Recent Progress on the Synthesis of Graphene-Based Nanostructures as Counter Electrodes in DSSCs Based on Iodine/Iodide Electrolytes
Abstract
:1. Introduction
2. Synthetic Approaches
2.1. Liquid-Phase Exfoliated Graphene
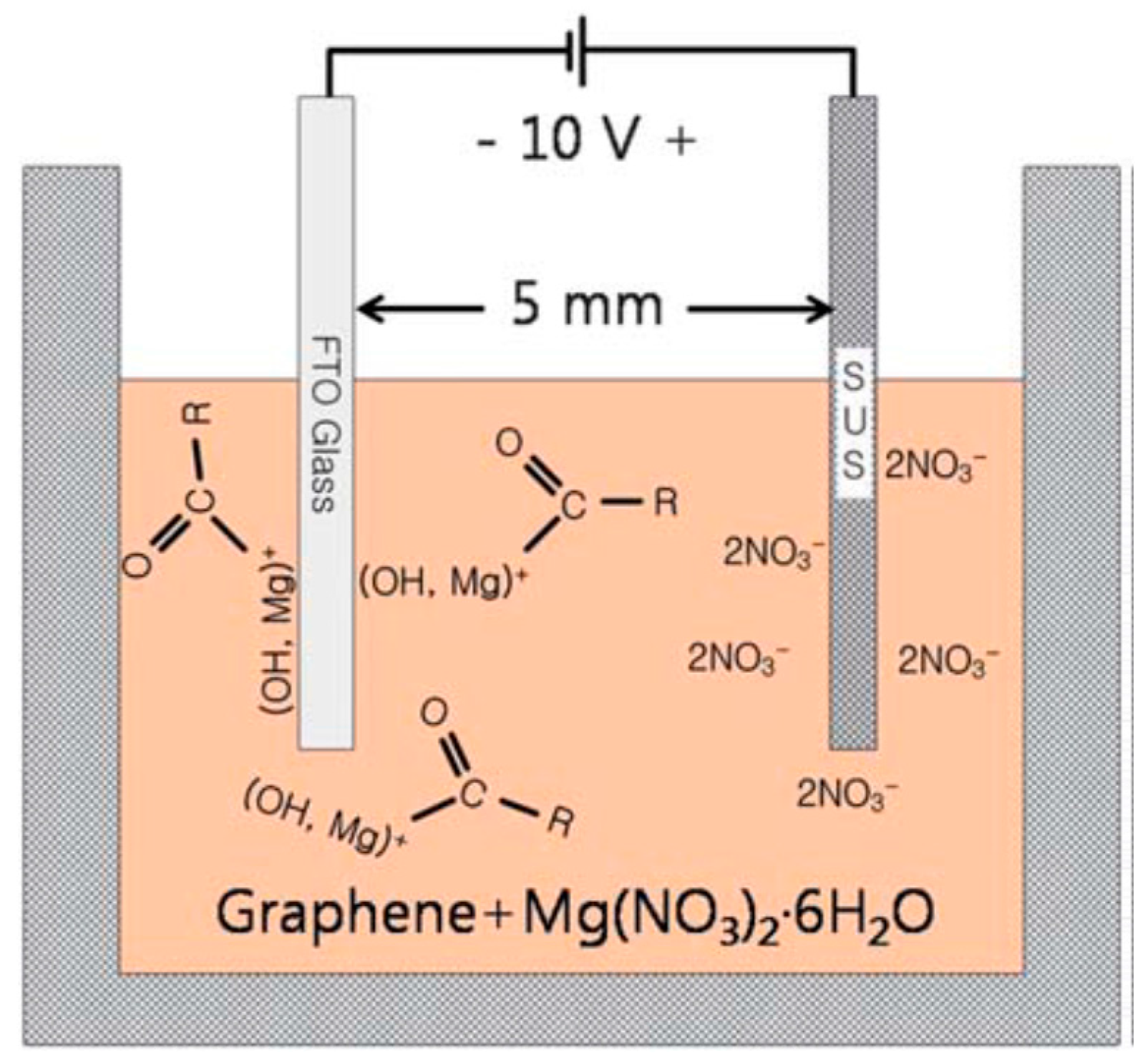
2.1.1. Electrophoretic Deposition
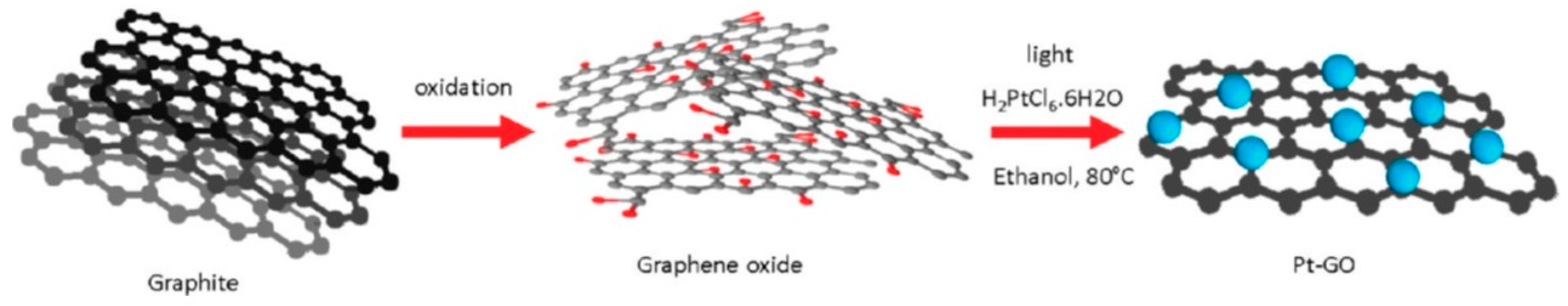
2.1.2. Graphene/Pt Hybrids by Reduction of Precursor Salt
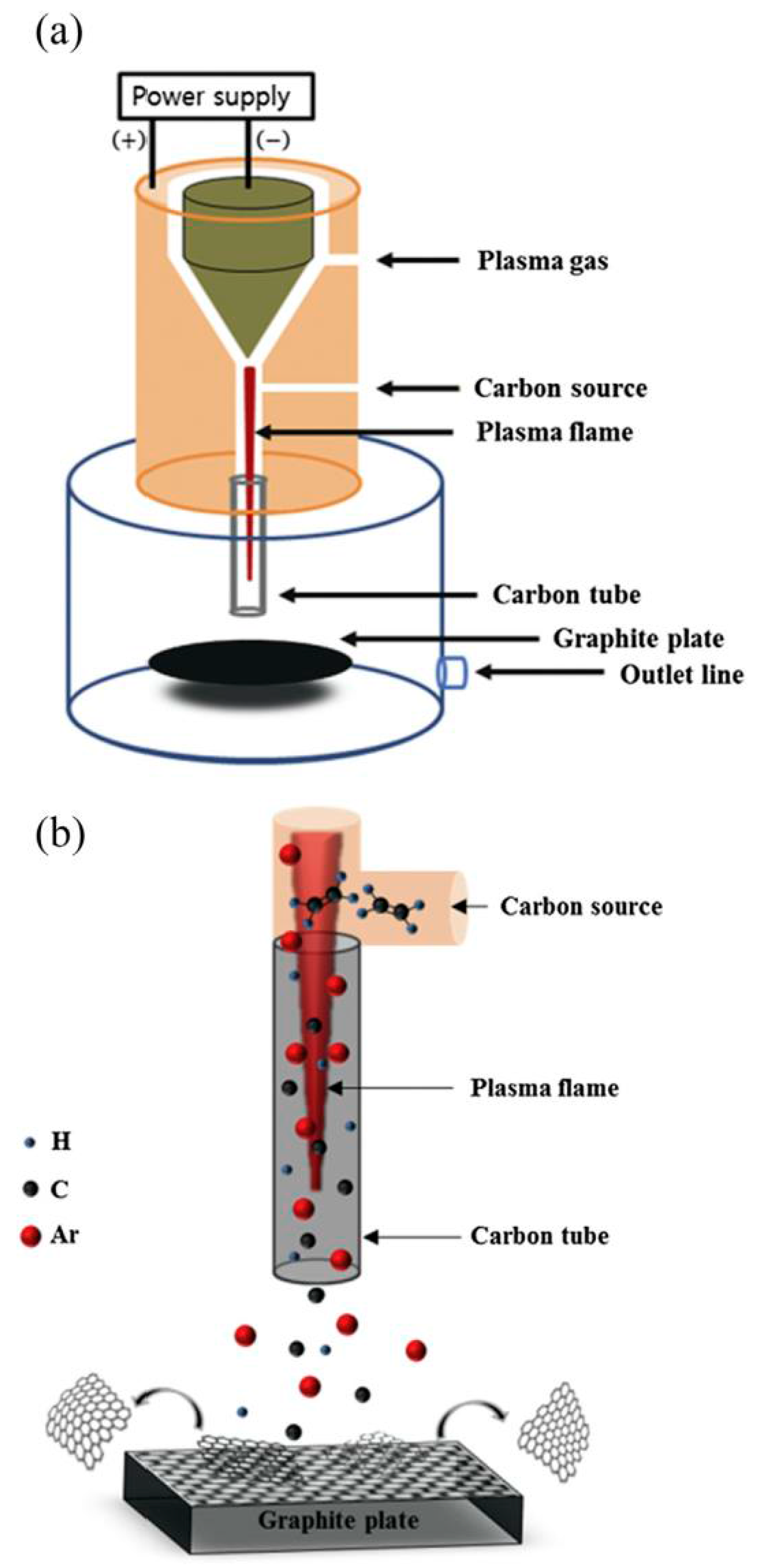
2.1.3. Plasma-Induced Processes
2.1.4. Heteroatom-Doped Graphenes by Annealing Process
2.1.5. Chemical Doping by Ball Milling
2.1.6. Hydrothermal Synthesis
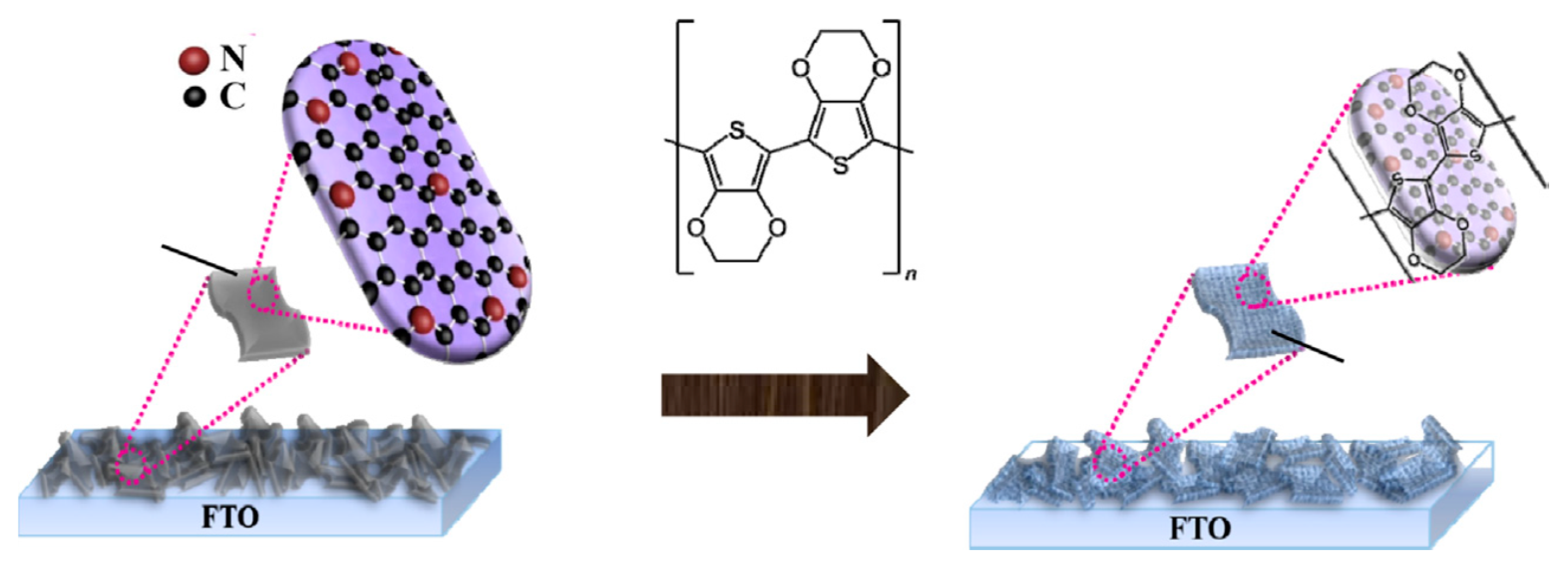
2.1.7. Polymer-Mediated Functionalization
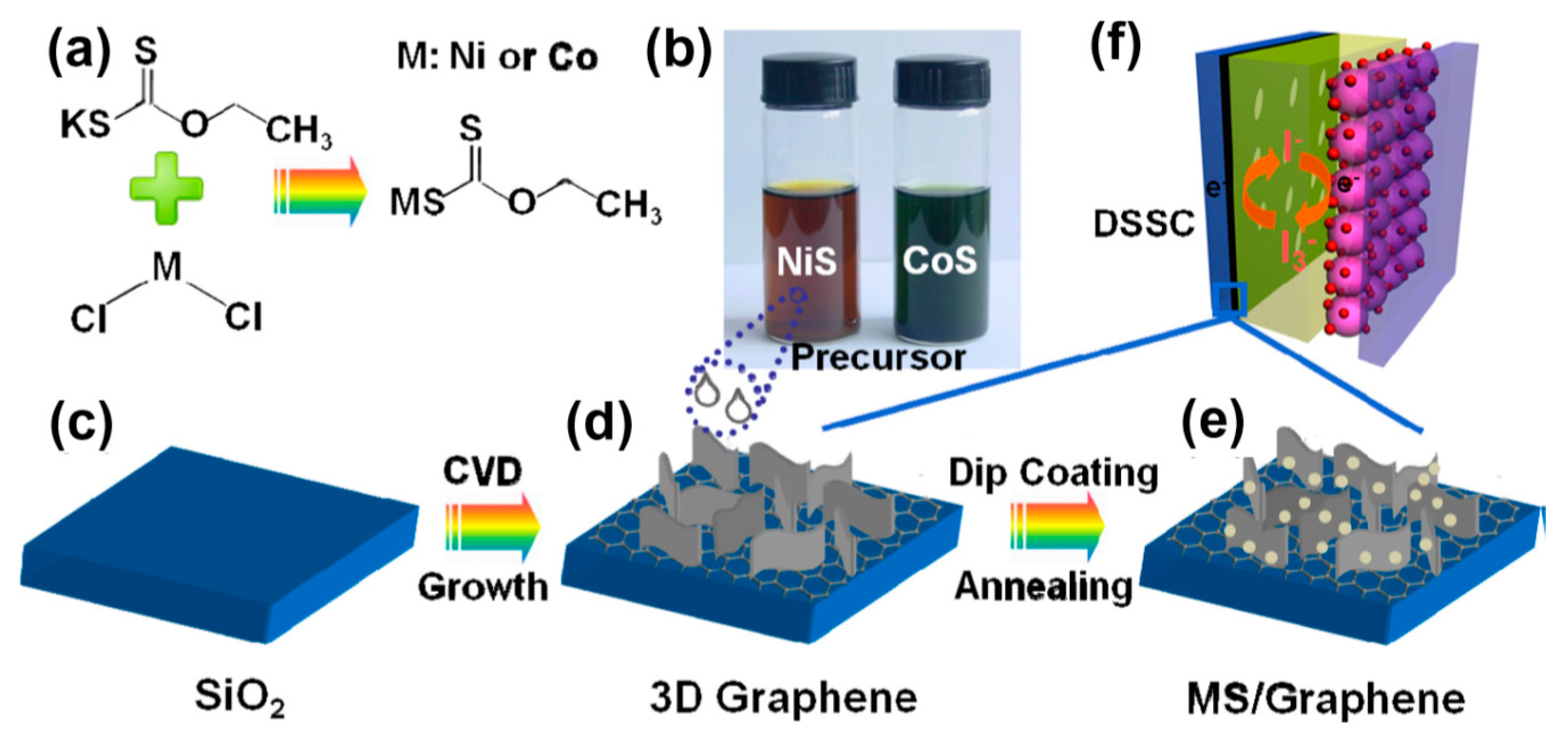
2.2. Chemical Vapor Deposition-Grown Graphene
3. Selected Literature Data and Conclusions
Acknowledgments
Conflicts of Interest
References
- Hagfeldt, A.; Boschloo, G.; Sun, L.; Kloo, L.; Pettersson, H. Dye-sensitized solar cells. Chem. Rev. 2010, 110, 6595–6663. [Google Scholar]
- Wu, M.; Ma, T. Recent progress of counter electrode catalysts in dye-sensitized solar cells. J. Phys. Chem. C 2014, 118, 16727–16742. [Google Scholar] [CrossRef]
- Irani, R.; Naseri, N.; Beke, S. A review of 2D-based counter electrodes applied in solar-assisted devices. Coord. Chem. Rev. 2016, 324, 54–81. [Google Scholar] [CrossRef]
- Shearer, C.J.; Cherevan, A.; Eder, D. Application and Future Challenges of Functional Nanocarbon Hybrids. Adv. Mater. 2014, 26, 2295–2318. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Rutledge, G.C.; Hatton, T.A. Nanocarbon-based electrochemical systems for sensing, electrocatalysis, and energy storage. Nano Today 2014, 9, 405–432. [Google Scholar] [CrossRef]
- Strauss, V.; Roth, A.; Sekita, M.; Guldi, D.M. Efficient energy-conversion materials for the future: Understanding and tailoring charge-transfer processes in carbon nanostructures. Chem 2016, 1, 531–556. [Google Scholar] [CrossRef]
- Mathur, R.B.; Singh, B.P.; Pande, S. Carbon Nanomaterials: Synthesis, Structure, Properties and Applications; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and graphene oxide: Synthesis, properties, and applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef] [PubMed]
- Hong, W.; Xu, Y.; Lu, G.; Li, C.; Shi, G. Transparent graphene/PEDOT–PSS composite films as counter electrodes of dye-sensitized solar cells. Electrochem. Commun. 2008, 10, 1555–1558. [Google Scholar] [CrossRef]
- Roy-Mayhew, J.D.; Aksay, I.A. Graphene materials and their use in dye-sensitized solar cells. Chem. Rev. 2014, 114, 6323–6348. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Wu, D.; Li, S.; Zhang, F.; Feng, X. Porous graphene materials for advanced electrochemical energy storage and conversion devices. Adv. Mater. 2014, 26, 849–864. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Shi, G. Flexible graphene devices related to energy conversion and storage. Energy Environ. Sci. 2015, 8, 790–823. [Google Scholar] [CrossRef]
- Yang, Y.; Han, C.; Jiang, B.; Iocozzia, J.; He, C.; Shi, D.; Jiang, T.; Lin, Z. Graphene-based materials with tailored nanostructures for energy conversion and storage. Mater. Sci. Eng. R 2016, 102, 1–72. [Google Scholar] [CrossRef]
