Highly Effective Dual Transition Metal Macrocycle Based Electrocatalyst with Macro-/Mesoporous Structures for Oxygen Reduction Reaction
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
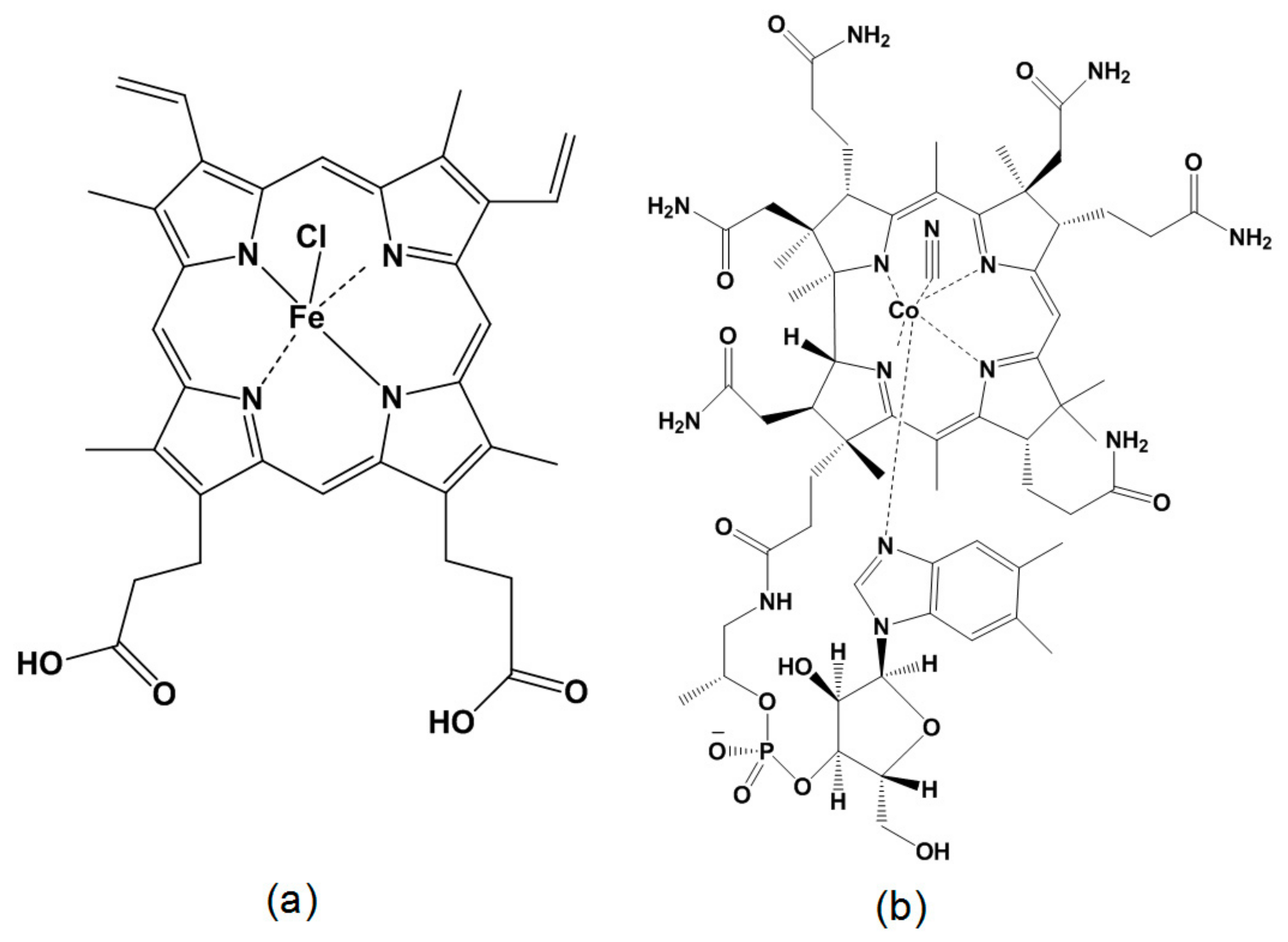
4.1. Materials
4.2. Synthesis of Metal-Macrocycle Based Porous Carbon (MMPC)
4.3. Characterization
4.4. Electrocatalytic Activity Measurements
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- James, B.; Kalinoski, J. Mass-production cost estimation of automotive fuel cell system. In Proceedings of the DOE-EERE Fuel Cell Technologies Program-2009 DOE Hydrogen Program Review, Arlington, VA, USA, 21 May 2009. [Google Scholar]
- Sinha, J.; Lasher, S.; Yang, Y. Direct Hydrogen PEMFC Manufacturing Cost Estimation for Automotive Application. In Proceedings of the DOE-EERE Fuel Cell Technologies Program-2009 DOE Hydrogen Program Review, Arlington, VA, USA, 21 May 2009. [Google Scholar]
- Liu, H.S.; Song, C.J.; Tang, Y.H.; Zhang, J.L.; Zhang, J.J. High-surface-area CoTMPP/C synthesized by ultrasonic spray pyrolysis for PEM fuel cell electrocatalysts. Electrochim. Acta 2007, 52, 4532–4538. [Google Scholar] [CrossRef]
- Wu, Z.S.; Chen, L.; Liu, J.Z.; Parvez, K.; Liang, H.W.; Shu, J.; Sachdev, H.; Graf, R.; Feng, X.L.; Müllen, K. High-performance electrocatalysts for oxygen reduction derived from cobalt porphyrin-based conjugated mesoporous polymers. Adv. Mater. 2014, 26, 1450–1455. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.Y.; Li, Y.G.; Wang, H.L.; Zhou, J.G.; Wang, J.; Regier, T.; Dai, H.J. Co3O4 nanocrystals on graphene as a synergistic catalyst for oxygen reduction reaction. Nat. Mater. 2011, 10, 780–786. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.Y.; Wang, H.L.; Zhou, J.G.; Li, Y.G.; Wang, J.; Regier, T.; Dai, H.J. Covalent hybrid of spinel manganese-cobalt oxide and graphene as advanced oxygen reduction electrocatalysts. J. Am. Chem. Soc. 2012, 134, 3517–3523. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Jensen, J.O.; Zhang, W.; Cleemann, L.N.; Xing, W.; Bjerrum, N.J.; Li, Q.F. Hollow spheres of iron carbide nanoparticles encased in graphitic layers as oxygen reduction catalysts. Angew. Chem. Int. Ed. 2014, 53, 3675–3679. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.L.; Zhu, J.B.; Feng, L.G.; Liu, C.P.; Xing, W. Meso/macroporous nitrogen-doped carbon architectures with iron carbide encapsulated in graphitic layers as an efficient and robust catalyst for the oxygen reduction reaction in both acidic and alkaline solutions. Adv. Mater. 2015, 27, 2521–2527. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.S.; Zhang, B.S.; Liu, X.; Wang, D.W.; Su, D.S. Unravelling the structure of electrocatalytically active Fe-N complexes in carbon for the oxygen reduction reaction. Angew. Chem. Int. Ed. 2014, 53, 10673–10677. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.J.; Jiang, S.J.; Zhao, Y.; Zhu, L.; Chen, S.; Wang, X.Z.; Wu, Q.; Ma, J.; Ma, Y.W.; Hu, Z. Boron-doped carbon nanotubes as metal-free electrocatalysts for the oxygen reduction reaction. Angew. Chem. Int. Ed. 2011, 50, 7132–7135. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.S.; Bhattacharjya, D.; Inamdar, S.; Park, J.; Yu, J.S. Phosphorus-doped ordered mesoporous carbons with different lengths as efficient metal-free electrocatalysts for oxygen reduction reaction in alkaline media. J. Am. Chem. Soc. 2012, 134, 16127–16130. [Google Scholar] [CrossRef] [PubMed]
- Gong, K.P.; Du, F.; Xia, Z.H.; Durstock, M.; Dai, L.M. Nitrogen-doped carbon nanotube arrays with high electrocatalytic activity for oxygen reduction. Science 2009, 323, 760–764. [Google Scholar] [CrossRef] [PubMed]
- Jasinski, R. A new fuel cell cathode catalyst. Nature 1964, 201, 1212–1213. [Google Scholar] [CrossRef]
- Scherson, D.A.; Gupta, S.L.; Fierro, C.; Yeager, E.B.; Kordesch, M.E.; Eldridge, J.; Hoffman, R.W.; Blue, J. Cobalt tetramethoxyphenyl porphyrin-emission Mossbauer spectroscopy and O2 reduction electrochemical studies. Electrochim. Acta 1983, 28, 1205–1209. [Google Scholar] [CrossRef]
- Jiang, Y.; Xie, Y.; Jin, X.X.; Hu, Q.; Chen, L.; Xu, L.; Huang, J.H. Highly efficient iron phthalocyanine based porous carbon electrocatalysts for the oxygen reduction reaction. RSC Adv. 2016, 6, 78737–78742. [Google Scholar] [CrossRef]
