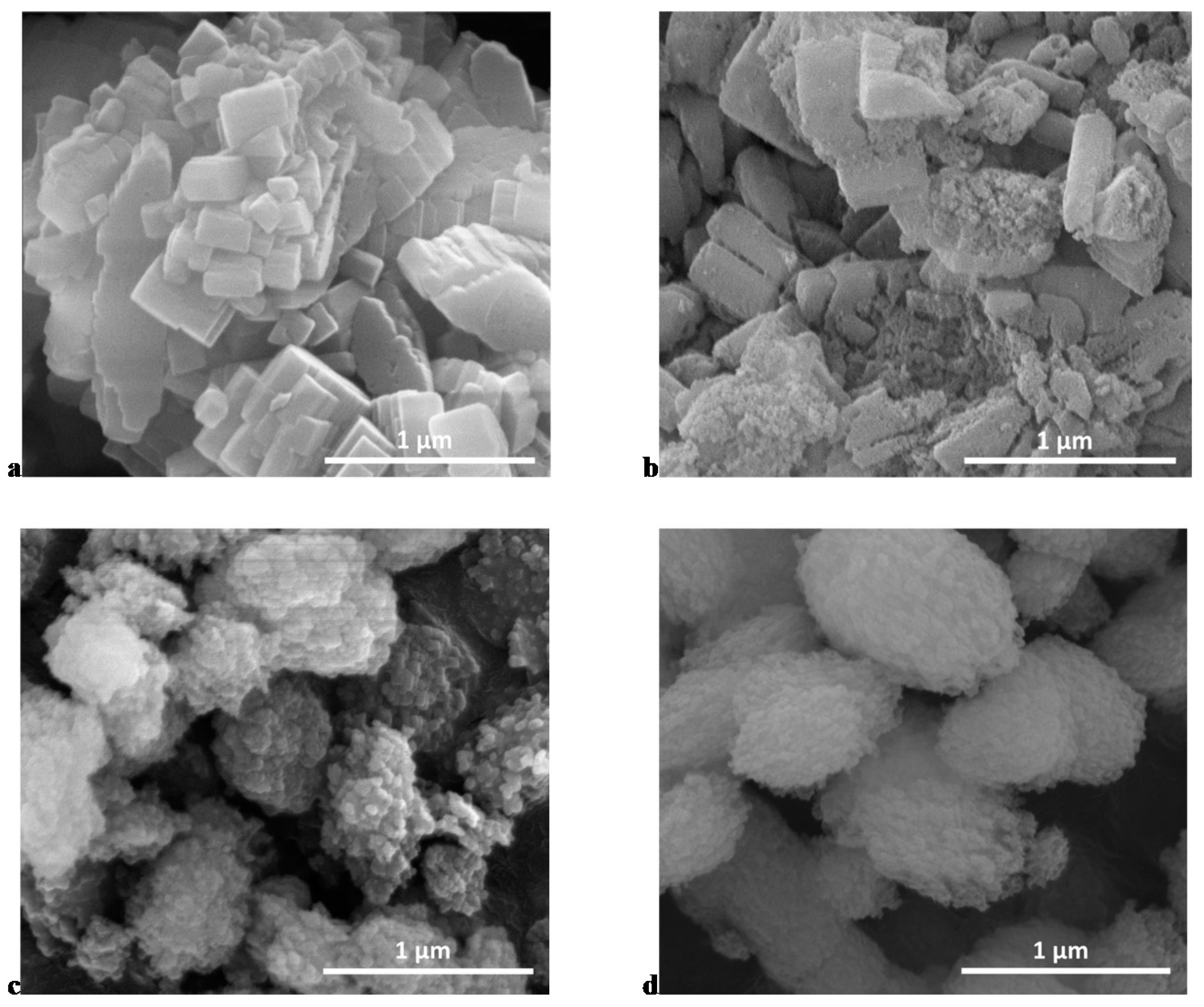
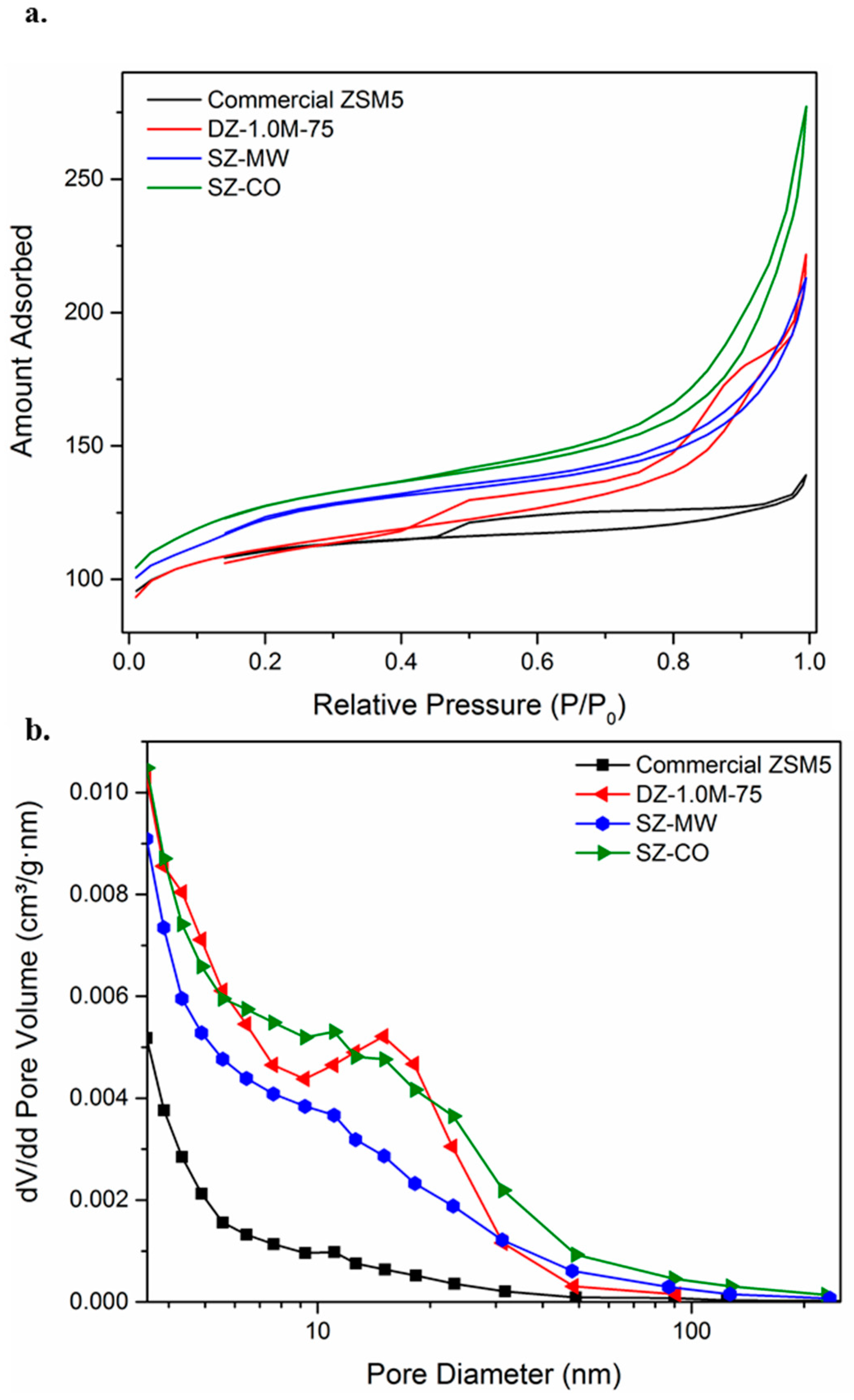