- Choi, H.; Kim, H.; Hwang, S.; Han, Y.; Jeon, M. Graphene counter electrodes for dye-sensitized solar cells prepared by electrophoretic deposition. J. Mater. Chem. 2011, 21, 7548–7551. [Google Scholar] [CrossRef]
- Miao, X.; Pan, K.; Wang, G.; Liao, Y.; Wang, L.; Zhou, W.; Jiang, B.; Pan, Q.; Tian, G. Well-dispersed CoS nanoparticles on a functionalized graphene nanosheet Surface: A counter electrode of dye-sensitized solar cells. Chem. Eur. J. 2014, 20, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Huo, J.; Wu, J.; Zheng, M.; Tu, Y.; Lan, Z. High performance sponge-like cobalt sulfide/reduced graphene oxide hybrid counter electrode for dye-sensitized solar cells. J. Power Sources 2015, 293, 570–576. [Google Scholar] [CrossRef]
- Yen, M.-Y.; Teng, C.-C.; Hsiao, M.-C.; Liu, P.-I.; Chuang, W.-P.; Ma, C.-C.M.; Hsieh, C.-K.; Tsai, M.-C.; Tsai, C.-H. Platinum nanoparticles/graphene composite catalyst as a novel composite counter electrode for high performance dye-sensitized solar cells. J. Mater. Chem. 2011, 21, 12880–12888. [Google Scholar] [CrossRef]
- Tjoa, V.; Chua, J.; Pramana, S.S.; Wei, J.; Mhaisalkar, S.G.; Mathews, N. Facile photochemical synthesis of graphene-Pt nanoparticle composite for counter electrode in dye sensitized solar cell. ACS Appl. Mater. Interfaces 2012, 4, 3447–3452. [Google Scholar] [CrossRef] [PubMed]
- Dao, V.-D.; Larina, L.L.; Jung, K.-D.; Leed, J.-K.; Choi, H.-S. Graphene–NiO nanohybrid prepared by dry plasma reduction as a low-cost counter electrode material for dye-sensitized solar cells. Nanoscale 2014, 6, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Dao, V.-D.; Jin, I.-K.; Choi, H.-S. Design of PtRu alloy/reduced graphene oxide nanohybrid counter electrodes for highly efficient dye-sensitized solar cells. Electrochim. Acta 2016, 201, 1–7. [Google Scholar] [CrossRef]
- Yoon, S.-W.; Dao, V.-D.; Larina, L.L.; Lee, J.-K.; Choi, H.-S. Optimum strategy for designing PtCo alloy/reduced graphene oxide nanohybrid counter electrode for dye-sensitized solar cells. Carbon 2016, 96, 229–236. [Google Scholar] [CrossRef]
- Dao, V.-D.; Larina, L.L.; Tran, Q.C.; Bui, V.-T.; Nguyen, V.-T.; Pham, T.-D.; Mohamed, I.M.A.; Barakat, N.A.M.; Huy, B.T.; Choi, H.-S. Evaluation of Pt-based alloy/graphene nanohybrid electrocatalysts for triiodide reduction in photovoltaics. Carbon 2017, 116, 294–302. [Google Scholar] [CrossRef]
- Lee, M.W.; Kim, H.-Y.; Yoon, H.; Kim, J.; Suh, J.S. Fabrication of dispersible graphene flakes using thermal plasma jet and their thin films for solar cells. Carbon 2016, 106, 48–55. [Google Scholar] [CrossRef]
- Wen, Z.; Cui, S.; Pu, H.; Mao, S.; Yu, K.; Feng, X.; Chen, J. Metal nitride/graphene nanohybrids: General synthesis and multifunctional titanium nitride/graphene electrocatalyst. Adv. Mater. 2011, 23, 5445–5450. [Google Scholar] [CrossRef] [PubMed]
- Balamurugan, J.; Thanh, T.D.; Kim, N.H.; Lee, J.H. Nitrogen-doped graphene nanosheets with FeN core–shell nanoparticles as high-performance counter electrode materials for dye-sensitized solar cells. Adv. Mater. Interfaces 2016, 3. [Google Scholar] [CrossRef]