- Liang, H.W.; Wei, W.; Wu, Z.S.; Feng, X.L.; Müllen, K. Mesoporous metal-nitrogen-doped carbon electrocatalysts for highly efficient oxygen reduction reaction. J. Am. Chem. Soc. 2013, 135, 16002–16005. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Song, Y.J.; Zhang, G.X.; Liu, H.Y.; Wang, Y.R.; Sun, S.H.; Guo, X.W. Pyrolysis of selfassembled iron porphyrin on carbon black as core/shell structured electrocatalysts for highly efficient oxygen reduction in both alkaline and acidic medium. Adv. Funct. Mater. 2017, 27, 1604356. [Google Scholar] [CrossRef]
- Cheon, J.Y.; Kim, T.; Choi, Y.M.; Jeong, H.Y.; Kim, M.G.; Sa, Y.J.; Kim, J.; Lee, Z.; Yang, T.H.; Kwon, K.; et al. Ordered mesoporous porphyrinic carbons with very high electrocatalytic activity for the oxygen reduction reaction. Sci. Rep. 2013, 3. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Tang, C.Z.; Hao, Z.Q.; Lv, Y.; Yang, R.X.; Wei, X.M.; Deng, W.Q.; Wang, A.J.; Yi, B.L.; Song, Y.J. Carbonization of self-assembled nanoporous hemin with a significantly enhanced activity for the oxygen reduction reaction. Faraday Discuss. 2014, 176, 393–408. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Zhu, D.; Fu, Y.; Wang, X. CoFe2O4-graphene nanocomposite as a high-capacity anode material for lithium-ion batteries. Electrochim. Acta 2012, 83, 166–174. [Google Scholar] [CrossRef]
- Liu, J.X.; Liang, J.; Zhou, H.; Xiao, C.H.; Liang, F.X.; Ding, S.J. CoFe2O4 nanoparticles anchored on bowl-like carbon backbone for enhanced reversible lithium storage. RSC Adv. 2016, 6, 50153–50157. [Google Scholar] [CrossRef]