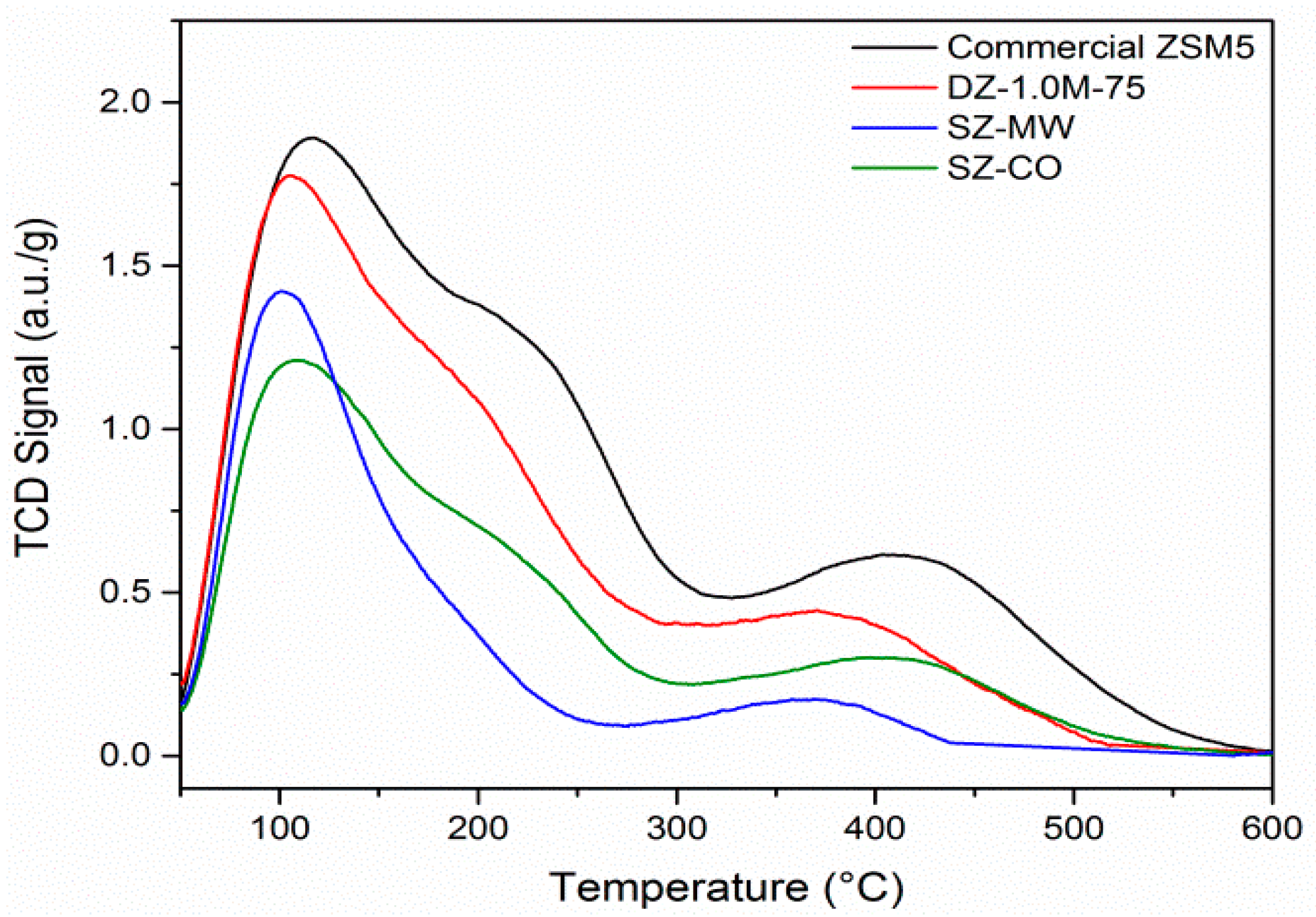
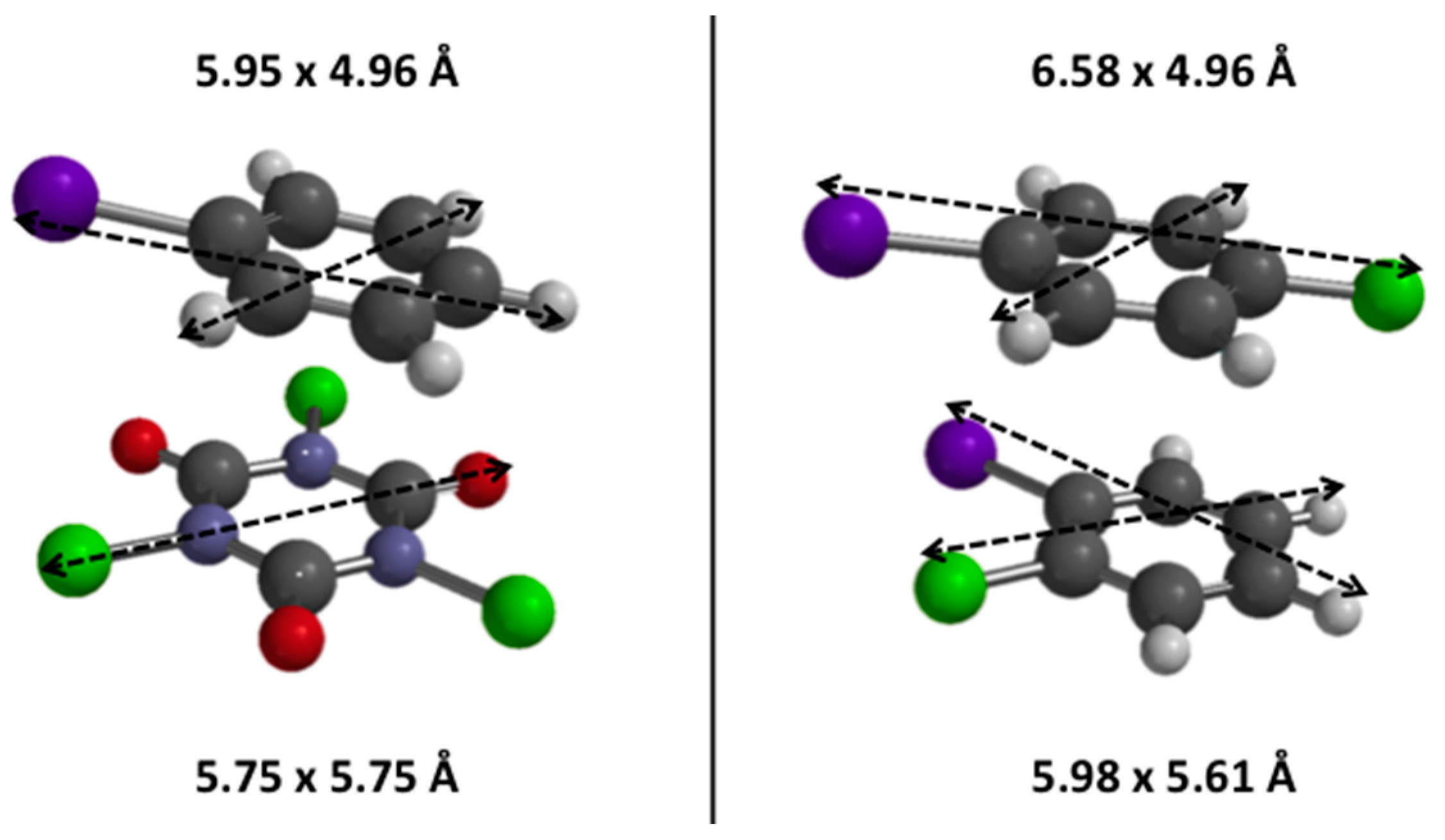
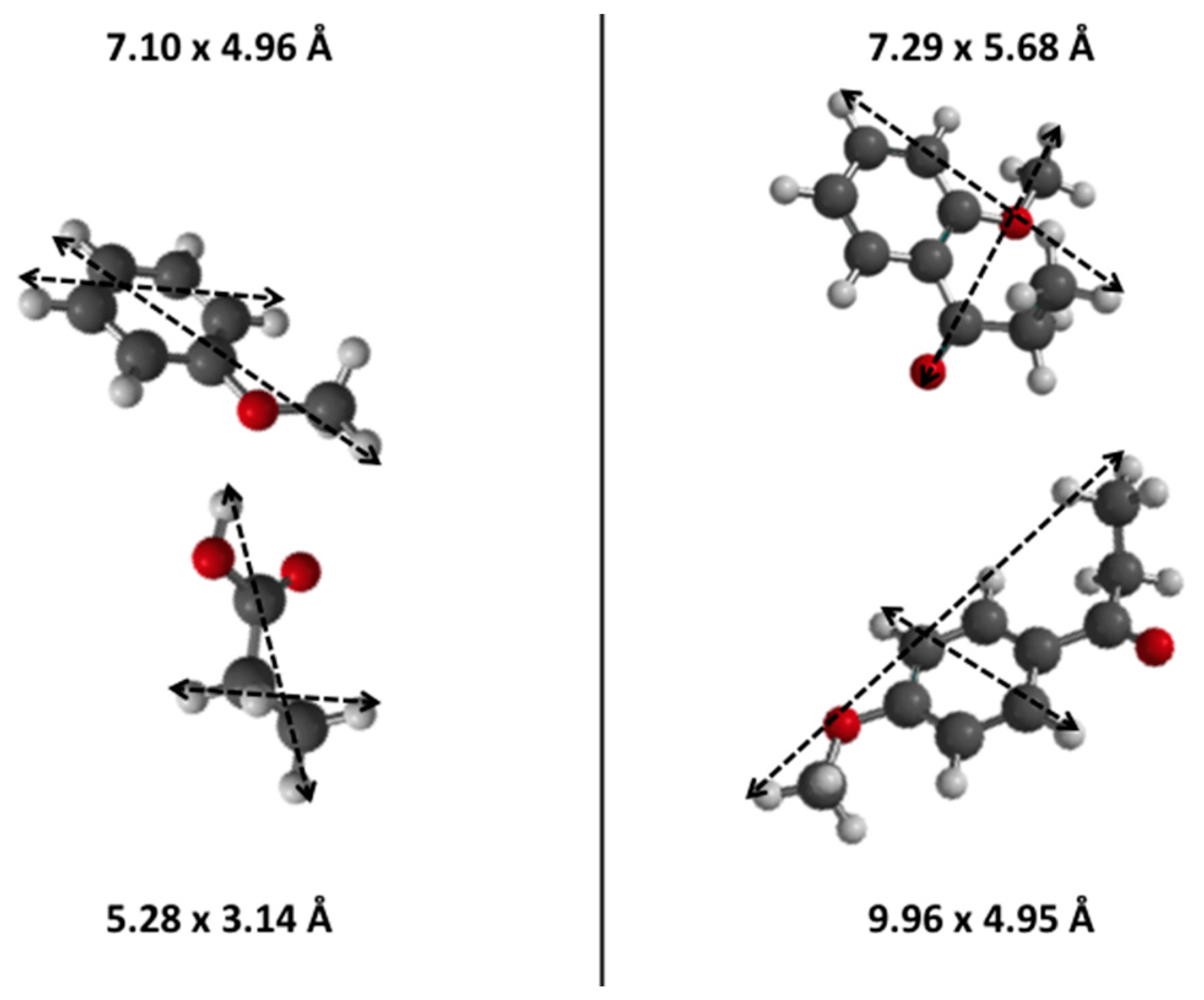