- Hou, S.; Cai, X.; Wu, H.; Yu, X.; Peng, M.; Yan, K.; Zou, D. Nitrogen-doped graphene for dye-sensitized solar cells and the role of nitrogen states in triiodide reduction. Energy Environ. Sci. 2013, 6, 3356–3362. [Google Scholar] [CrossRef]
- Zhai, P.; Wei, T.-C.; Chang, Y.-H.; Huang, Y.-T.; Yeh, W.-T.; Su, H.; Feng, S.-P. High electrocatalytic and wettable nitrogen-doped microwave-exfoliated graphene nanosheets as counter electrode for dye-sensitized solar cells. Small 2014, 10, 3347–3353. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Yu, C.; Ma, T.; Qiu, J. Boron-doped graphene as high-efficiency counter electrode for dye-sensitized solar cells. Chem. Commun. 2014, 50, 3328–3330. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, P.; Chen, Y.; He, J.; Liu, J.; Zhang, W.; Li, Y. Phosphorus-doped reduced graphene oxide as an electrocatalyst counter electrode in dye-sensitized solar cells. J. Power Sources 2014, 263, 246–251. [Google Scholar] [CrossRef]
- Belekoukia, M.; Ploumistos, A.; Sygellou, L.; Nouri, E.; Tasis, D.; Lianos, P. Co-N doped reduced graphene oxide used as efficient electrocatalyst for dye-sensitized solar cells. Sol. Energy Mater. Sol. Cells 2016, 157, 591–598. [Google Scholar] [CrossRef]
- Meng, X.; Yu, C.; Song, X.; Liu, Z.; Lu, B.; Hao, C.; Qiu, J. Rational design and fabrication of sulfur-doped porous graphene with enhanced performance as counter electrode in dye sensitized solar cells. J. Mater. Chem. A 2017, 5, 2280–2287. [Google Scholar] [CrossRef]
- Yu, C.; Fang, H.; Liu, Z.; Hu, H.; Meng, X.; Qiu, J. Chemically grafting graphene oxide to B,N co-doped graphene via ionic liquid and their superior performance for triiodide reduction. Nano Energy 2016, 25, 184–192. [Google Scholar] [CrossRef]
- Jeon, I.-Y.; Shin, Y.-R.; Sohn, G.-J.; Choi, H.-J.; Bae, S.-Y.; Mahmood, J.; Jung, S.-M.; Seo, J.-M.; Kim, M.-J.; Chang, D.W.; et al. Edge-carboxylated graphene nanosheets via ball milling. Proc. Natl. Acad. Sci. USA 2012, 109, 5588–5593. [Google Scholar] [CrossRef] [PubMed]
- Ju, M.J.; Jeon, I.-Y.; Lim, K.; Kim, J.C.; Choi, H.-J.; Choi, I.T.; Eom, Y.K.; Kwon, Y.J.; Ko, J.; Lee, J.-J.; et al. Edge-carboxylated graphene nanoplatelets as oxygen-rich metal-free cathodes for organic dye-sensitized solar cells. Energy Environ. Sci. 2014, 7, 1044–1052. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Zhang, J.; Hou, S.; Zhang, W.; Zhao, Z. Edge-nitrogenated graphene nanoplatelets as high-efficiency counter electrodes for dye-sensitized solar cells. Nanoscale 2016, 8, 9676–9681. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Xiao, J.; Wu, Y.; Du, P.; Si, R.; Yang, H.; Tian, H.; Li, J.; Zhang, W.-H.; Deng, D.; et al. A graphene composite material with single cobalt active sites: A highly efficient counter electrode for dye-sensitized solar cells. Angew. Chem. Int. Ed. 2016, 55, 6708–6712. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Liu, Z.; Meng, X.; Lu, B.; Cui, D.; Qiu, J. Nitrogen and phosphorus dual-doped graphene as a metal-free high-efficiency electrocatalyst for triiodide reduction. Nanoscale 2016, 8, 17458–17464. [Google Scholar] [CrossRef] [PubMed]