- Su, Y.H.; Jiang, H.L.; Zhu, Y.H.; Yang, X.L.; Shen, J.H.; Zou, W.J.; Chen, J.D.; Li, C.Z. Enriched graphitic N-doped carbon-supported Fe3O4 nanoparticles as efficient electrocatalysts for oxygen reduction reaction. J. Mater. Chem. A 2014, 2, 7281–7287. [Google Scholar] [CrossRef]
- Ferrero, G.A.; Preuss, K.; Marinovic, A.; Jorge, A.B.; Mansor, N.; Brett, D.J.; Fuertes, A.B.; Sevilla, M.; Titirici, M.M. Fe-N-doped carbon capsules with outstanding electrochemical performance and stability for the oxygen reduction reaction in both acid and alkaline conditions. ACS Nano 2016, 10, 5922–5932. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Hu, J.; Weng, M.; Tan, R.; Tian, L.; Yang, J.; Amine, J.; Zheng, J.; Chen, H.; Pan, F. Fe-cluster pushing electrons to N-doped graphitic layers with Fe3C (Fe) hybrid nanostructure to enhance O2 reduction catalysis of Zn-Air batteries. ACS Appl. Mater. Interfaces 2017, 9, 4587–4596. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Zhu, Q.; Xu, A.W. Noble-metal-free Fe-N/C catalyst for highly efficient oxygen reduction reaction under both alkaline and acidic conditions. J. Am. Chem. Soc. 2014, 136, 11027–11033. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.L.; Wu, D.Q.; Feng, X.L.; Müllen, K. Nitrogen-doped ordered mesoporous graphitic arrays with high electrocatalytic activity for oxygen reduction. Angew. Chem. Int. Ed. 2010, 49, 2565–2569. [Google Scholar] [CrossRef] [PubMed]