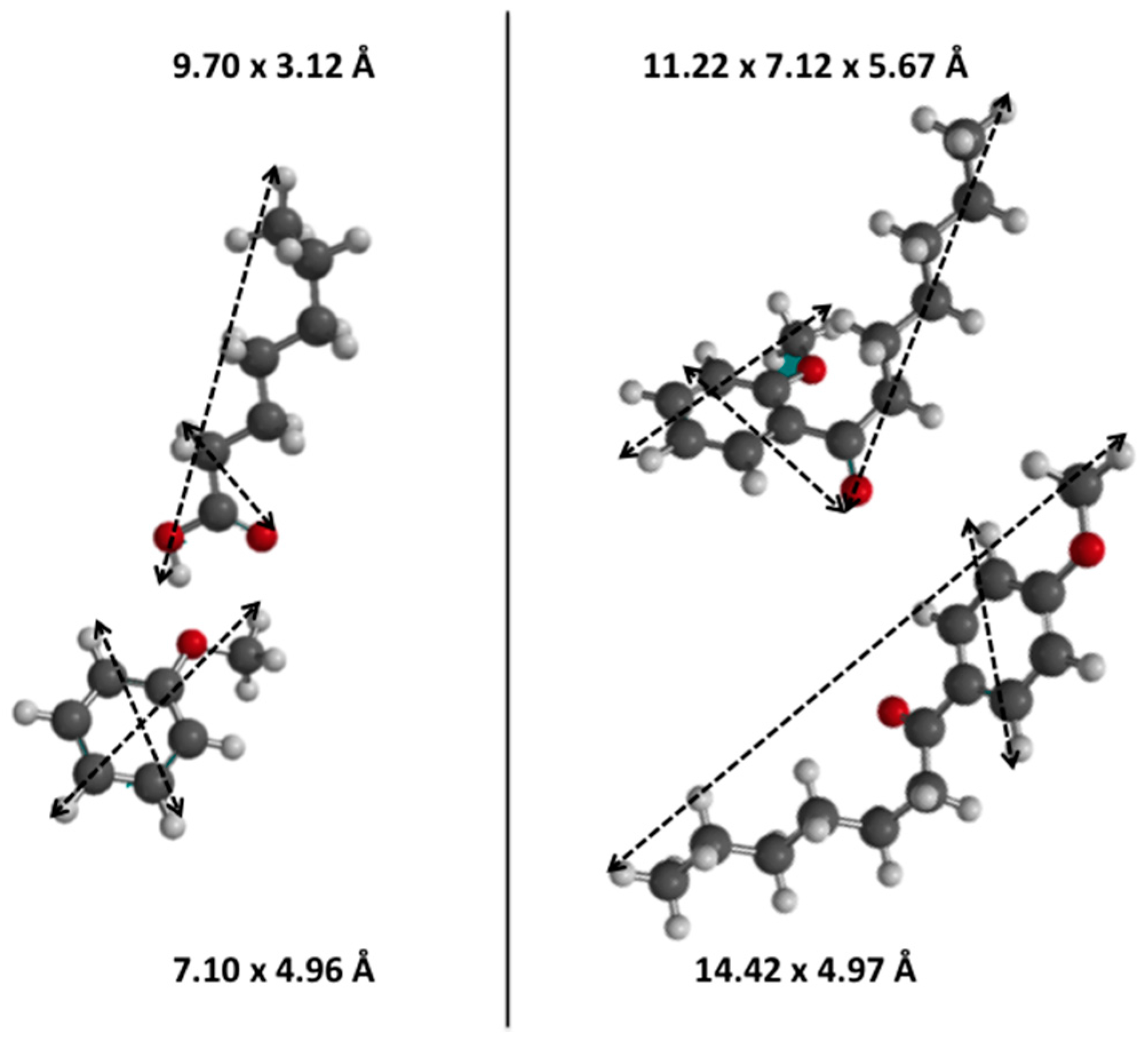
3.1. Diels Alder
The Diels Alder cyclisation reaction between isoprene and methylacrylate was used as a model reaction known to require only mild and medium acidity to achieve high yields in cycloadduct products (
Table 2).