- Long, D.; Li, W.; Ling, L.; Miyawaki, J.; Mochida, I.; Yoon, S.-H. Preparation of nitrogen-doped graphene sheets by a combined chemical and hydrothermal reduction of graphene oxide. Langmuir 2010, 26, 16096–16102. [Google Scholar] [CrossRef] [PubMed]
- Yen, M.-Y.; Hsieh, C.-K.; Teng, C.-C.; Hsiao, M.-C.; Liu, P.-I.; Ma, C.-C.M.; Tsai, M.-C.; Tsai, C.-H.; Lin, Y.-R.; Chou, T.-Y. Metal-free, nitrogen-doped graphene used as a novel catalyst for dye-sensitized solar cell counter electrodes. RSC Adv. 2012, 2, 2725–2728. [Google Scholar] [CrossRef]
- Wang, G.; Xing, W.; Zhuo, S. Nitrogen-doped graphene as low-cost counter electrode for high-efficiency dye-sensitized solar cells. Electrochim. Acta 2013, 92, 269–275. [Google Scholar] [CrossRef]
- Kannan, A.G.; Zhao, J.; Jo, S.G.; Kang, Y.S.; Kim, D.-W. Nitrogen and sulfur co-doped graphene counter electrodes with synergistically enhanced performance for dye-sensitized solar cells. J. Mater. Chem. A 2014, 2, 12232–12239. [Google Scholar] [CrossRef]
- Yu, Z.; Bai, Y.; Wang, Y.; Liu, Y.; Zhao, Y.; Liu, Y.; Sun, K. One-step synthesis of three-dimensional nitrogen and sulfur co-doped graphene networks as low cost metal-free counter electrodes for dye-sensitized solar cells. Chem. Eng. J. 2017, 311, 302–309. [Google Scholar] [CrossRef]
- Dou, Y.Y.; Li, G.R.; Song, J.; Gao, X.P. Nickel phosphide-embedded graphene as counter electrode for dye-sensitized solar cells. Phys. Chem. Chem. Phys. 2012, 14, 1339–1342. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Gong, F.; Zhou, G.; Wang, Z.-S. NiS2/reduced graphene oxide nanocomposites for efficient dye- sensitized solar cells. J. Phys. Chem. C 2013, 117, 6561–6566. [Google Scholar] [CrossRef]
- Li, G.; Chen, X.; Gao, G. Bi2S3 microspheres grown on graphene sheets as low-cost counter-electrode materials for dye sensitized solar cells. Nanoscale 2014, 6, 3283–3288. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yang, Y.; Guo, S.; Hu, F.; Liu, L. Mesoporous Ni0.85Se nanospheres grown in situ on graphene with high-performance in dye sensitized solar cells. ACS Appl. Mater. Interfaces 2015, 7, 8457–8464. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Zuo, X.; Chen, P.; Zhou, L.; Yang, X.; Zhang, H.; Li, G.; Wu, M.; Ma, Y.; Jin, S.; et al. Nanocomposite of tin sulfide nanoparticles with reduced graphene oxide in high-efficiency dye-sensitized solar cells. ACS Appl. Mater. Interfaces 2015, 7, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Huo, J.; Wu, J.; Zheng, M.; Tu, Y.; Lan, Z. A transparent cobalt sulfide/reduced graphene oxide nanostructure counter electrode for high efficient dye-sensitized solar cells. Electrochim. Acta 2016, 187, 210–217. [Google Scholar] [CrossRef]
- Dong, J.; Wu, J.; Jia, J.; Fan, L.; Lan, Z.; Lin, J.; Wei, Y. Cobalt selenite dihydrate as an effective and stable Pt-free counter electrode in dye-sensitized solar cells. J. Power Sources 2016, 336, 83–90. [Google Scholar] [CrossRef]
- Yang, W.; Xu, X.; Li, Z.; Yang, F.; Zhang, L.; Li, Y.; Wang, A.; Chen, S. Construction of efficient counter electrodes for dye-sensitized solar cells: Fe2O3 nanoparticles anchored onto graphene frameworks. Carbon 2016, 96, 947–954. [Google Scholar] [CrossRef]