- Niu, W.H.; Li, L.G.; Liu, X.J.; Wang, N.; Liu, J.; Zhou, W.J.; Tang, Z.H.; Chen, S.W. Mesoporous N-doped carbons prepared with thermally removable nanoparticle templates: An efficient electrocatalyst for oxygen reduction reaction. J. Am. Chem. Soc. 2015, 137, 5555–5562. [Google Scholar] [CrossRef] [PubMed]
- Long, N.V.; Yang, Y.; Teranishi, T.; Thi, C.M.; Cao, Y.Q.; Nogami, M. Related magnetic properties of CoFe2O4 cobalt ferrite particles synthesised by the polyol method with NaBH4 and heat treatment: new micro and nanoscale structures. RSC Adv. 2015, 5, 56560–56569. [Google Scholar] [CrossRef]
- Wu, G.; Chen, Z.W.; Artyushkova, K.; Garzon, F.H.; Zelenay, P. Polyaniline-derived non-precious catalyst for the polymer electrolyte fuel cell cathode. ECS Trans. 2008, 16, 159–170. [Google Scholar]
- Li, S.; Wu, D.Q.; Liang, H.W.; Wang, J.Z.; Zhuang, X.D.; Mai, Y.Y.; Su, Y.Z.; Feng, X.L. Metal-nitrogen doping of mesoporous carbon/graphene nanosheets by self-templating for oxygen reduction electrocatalysts. ChemSusChem 2014, 7, 3002–3006. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; More, K.L.; Johnston, C.M.; Zelenay, P. High-performance electrocatalysts for oxygen reduction derived from polyaniline, iron, and cobalt. Science 2011, 332, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, H.H.; Gao, D.F.; Miao, S.; Wang, G.X.; Bao, X.H. High-density iron nanoparticles encapsulated within nitrogen-doped carbon nanoshell as efficient oxygen electrocatalyst for zinc-air battery. Nano Energy 2015, 13, 387–396. [Google Scholar] [CrossRef]
