Three-dimensionally ordered macroporous Au/CeO2-Co3O4 catalysts with nanoporous walls for enhanced catalytic oxidation of formaldehyde. Appl. Catal. B Environ. 2012, 127, 47–58. [Google Scholar] [CrossRef]
- Yan, H.W.; Blanford, C.F.; Holland, B.T.; Smyrl, W.H.; Stein, A. General synthesis of periodic macroporous solids by templated salt precipitation and chemical conversion. Chem. Mater. 2000, 12, 1134–1141. [Google Scholar] [CrossRef]
- Ji, K.; Dai, H.; Deng, J.; Zhang, L.; Wang, F.; Jiang, H.; Au, C.T. Three-dimensionally ordered macroporous SrFeO3−δ with high surface area: Active catalysts for the complete oxidation of toluene. Appl. Catal. A Gen. 2012, 425–426, 153–160. [Google Scholar] [CrossRef]
- Jalowiecki-Duhamel, L.; Pirez, C.; Capron, M.; Dumeignil, F.; Payen, E. Hydrogen production from ethanol steam reforming over cerium and nickel based oxyhydrides. Int. J. Hydrogen Energy 2010, 35, 12741–12750. [Google Scholar] [CrossRef]
- Lamonier, C.; Ponchel, A.; D’Huysser, A.; Jalowiecki-Duhamel, L. Studies of the cerium-metal-oxygen-hydrogen system (metal = Cu, Ni). Catal. Today 1999, 50, 247–259. [Google Scholar] [CrossRef]