- Zhang, X.; Zhen, M.; Bai, J.; Jin, S.; Liu, L. Efficient NiSe-Ni3Se2/graphene electrocatalyst in dye-sensitized solar cells: The role of hollow hybrid nanostructure. ACS Appl. Mater. Interfaces 2016, 8, 17187–17193. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Jiao, Q.; Liu, J.; Liu, X.; Yang, H.; Zhao, Y.; Wu, Q.; Shi, D.; Li, H. Ultrathin-walled Co9S8 nanotube/reduced graphene oxide composite as an efficient electrocatalyst for the reduction of triiodide. J. Power Sources 2016, 336, 132–142. [Google Scholar] [CrossRef]
- Yuan, H.; Liu, J.; Jiao, Q.; Li, Y.; Liu, X.; Shi, D.; Wu, Q.; Zhao, Y.; Li, H. Sandwich-like octahedral cobalt disulfide/reduced graphene oxide as an efficient Pt-free electrocatalyst for high-performance dye-sensitized solar cells. Carbon 2017, 119, 225–234. [Google Scholar] [CrossRef]
- Lu, M.-N.; Lin, J.-Y.; Wei, T.-C. Exploring the main function of reduced graphene oxide nano-flakes in a nickel cobalt sulfide counter electrode for dye-sensitized solar cell. J. Power Sources 2016, 332, 281–289. [Google Scholar] [CrossRef]
- Krishnapriya, R.; Praneetha, S.; Rabel, A.M.; Murugan, A.V. Energy efficient, one-step microwave-solvothermal synthesis of highly electro-catalytic thiospinel NiCo2S4/graphene nanohybrid as a novel sustainable counter electrode material for Pt-free dye sensitized solar cells. J. Mater. Chem. C 2017, 5, 3146–3155. [Google Scholar] [CrossRef]
- Bi, E.; Chen, H.; Yang, X.; Peng, W.; Gratzel, M.; Han, L. A quasi core–shell nitrogen-doped graphene/cobalt sulfide conductive catalyst for highly efficient dye-sensitized solar cells. Energy Environ. Sci. 2014, 7, 2637–2641. [Google Scholar] [CrossRef]
- Xu, Y.; Bai, H.; Lu, G.; Li, C.; Shi, G. Flexible Graphene Films via the Filtration of Water-Soluble Noncovalent Functionalized Graphene Sheets. J. Am. Chem. Soc. 2008, 130, 5856–5857. [Google Scholar] [CrossRef] [PubMed]
- Roy-Mayhew, J.D.; Bozym, D.J.; Punckt, C.; Aksay, I.A. Functionalized graphene as a catalytic counter electrode in dye-sensitized solar cells. ACS Nano 2010, 4, 6203–6211. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.W.; Li, X.D.; Li, H.B.; Chen, S.; Sun, Z.; Yin, X.J.; Huang, S.M. Graphene-based counter electrode for dye-sensitized solar cells. Carbon 2011, 49, 5382–5388. [Google Scholar] [CrossRef]
- Roy-Mayhew, J.D.; Boschloo, G.; Hagfeldt, A.; Aksay, I.A. Functionalized graphene sheets as a versatile replacement for platinum in dye-sensitized solar cells. ACS Appl. Mater. Interfaces 2012, 4, 2794–2800. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Yu, C.; Song, X.; Liu, Y.; Liang, S.; Liu, Z.; Hao, C.; Qiu, J. Nitrogen-doped graphene nanoribbons with surface enriched active sites and enhanced performance for dye-sensitized solar cells. Adv. Energy Mater. 2015, 5. [Google Scholar] [CrossRef]
- Zhang, X.; Bai, J.; Yang, B.; Li, G.; Liu, L. Self-assembled mesoporous Ni0.85Se spheres as high performance counter cells of dye-sensitized solar cells. RSC Adv. 2016, 6, 58925–58932. [Google Scholar] [CrossRef]