- Sa, Y.J.; Park, C.Y.; Jeong, H.Y.; Park, S.H.; Lee, Z.H.; Kim, K.T.; Park, G.G.; Joo, S.H. Carbon nanotubes/heteroatom-doped carbon core-sheath nanostructures as highly active, metal-free oxygen reduction electrocatalysts for alkaline fuel cells. Angew. Chem. Int. Ed. 2014, 53, 4102–4106. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.G.; Zhou, W.; Wang, H.L.; Xie, L.M.; Liang, Y.Y.; Wei, F.; Idrobo, J.C.; Pennycook, S.J.; Dai, H.J. An oxygen reduction electrocatalyst based on carbon nanotube-graphene complexes. Nat. Nanotechnol. 2012, 7, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.W.; Zhuang, X.D.; Brüller, S.; Feng, X.L.; Müllen, K. Hierarchically porous carbons with optimized nitrogen doping as highly active electrocatalysts for oxygen reduction. Nat. Commun. 2014, 5, 4973. [Google Scholar] [CrossRef] [PubMed]
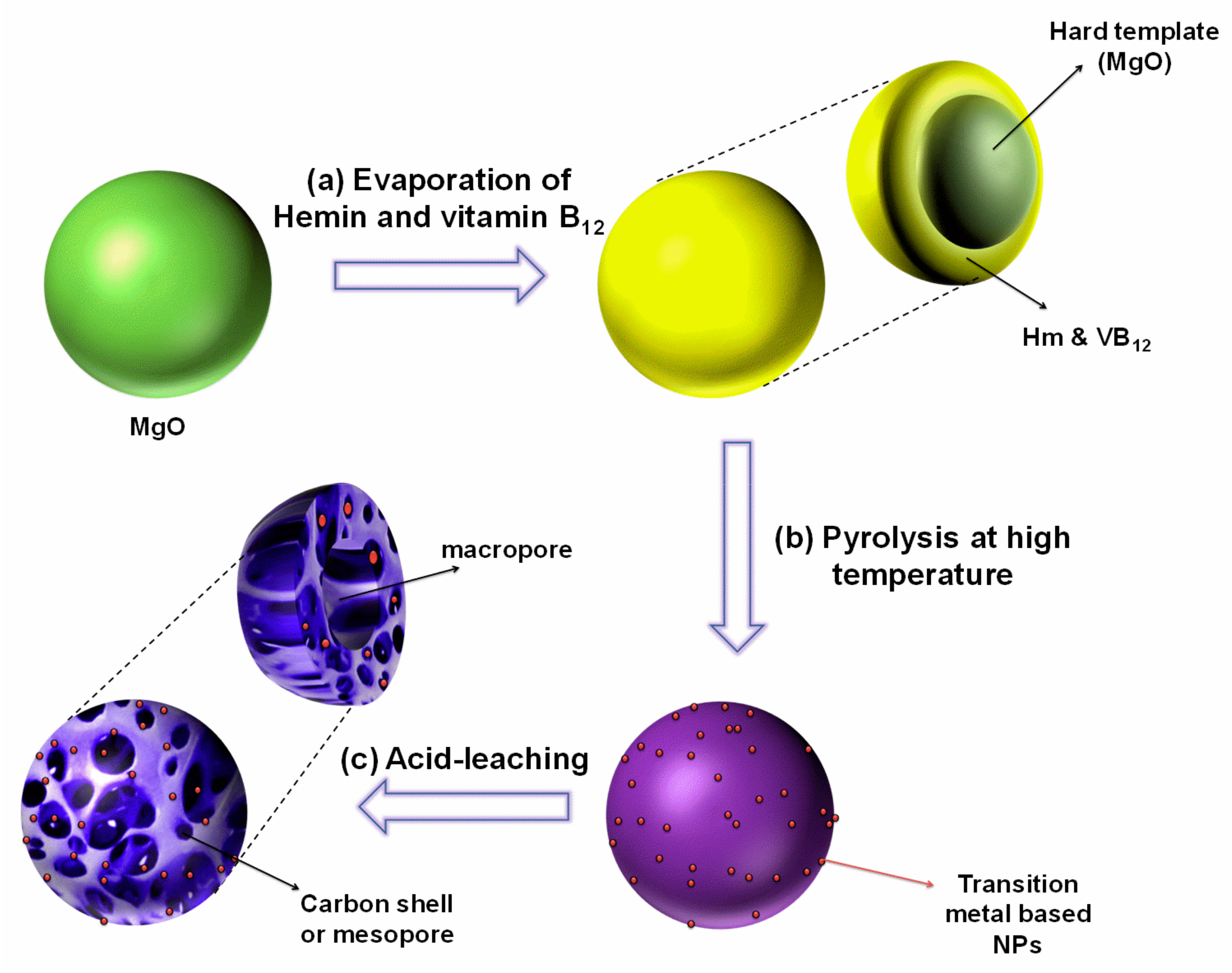
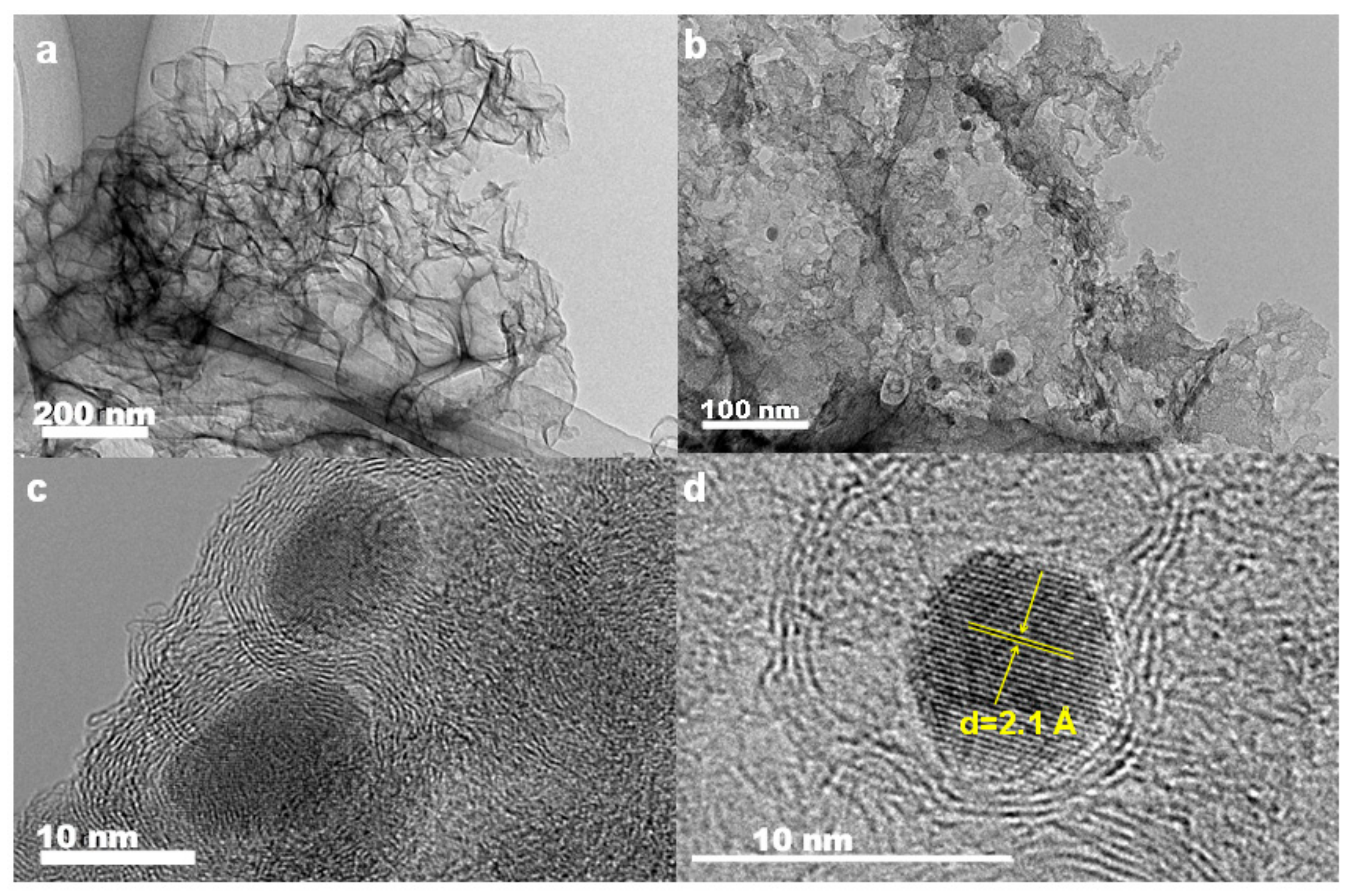



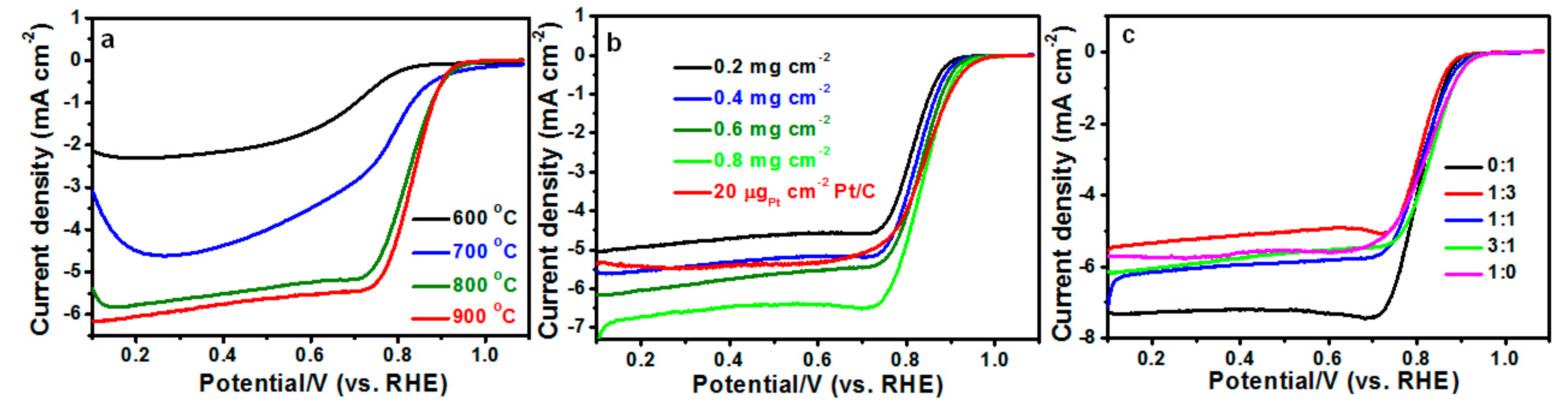
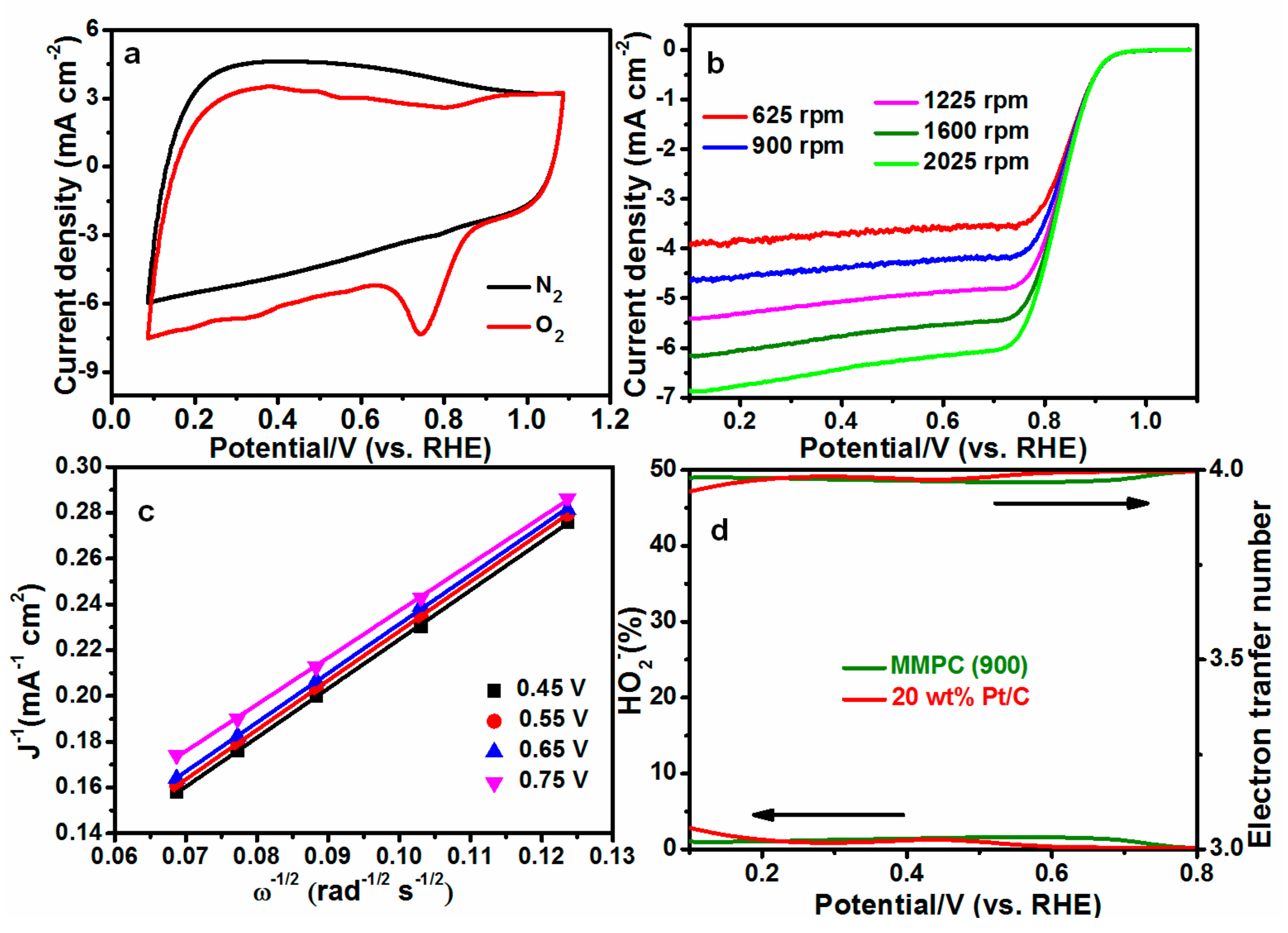
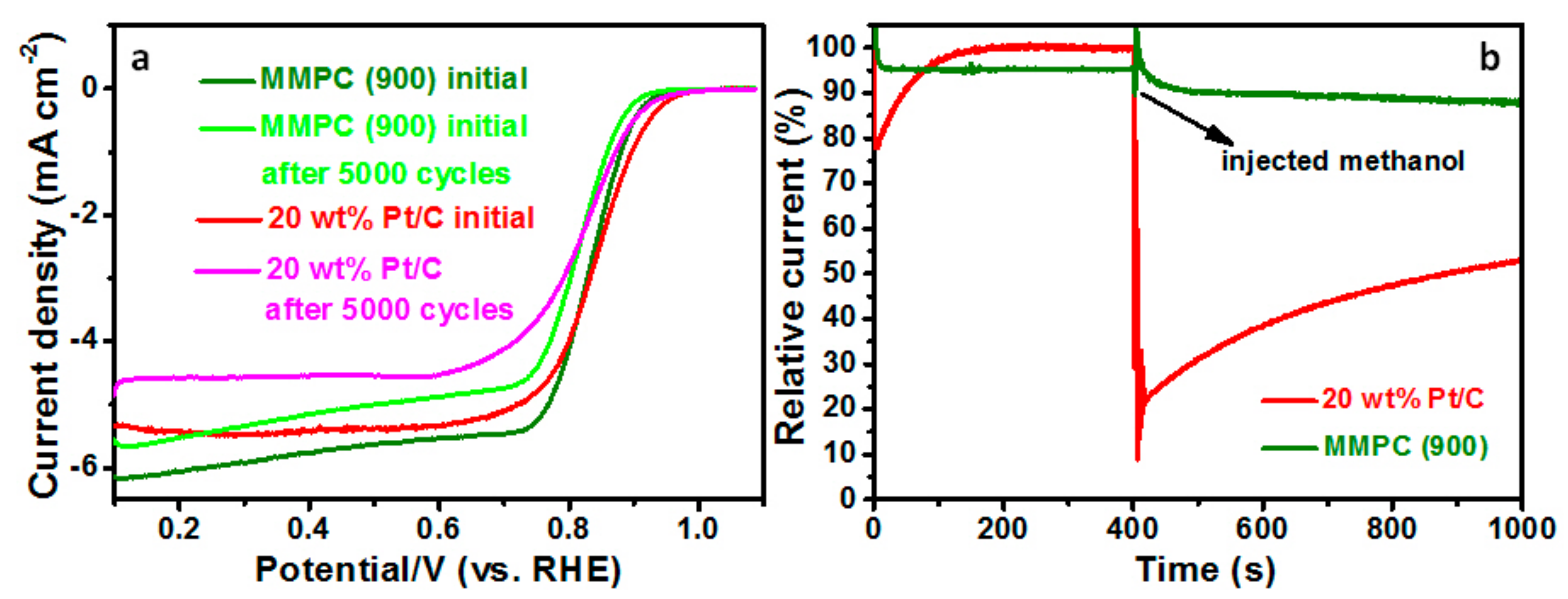
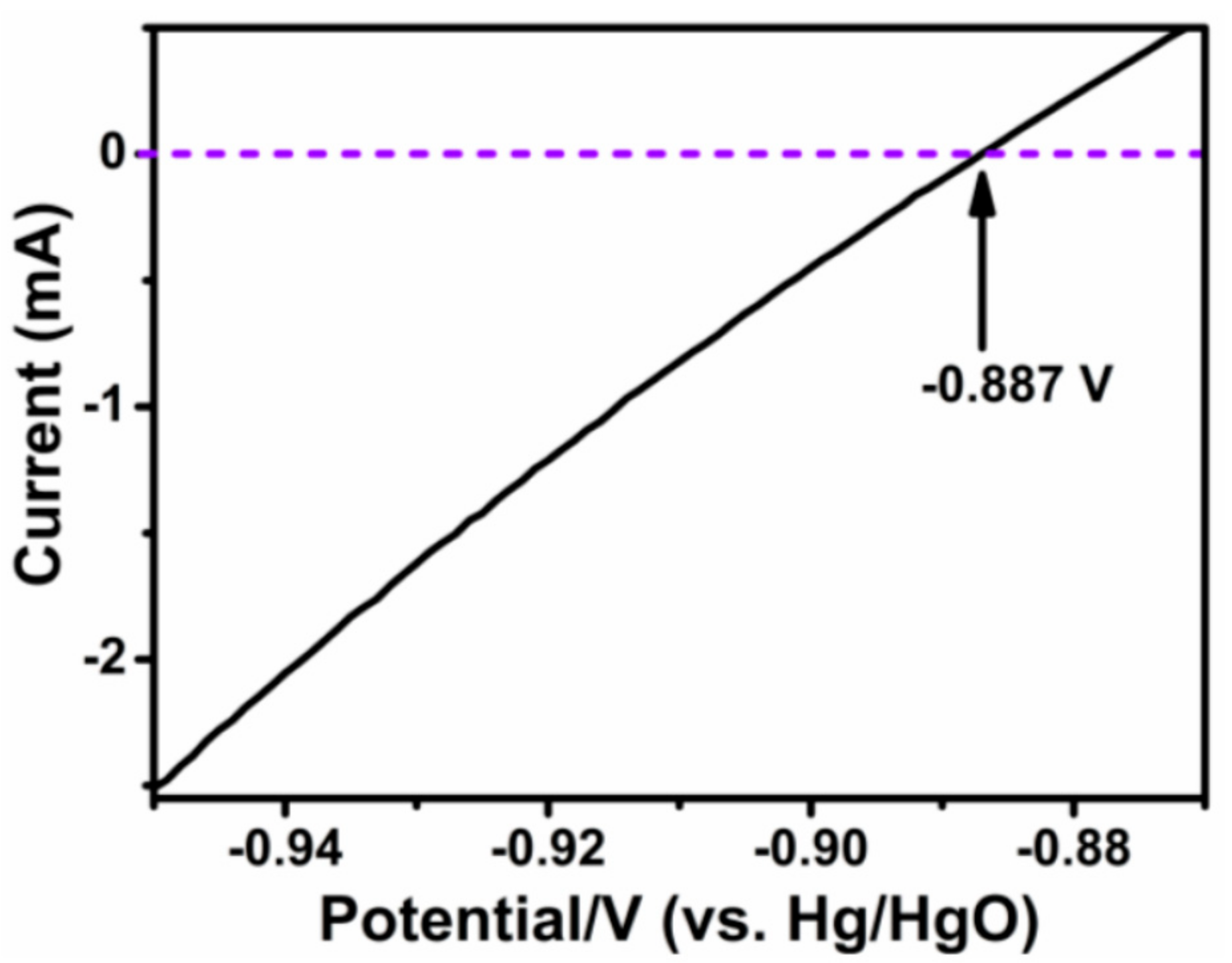
| MMPC (T) | SBET (m2·g−1) | Smic (m2·g−1) | Vt (cm3·g−1) | Dave (nm) |
|---|---|---|---|---|
| MMPC (600) | 168 | 102 | 0.26 | 6.97 |
| MMPC (700) | 541 | 163 | 0.83 | 6.51 |
| MMPC (800) | 627 | 236 | 0.96 | 6.63 |
| MMPC (900) | 429 | 179 | 0.50 | 5.25 |