- Reddy, B.M.; Khan, A.; Lakshmanan, P.; Aouine, M.; Loridant, S.; Volta, J.-C. Structural characterization of nanosized CeO2-SiO2, CeO2-TiO2, and CeO2-ZrO2 catalysts by XRD, Raman, and HREM techniques. J. Phys. Chem. B 2005, 109, 3355–3363. [Google Scholar] [CrossRef] [PubMed]
- Heracleous, E.; Lemonidou, A.A. Ni–Nb–O mixed oxides as highly active and selective catalysts for ethene production via ethane oxidative dehydrogenation. Part I: Characterization and catalytic performance. J. Catal. 2006, 237, 162–174. [Google Scholar] [CrossRef]
- Savova, B.; Loridant, S.; Filkova, D.; Millet, J.M.M. Ni–Nb–O catalysts for ethane oxidative dehydrogenation. Appl. Catal. A Gen. 2010, 390, 148–157. [Google Scholar] [CrossRef]
- Solsona, B.; Concepción, P.; Hernández, S.; Demicol, B.; Nieto, J.M.L. Oxidative dehydrogenation of ethane over NiO–CeO2 mixed oxides catalysts. Catal. Today 2012, 180, 51–58. [Google Scholar] [CrossRef]
- Mahammadunnisa, S.; Manoj, K.P.; Lingaiah, N.; Subrahmanyam, C. NiO/Ce1−xNixO2−δ as an alternative to noble metal catalysts for CO oxidation. Catal. Sci. Technol. 2013, 3, 730–736. [Google Scholar] [CrossRef]
- Bortolozzi, J.P.; Weiss, T.; Gutierrez, L.B.; Ulla, M.A. Comparison of Ni and Ni–Ce/Al2O3 catalysts in granulated and structured forms: Their possible use in the oxidative dehydrogenation of ethane reaction. Chem. Eng. J. 2014, 246, 343–352. [Google Scholar] [CrossRef]
- Shan, W.; Luo, M.; Ying, P.; Shen, W.; Li, C. Reduction property and catalytic activity of Ce1−xNixO2 mixed oxide catalysts for CH4 oxidation. Appl. Catal. A Gen. 2003, 246, 1–9. [Google Scholar] [CrossRef]
- Lu, L.; Li, L.; Hu, T.; Zhang, W.; Huang, X.; Zhang, J.; Liu, X. Preparation, characterization, and photocatalytic activity of three-dimensionally ordered macroporous hybrid monosubstituted polyoxometalate K5[Co(H2O)PW11O39] amine functionalized titanium catalysts. J. Mol. Catal. A Chem. 2014, 394, 283–294. [Google Scholar] [CrossRef]
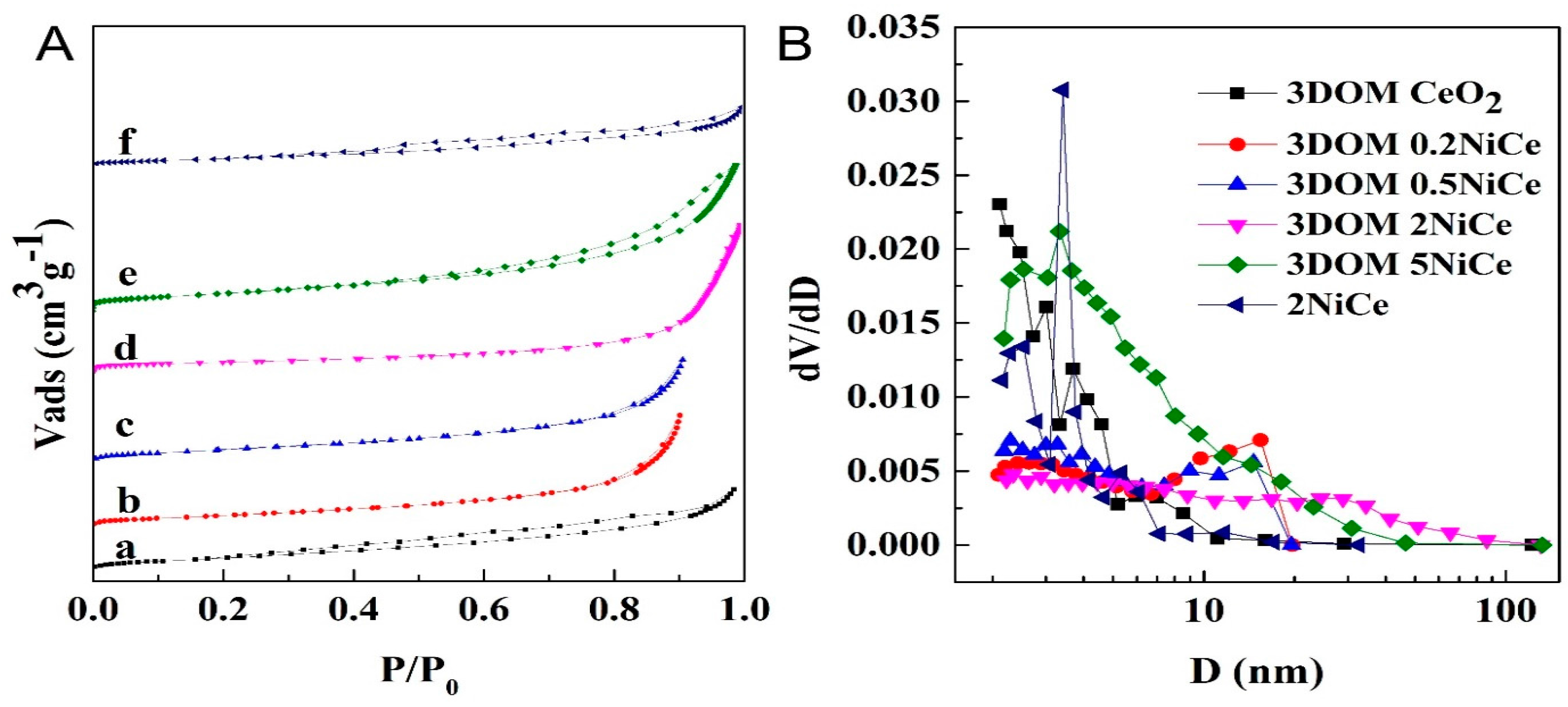
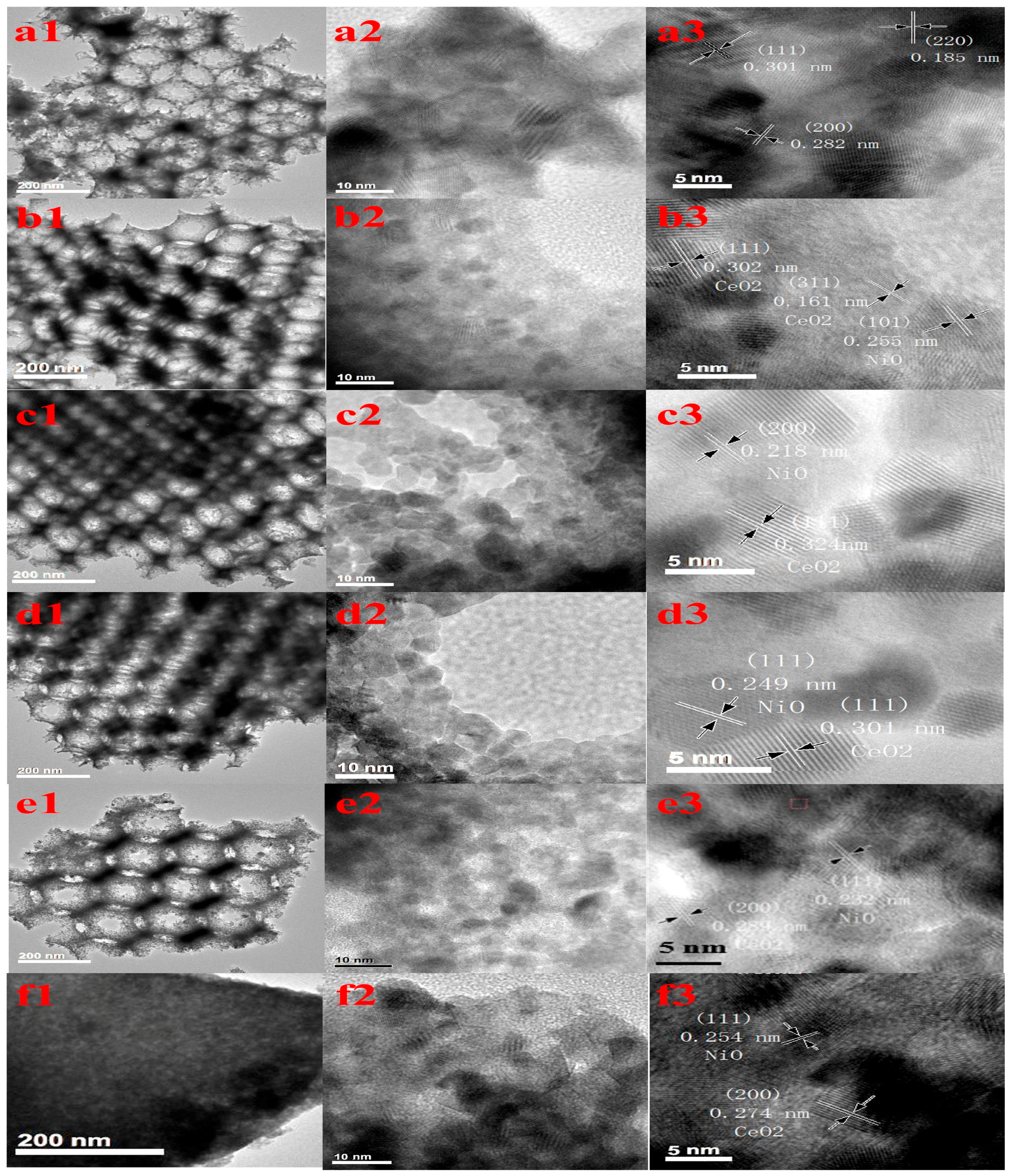
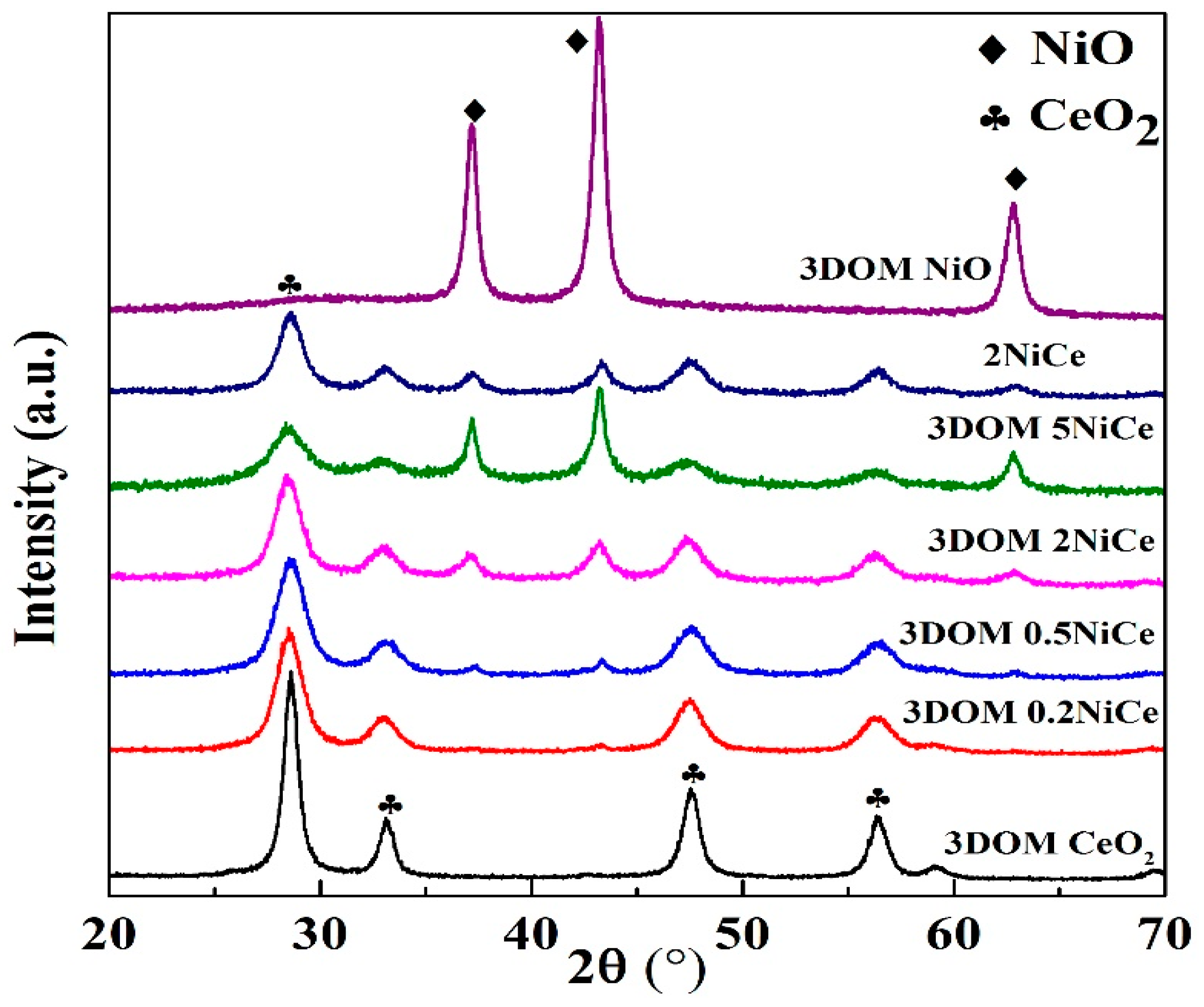