- Wang, G.; Xing, W.; Zhuo, S. The production of polyaniline/graphene hybrids for use as a counter electrode in dye-sensitized solar cells. Electrochim. Acta 2012, 66, 151–157. [Google Scholar] [CrossRef]
- Gao, Z.; Wang, L.; Chang, J.; Liu, X.; Wu, D.; Xu, F.; Guo, Y.; Jiang, K. Nitrogen doped porous graphene as counter electrode for efficient dye sensitized solar cell. Electrochim. Acta 2016, 188, 441–449. [Google Scholar] [CrossRef]
- Nikolakopoulou, A.; Tasis, D.; Sygellou, L.; Dracopoulos, V.; Galiotis, C.; Lianos, P. Study of the thermal reduction of graphene oxide and of its application as electrocatalyst in quasi-solid state dye-sensitized solar cells in combination with PEDOT. Electrochim. Acta 2013, 111, 698–706. [Google Scholar] [CrossRef]
- Ramasamy, M.S.; Nikolakapoulou, A.; Raptis, D.; Dracopoulos, V.; Paterakis, G.; Lianos, P. Reduced graphene oxide/Polypyrrole/PEDOT composite films as efficient Pt-free counter electrode for dye-sensitized solar cells. Electrochim. Acta 2015, 173, 276–281. [Google Scholar] [CrossRef]
- Chen, P.-Y.; Li, C.-T.; Lee, C.-P.; Vittal, R.; Ho, K.-C. PEDOT-decorated nitrogen-doped graphene as the transparent composite film for the counter electrode of a dye-sensitized solar cell. Nano Energy 2015, 12, 374–385. [Google Scholar] [CrossRef]
- Belekoukia, M.; Ramasamy, M.S.; Yang, S.; Feng, X.; Paterakis, G.; Dracopoulos, V.; Galiotis, C.; Lianos, P. Electrochemically exfoliated graphene/PEDOT composite films as efficient Pt-free counter electrode for dye-sensitized solar cells. Electrochim. Acta 2016, 194, 110–115. [Google Scholar] [CrossRef]
- Xu, X.; Huang, D.; Cao, K.; Wang, M.; Zakeeruddin, S.M.; Gratzel, M. Electrochemically reduced graphene oxide multilayer films as efficient counter electrode for dye-sensitized solar cells. Sci. Rep. 2013, 3, 1489. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Bai, Y.; Zhang, N.; Sun, K. Facile preparation of cobalt disulfide/reduced graphene oxide composite film as an efficient counter electrode for dye-sensitized solar cells. Chem. Commun. 2015, 51, 1846–1849. [Google Scholar] [CrossRef] [PubMed]
- Rani, A.; Chung, K.; Kwon, J.; Kim, S.J.; Jang, Y.H.; Jang, Y.J.; Quan, L.N.; Yoon, M.; Park, J.H.; Kim, D.H. Layer-by-layer self-assembled graphene multilayers as Pt-Free alternative counter electrodes in dye-sensitized solar cells. ACS Appl. Mater. Interfaces 2016, 8, 11488–11498. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Wen, Z.; Pu, H.; Lu, G.; Bo, Z.; Kim, H.; Qian, Y.; Andrew, E.; Mao, S.; Chen, J. Hierarchical vertically oriented graphene as a catalytic counter electrode in dye-sensitized solar cells. J. Mater. Chem. A 2013, 1, 188–193. [Google Scholar] [CrossRef]
- Wang, H.; Sun, K.; Tao, F.; Stacchiola, D.J.; Hu, Y.H. 3D Honeycomb-Like Structured Graphene and Its High Efficiency as a Counter-Electrode Catalyst for Dye-Sensitized Solar Cells. Angew. Chem. Int. Ed. 2013, 52, 9210–9214. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Sun, K.; Hu, Y.H. Synthesis of 3D cauliflower-fungus-like graphene from CO2 as a highly efficient counter electrode material for dye-sensitized solar cells. J. Mater. Chem. A 2014, 2, 16842–16846. [Google Scholar] [CrossRef]