| Cat. Loading | Eonset (vs. RHE) | E1/2 (V vs. RHE) | Current Density at 0.8 V (mA·cm−2) |
|---|---|---|---|
| 0.2 mg·cm−2 | 0.878 | 0.815 | 2.94 |
| 0.4 mg·cm−2 | 0.894 | 0.824 | 3.63 |
| 0.6 mg·cm−2 | 0.905 | 0.831 | 4.12 |
| 0.8 mg·cm−2 | 0.919 | 0.836 | 4.83 |
| 20 μgPt·cm−2 | 0.924 | 0.841 | 3.97 |
| Electrocatalyst | Cat. Loading(mg·cm−2) | E1/2 (V vs. RHE) | Current Density at 0.8 V (mA·cm−2) | Reference |
|---|---|---|---|---|
| Fe@N–C-700 | 0.3 | 0.83 | 4.15 | [32] |
| CNT/HDC-1000 | 0.6 | 0.82 | 3.25 | [33] |
| NT–G | 0.485 | 0.86 | 5.00 | [34] |
| Meso/micro-PoPD | 0.5 | 0.87 | 4.95 | [35] |
| Core/shell NPME | 0.6 | 0.87 | 5.40 | [17] |
| MMPC (900) | 0.6 | 0.83 | 4.12 | This work |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, X.; Xie, Y.; Huang, J. Highly Effective Dual Transition Metal Macrocycle Based Electrocatalyst with Macro-/Mesoporous Structures for Oxygen Reduction Reaction. Catalysts 2017, 7, 201. https://doi.org/10.3390/catal7070201
Jin X, Xie Y, Huang J. Highly Effective Dual Transition Metal Macrocycle Based Electrocatalyst with Macro-/Mesoporous Structures for Oxygen Reduction Reaction. Catalysts. 2017; 7(7):201. https://doi.org/10.3390/catal7070201
Chicago/Turabian StyleJin, Xinxin, Yan Xie, and Jiahui Huang. 2017. "Highly Effective Dual Transition Metal Macrocycle Based Electrocatalyst with Macro-/Mesoporous Structures for Oxygen Reduction Reaction" Catalysts 7, no. 7: 201. https://doi.org/10.3390/catal7070201