| Samples | Crystallite Size from XRD (nm) | SBET (m2g−1) | Dp (nm) | VP (cm3g−1) | |
|---|---|---|---|---|---|
| CeO2 (111) | NiO (200) | ||||
| 3DOM CeO2 | 12.8 | - | 29.2 | 6.8 | 0.08 |
| 3DOM 0.2NiCe | 9.7 | - | 34.1 | 9.0 | 0.11 |
| 3DOM 0.5NiCe | 9.2 | 14.8 | 37.3 | 11.0 | 0.15 |
| 3DOM 2NiCe | 9.0 | 16.9 | 41.6 | 19.1 | 0.24 |
| 3DOM 5NiCe | 8.8 | 12.9 | 76.04 | 9.6 | 0.26 |
| 2NiCe | 10.6 | 17.9 | 13.8 | 7.4 | 0.06 |
| 3DOM NiO | - | 15 | 49.17 | 15.4 | 0.21 |
| Samples | H2 Consumption (mmol·g−1) | Total Amount of H2 Consumption (mmol·g−1) | |||
|---|---|---|---|---|---|
| T1 Peak | T2 Peak | T3 Peak | CeO2 Peak | ||
| 3DOM NiO | - | 8.3 | 5.0 | - | 13.3 |
| 2NiCe | 0.1 | 4.6 | 3.3 | - | 8.0 |
| 3DOM 5NiCe | 0.2 | 4.4 | 5.4 | - | 9.9 |
| 3DOM 2NiCe | 0.1 | 3.9 | 3.8 | - | 7.8 |
| 3DOM 0.5NiCe | 0.1 | 2.5 | 1.9 | 0.2 | 4.8 |
| 3DOM 0.2NiCe | 0.1 | 1.8 | 1.1 | 0.5 | 3.5 |
| 3DOM CeO2 | - | - | - | 2.7 | 2.7 |
| Samples | Propane Conversion (%) | Selectivity (%) | Propene Yield (%) | ||
|---|---|---|---|---|---|
| Propene | CO2 | Crack a | |||
| 2NiCe | 36.0 | 0.1 | 92.5 | 7.4 | 0.04 |