- Wei, W.; Sun, K.; Hu, Y.H. Direct conversion of CO2 to 3D graphene and its excellent performance for dye-sensitized solar cells with 10% efficiency. J. Mater. Chem. A 2016, 4, 12054–12057. [Google Scholar] [CrossRef]
- Ahn, H.-J.; Kim, I.-H.; Yoon, J.-C.; Kim, S.-I.; Jang, J.-H. p-Doped three-dimensional graphene nanonetworks superior to platinum as a counter electrode for dye-sensitized solar cells. Chem. Commun. 2014, 50, 2412–2415. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Xu, X.; Gao, Y.; Li, Z.; Li, C.; Wang, W.; Chen, Y.; Ning, G.; Zhang, L.; Yang, F.; et al. High-surface-area nanomesh graphene with enriched edge sites as efficient metal-free cathodes for dye-sensitized solar cells. Nanoscale 2016, 8, 13059–13066. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Sudhagar, P.; Verma, V.; Song, D.; Ito, E.; Lee, S.Y.; Kang, Y.S.; Choi, W. Amplifying charge-transfer characteristics of graphene for triiodide reduction in dye-sensitized solar cells. Adv. Funct. Mater. 2011, 21, 3729–3736. [Google Scholar] [CrossRef]
- Yang, W.; Xu, X.; Hou, L.; Ma, X.; Yang, F.; Wang, Y.; Li, Y. Insight into the topological defects and dopants in metal-free holey graphene for triiodide reduction in dye-sensitized solar cells. J. Mater. Chem. A 2017, 5, 5952–5960. [Google Scholar] [CrossRef]
- Bi, H.; Zhao, W.; Sun, S.; Cui, H.; Lin, T.; Huang, F.; Xie, X.; Jiang, M. Graphene films decorated with metal sulfide nanoparticles for use as counter electrodes of dye-sensitized solar cells. Carbon 2013, 61, 116–123. [Google Scholar] [CrossRef]
- Bi, H.; Cui, H.; Lin, T.; Huang, F. Graphene Wrapped copper-nickel nanospheres on highly conductive graphene film for use as counter electrodes of dye-sensitized solar cells. Carbon 2015, 91, 153–160. [Google Scholar] [CrossRef]
- Wei, W.; Chang, L.; Sun, K.; Pak, A.J.; Paek, E.; Hwang, G.S.; Hu, Y.H. The Bright Future for Electrode Materials of Energy Devices Highly Conductive Porous Na-Embedded Carbon. Nano Lett. 2016, 16, 8029–8033. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.S.; Lee, Y.; Lee, J.Y.; Ahn, J.-H.; Park, J.H. Flexible and platinum-free dye-sensitized solar cells with conducting-polymer-coated graphene counter electrodes. ChemSusChem 2012, 5, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Liu, J.; Chen, H.; Wang, R.; Li, D.; Qu, J.; Dai, L. Nitrogen-doped fraphene foams as metal-free counter electrodes in high-performance dye-sensitized solar cells. Angew. Chem. Int. Ed. 2012, 51, 12124–12127. [Google Scholar] [CrossRef] [PubMed]
- Di Bartolomeo, A. Graphene schottky diodes: An experimental review of the rectifying graphene/semiconductor heterojunction. Phys. Rep. 2016, 606, 1–58. [Google Scholar] [CrossRef]
- Chen, Y.-M.; Liang, W.; Li, S.; Zou, F.; Bhaway, S.M.; Qiang, Z.; Gao, M.; Vogt, B.D.; Zhu, Y. Nitrogen doped carbonized metal organic framework for high stability room temperature sodium-sulfur battery. J. Mater. Chem. A 2016, 4, 12471–12478. [Google Scholar] [CrossRef]