It is noteworthy that mild desilication treatments led to considerable improvements in turnover frequencies (TOF); up to twice as high (entries 1 vs. 3), while maintaining a high selectivity towards the shape selectivity induced para product (
Figure 5). In contrast, harsh treatments led to a decrease in TOF and a loss in micropores linked shape selectivity (entry 6). Interestingly, DZ-1.0M-75 (entry 6) behaved similarly to amorphous silica-alumina considering conversion and TOF values (entry 7), indicating that the reaction occurred in the mesopores rather than in microporous shape selective network, which might have been damaged in this sample. Additionally, this is in agreement with XRD data while comparing crystalline qualities of the different samples. The lower ‘para’ selectivity observed over DZ-1.0M-75 (entry 6,
Table 2) might also be attributed to the presence of Al debris in the vicinity of BA sites, acting as Lewis acid sites, being able to guide the activation of the diene towards the formation of the ‘ortho’ cycloadduct. This observation is line with the similar trend of amorphous SiO
2/Al
2O
3 catalyst (entry 7,
Table 2).
The spectacular increase in TOF values achieved over DZ-0.2M-75 and DZ-0.5M-45 zeolites, from 14 up to 28 × 10−2 [h−1], strongly suggests the favorable occurrence of the cycloaddition reaction within the microporous network. Indeed, DZ-0.2M-75 and DZ-0.5M-45 materials possess the highest internal surface areas among the top down desilicated samples.
Finally, one may also assume that this Diels Alder reaction can be used as a manner to compare the impact of the desilication treatment, i.e., duration versus alkaline medium concentration on the selectivity towards ‘endo’ product and in terms of turnover frequency.
3.2. Methanol-to-Hydrocarbons
The series of ZSM-5 zeolites were compared in the gas-solid Methanol-To-Olefins (MTO) reaction. It has to be mentioned that none of the tested Al-rich ZSM-5 zeolites can a priori be considered as a promising catalyst for this reaction. However, the conversion of methanol to hydrocarbons may give precious insights regarding the interconnectivity between micro- and mesopores. Indeed, we could observe that the most harshly treated samples exhibited higher selectivities towards the formation of light olefins (ethylene-propylene-butylenes) than pristine sample (
Table 3). The parent zeolite (entry 1) exhibits a 42% selectivity towards C
2-C
4 light olefins, while for mildly and harshly treated samples (entries 2–5) this selectivity is close to (or above) 50%. In contrast, the two synthesised samples (entries 6 and 7) can be really considered as MTO catalysts with selectivities towards C
2-C
4 olefins approaching 60%.
Surprisingly, the SZ-MW sample led to a high selectivity (78%) towards ethylene, propylene, butylenes and pentenes. This catalyst produced by far the highest amount of pentenes (18%), propylene (38%) and only few C1-C4 alkanes (6%). One may expect a similar behaviour of the SZ-CO sample (which exhibits even higher Si/Al), but that was not the case. Hence, considering such low hydrogen transfer indices (HTI), the mesoporous mildly acidic SZ-MW sample was the only MFI-catalyst (among tested samples) favoring the occurrence of an olefin-propagation-cracking regime. The latter material behaves therefore as a true MTO catalyst, whilst the rest of the series led to MTG typical product distribution. Only slight improvements in terms of selectivities in light olefins depending on the harshness of the desilication treatment could be conclusively observed.