| 3DOM 5NiCe | 34.6 | 0.2 | 97.7 | 2.1 | 0.06 |
| 3DOM 2NiCe | 30.3 | 39.4 | 57.2 | 3.4 | 11.9 |
| 3DOM 0.5NiCe | 27.3 | 38.3 | 57.3 | 4.4 | 10.5 |
| 3DOM 0.2NiCe | 26.2 | 28.8 | 69.5 | 1.7 | 7.55 |
| 3DOM CeO2 | 7.2 | 22.9 | 76.8 | 0.3 | 1.65 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, K.; Liu, L.; Zhang, M.; Zhao, L.; Zhou, J.; Li, W.; Mu, X.; Yang, C. Synthesis of Three-Dimensionally Ordered Macroporous NiCe Catalysts for Oxidative Dehydrogenation of Propane to Propene. Catalysts 2018, 8, 19. https://doi.org/10.3390/catal8010019
Fang K, Liu L, Zhang M, Zhao L, Zhou J, Li W, Mu X, Yang C. Synthesis of Three-Dimensionally Ordered Macroporous NiCe Catalysts for Oxidative Dehydrogenation of Propane to Propene. Catalysts. 2018; 8(1):19. https://doi.org/10.3390/catal8010019
Chicago/Turabian StyleFang, Kegong, Lulu Liu, Mingwei Zhang, Lu Zhao, Juan Zhou, Wenbin Li, Xiaoliang Mu, and Cheng Yang. 2018. "Synthesis of Three-Dimensionally Ordered Macroporous NiCe Catalysts for Oxidative Dehydrogenation of Propane to Propene" Catalysts 8, no. 1: 19. https://doi.org/10.3390/catal8010019