- Qiang, Z.; Chen, Y.-M.; Xia, Y.; Liang, W.; Zhu, Y.; Vogt, B.D. Ultra-long cycle life, low-cost room temperature sodium-sulfur batteries enabled by highly doped (N,S) nanoporous carbons. Nano Energy 2017, 32, 59–66. [Google Scholar] [CrossRef]

| Year | n (%) | nPt (%) | VOC (V) | JSC (mA·cm−2) | Counter Electrode Material | Ref. |
|---|---|---|---|---|---|---|
| 2011 | 5.78 | 5.03 | 0.728 | 12.34 | Ti nitride–N-doped rGO (TiN/NG) hybrid | 25 |
| 2011 | 6.35 | 5.27 | 0.79 | 12.06 | Pt nanoparticles/rGO hybrid | 16 |
| 2013 | 9.54 | 9.14 | 0.692 | 18.77 | Electrochemically Reduced Graphene Oxide/PDDA Multilayer Films (LbL) | 70 |
| 2013 | 8.55 | 8.15 | 0.749 | 16.55 | NiS2/rGO nanocomposites | 45 |
| 2014 | 10.71 | 9.73 | 0.71 | 20.38 | Quasi core-shell nitrogen-doped rGO/cobalt sulfide | 57 |
| 2014 | 9.31 | 8.67 | 0.889 * | 14.07 | Edge-carboxylated graphene nanoplatelets (ball milling) | 35 |
| 2014 | 8.46 | 7.98 | 0.713 | 17.2 | p-Doped (N and O) 3D graphene networks | 77 |
| 2014 | 8.07 | 7.5 | 0.79 | 19.04 | 3D cauliflower-fungus-like rGO | 75 |
| 2015 | 9.39 | 7.34 | 0.764 | 19.42 | CoS/rGO hybrid film | 17 |
| 2015 | 8.57 | 7.84 | 0.78 | 15.18 | N-doped rGO nanoribbons | 62 |
| 2015 | 8.3 | 8.17 | 0.739 | 15.60 | PEDOT-decorated nitrogen-doped graphene | 68 |
| 2016 | 10.86 | 9.93 | 0.74 | 18.83 | N-doped rGO–FeN core-shell nanoparticles | 26 |
| 2016 | 8.4 | 7.98 | 0.72 | 17.32 | N-doped graphene nanosheets with active metal (Co) sites | 37 |
| 2016 | 9.82 | 8.24 | 0.767 | 18.903 | CoS/rGO hybrid | 49 |
| 2016 | 8.08 | 6.34 | 0.77 | 15.3 | B,N co-doped rGO | 33 |
| 2016 | 9.89 | 8.39 | 0.747 | 19.94 | CoSeO3·2H2O/rGO | 50 |
| 2016 | 8.44 | 7.54 | 0.745 | 16.25 | Pt-Ru nanoparticles supported on rGO | 21 |
| 2016 | 10.1 | 7.7 | 0.78 | 19.29 | 3D flower-like graphene made from CO2 (CVD) | 76 |
| 2016 | 8 | 7.7 | 0.64 | 22.8 * | Electrochemically exfoliated graphene/PEDOT composite films | 69 |
| 2016 | 9.03 | 9.07 | 0.78 | 16.25 | Thin films of graphene flakes produced by thermal plasma jet | 24 |
| 2016 | 8.57 | 7.58 | 0.77 | 15.91 | N and P co-doped rGO | 38 |
| 2016 | 11.03 * | 7.89 | 0.8 | 20.95 | Highly conductive porous Na-embedded carbon | 83 |
| 2017 | 9.11 | 8.03 | 0.745 | 18.73 | PtMo alloy on rGO | 23 |
| 2017 | 9.07 | 8.19 | 0.744 | 17.19 | N-doped holey graphene | 80 |
| 2017 | 9.4 | 9.1 | 0.744 | 16.86 | 3D N and S co-doped rGO networks | 43 |
| 2017 | 8.67 | 7.88 | 0.75 | 16.70 | porous S-doped rGO | 32 |
© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tasis, D. Recent Progress on the Synthesis of Graphene-Based Nanostructures as Counter Electrodes in DSSCs Based on Iodine/Iodide Electrolytes. Catalysts 2017, 7, 234. https://doi.org/10.3390/catal7080234
Tasis D. Recent Progress on the Synthesis of Graphene-Based Nanostructures as Counter Electrodes in DSSCs Based on Iodine/Iodide Electrolytes. Catalysts. 2017; 7(8):234. https://doi.org/10.3390/catal7080234
Chicago/Turabian StyleTasis, Dimitrios. 2017. "Recent Progress on the Synthesis of Graphene-Based Nanostructures as Counter Electrodes in DSSCs Based on Iodine/Iodide Electrolytes" Catalysts 7, no. 8: 234. https://doi.org/10.3390/catal7080234