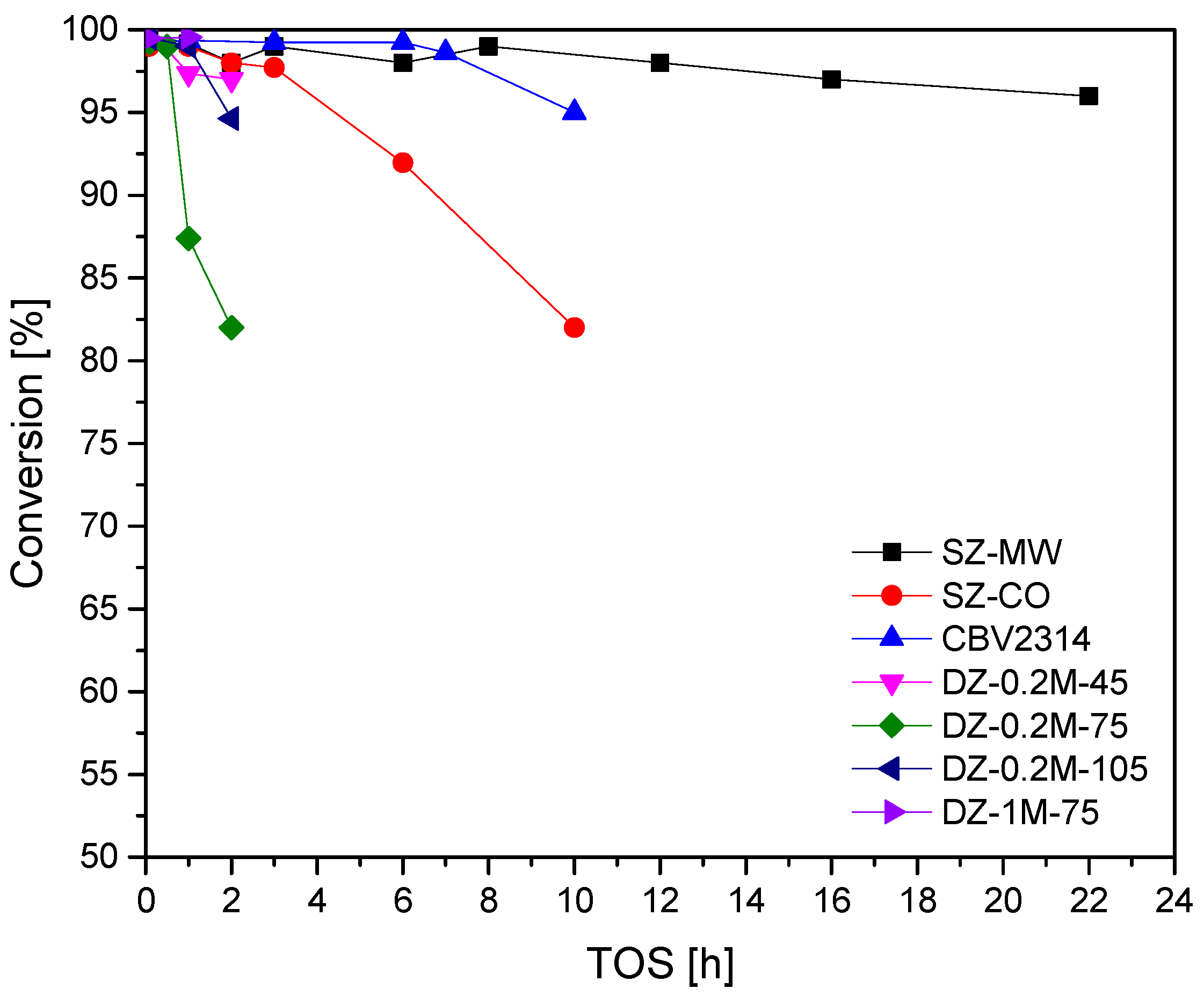
Figure 6 presents the conversion profile as a function of time on stream for all catalysts. In line with aforementioned observations, SZ-MW catalyst demonstrated the highest stability with almost no drop in conversion during 22 h on stream. It is noteworthy that all desilicated samples exhibit a faster deactivation with respect to pristine CBV2314 zeolite. The introduction of mesoporosity seems therefore to alter the stability for the MTO reaction. This is probably due to the easy formation and growth of coke in the generated mesopores. Consecutive reactions are occurring, thus favouring hydrogen transfer reactions. Both the production of gasoline and C
1-C
4 alkanes are warranted over top-down prepared samples in line selectivity results (
Table 3).
3.4. Friedel-Crafts Acylation of Anisole
The Friedel-Crafts acylation of anisole with two different carboxylic acids was also investigated, usually requiring a strong BrØnsted acid strength. The smaller propanoic acid is able to diffuse within the microporous network of ZSM-5 zeolites and, thus, its acylation reaction should occur in such shape selective environments. The opposite is expected for the larger heptanoic acid, which may barely react within the microporous network to form acylation products (
Figure 8). In
Table 5, it can be seen that DZ-0.2M-75 (entry 3) behaves as the best catalyst with a 50% relative improvement in TOF values with respect to pristine zeolite and, more interestingly, producing the main ortho-substituted product with a three times higher selectivity.
The degree of carboxylic acid conversion can be assumed nearly constant, only varying between 62–80% among all zeolites. The selectivity towards the ortho regioisomer increases with the extent of the desilication treatment, thus being favoured in the mesopores. This assumption is supported by the higher mesoporous volumes present in DZ-0.2M-75 and DZ-1.0M-75.
Again, it is important to mention that the highest TOF values were achieved over as-synthesised ZSM-5 zeolites (entries 7 and 8) with almost twice as high as parent zeolite. Amorphous silica-alumina did not exhibit any catalytic activity, thus suggesting the preferential occurrence of the acylation reaction in the zeolite micropores. Besides, the creation of (probably) connected mesopores with micropores led to higher TOF values.
In contrast, the reaction performed with sulphuric acid (entry 10) led to a complete inversion in ortho/para regioisomers selectivity, corresponding to the thermodynamic equilibrium obtained under non-shape selective conditions.
The acylation with heptanoic acid has to be considered to be favoured either inside the mesoporous network or (perhaps) at the micropores pore-mouth entrances, which are in turn more present in mesoporous zeolites. Indeed, the TOF values could be quadrupled over mesoporous zeolites in mildly treated DZ-0.2M-75 while even a 5-times higher TOF could be measured for harsher treated DZ-0.5M-45 (entry 5,
Table 6) with respect to parent zeolite. Again, the reaction catalysed by sulphuric acid indicates the thermodynamic equilibrium (entry 10).
The bottom-up designed SZ-MW (entry 7) exhibiting the highest TOF value of 19 is therefore interesting in terms of selectivity, since an inversion of the thermodynamically favoured product from ortho-, under non-shape selective conditions, to para- in shape selective micro/mesoporous zeolites could be observed (
Figure 9).
Figure 10 aims to summarise all the catalytic results being expressed as TOF versus surface acid sites density. The H/D isotope exchange technique was used for the estimation of the total surface acidity since all hydrons (silanol groups, bridging Al-(OH)-Si) might be exchanged [
46]. As desilication may create silanol defects, which can be either internal or external, the total BET surface area was chosen to express the surface Brønsted acid site density. The dependence between TOF values and BrØnsted surface acidity depends on the type of reaction chosen (
Figure 10).
The model reactions, which probably occurred within the microporous network, i.e., the chlorination of iodobenzene and the Friedel-Crafts acylation of anisole with propionic acid follow a tight linear fit. Hence, these highly acid strength-demanding reactions are favoured over ZSM-5 zeolites possessing more isolated Brønsted acid sites.
In contrast, for Diels-Alder cyclisation this linear relationship seems more volatile. Likewise, the Friedel-Crafts acylation of anisole with heptanoic acid seems more affected by the presence of mesopores rather than surface acidity. It is noteworthy that the slope of the Friedel-Crafts acylation of anisole with heptanoic acid (FC-HA) fit is much smaller than the slope of Friedel-Crafts acylation of anisole with propionic acid (FC-PA), thus indicating a greater impact for the latter involvement of micropores in the catalysed reaction.
Likewise, the microporous network appears as a governing parameter for the methanol conversion into hydrocarbons in terms of selectivity towards light olefins (
Figure 11). The higher the acid site dispersion is, the higher the selectivity towards C
2-C
4 olefins is.
The presence of higher surface acid density induces a drop in C
2-C
4 olefins selectivity, from 80% [
47] to below 50% as reported herein. Indeed, the more the acid sites, the more consecutive reactions are occurring, thus favouring hydrogen transfer reactions hence both the production of gasoline and C
1-C
4 alkanes.
The bottom-up synthesised mesoporous (SZ-MW and SZ-CO) samples behave in most cases better than top-down as-prepared catalysts. This can be explained by a higher crystallinity with respect to desilicated zeolites that exhibit a loss in crystal quality. Besides, a higher Si/Al ratio is also observed for the former two zeolites, thus suggesting a better resistance to deactivation by coking. Among SZ-MW and SZ-CO, the latter exhibits a higher Si/Al ratio that underlines the impact of possessing more disperse acid sites which positively impacts its reactivity in MTO. In contrast, SZ-MW (with more acid sites) allows achieving a higher conversion in FC reaction.
By properly selecting the desilication treatment (duration, concentration, temperature) or the constructive approach to introduce mesoporosity, it is possible to design an optimal acid catalyst depending on the targeted catalytic reaction. The herein presented cheap catalytic reactions are sensitive tools for qualitatively characterising meso- and micropores connectivity.