Preparation of ZnO-Loaded Lignin-Based Carbon Fiber for the Electrocatalytic Oxidation of Hydroquinone
Abstract
:1. Introduction
2. Results and Discussion
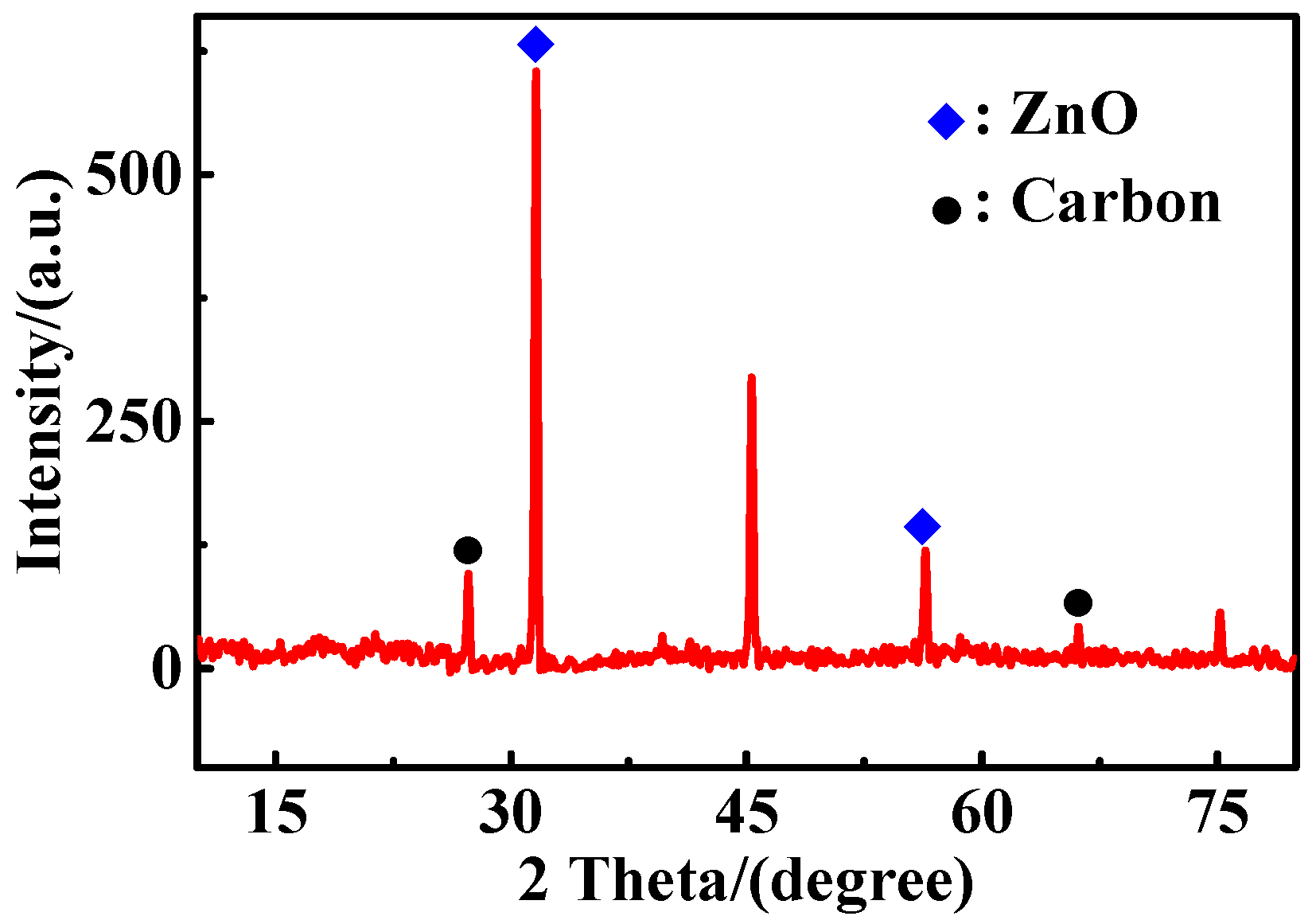
2.1. The XRD Pattern of ZCF
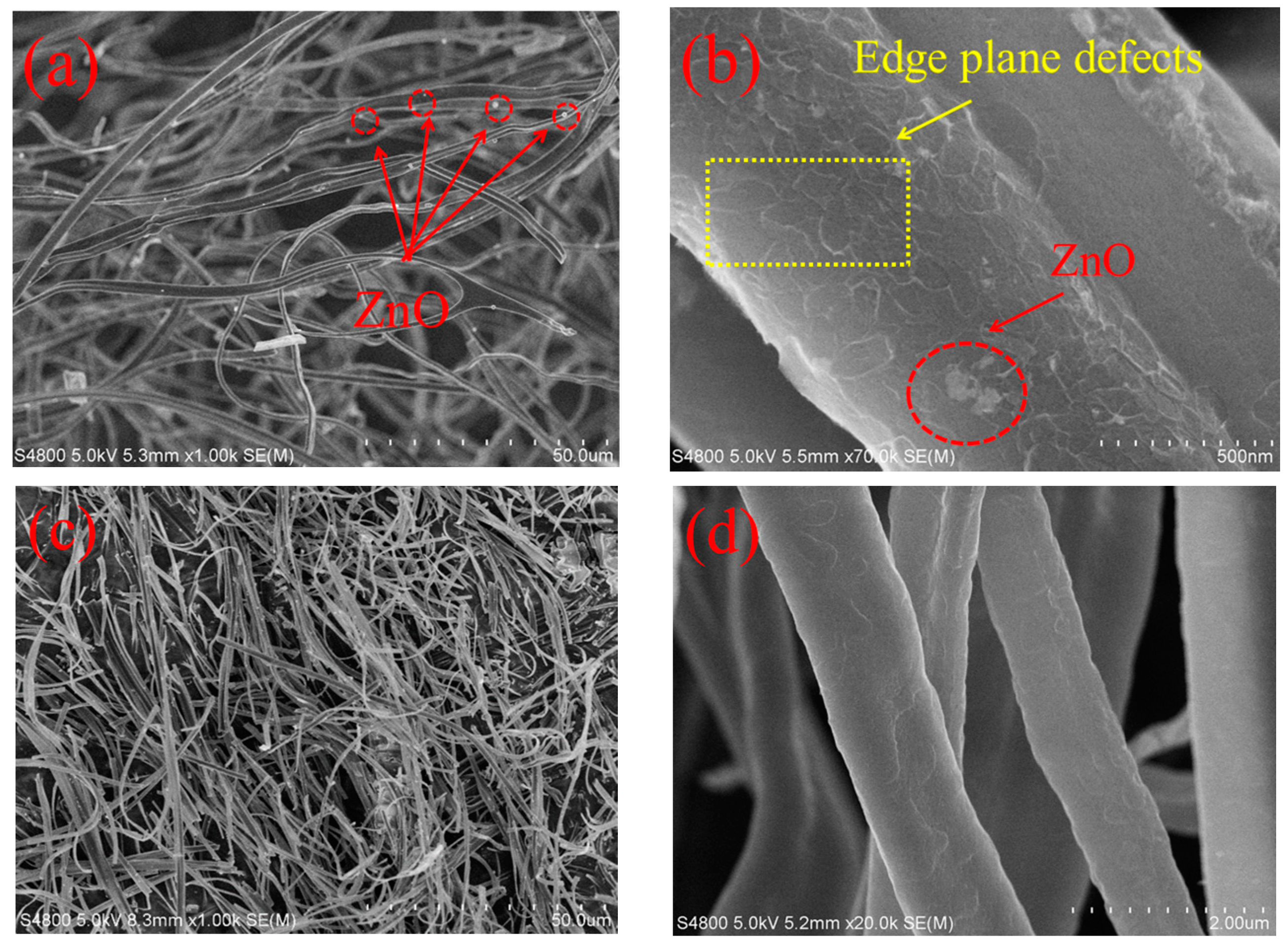
2.2. The Surface Morphology of ZCF and CF
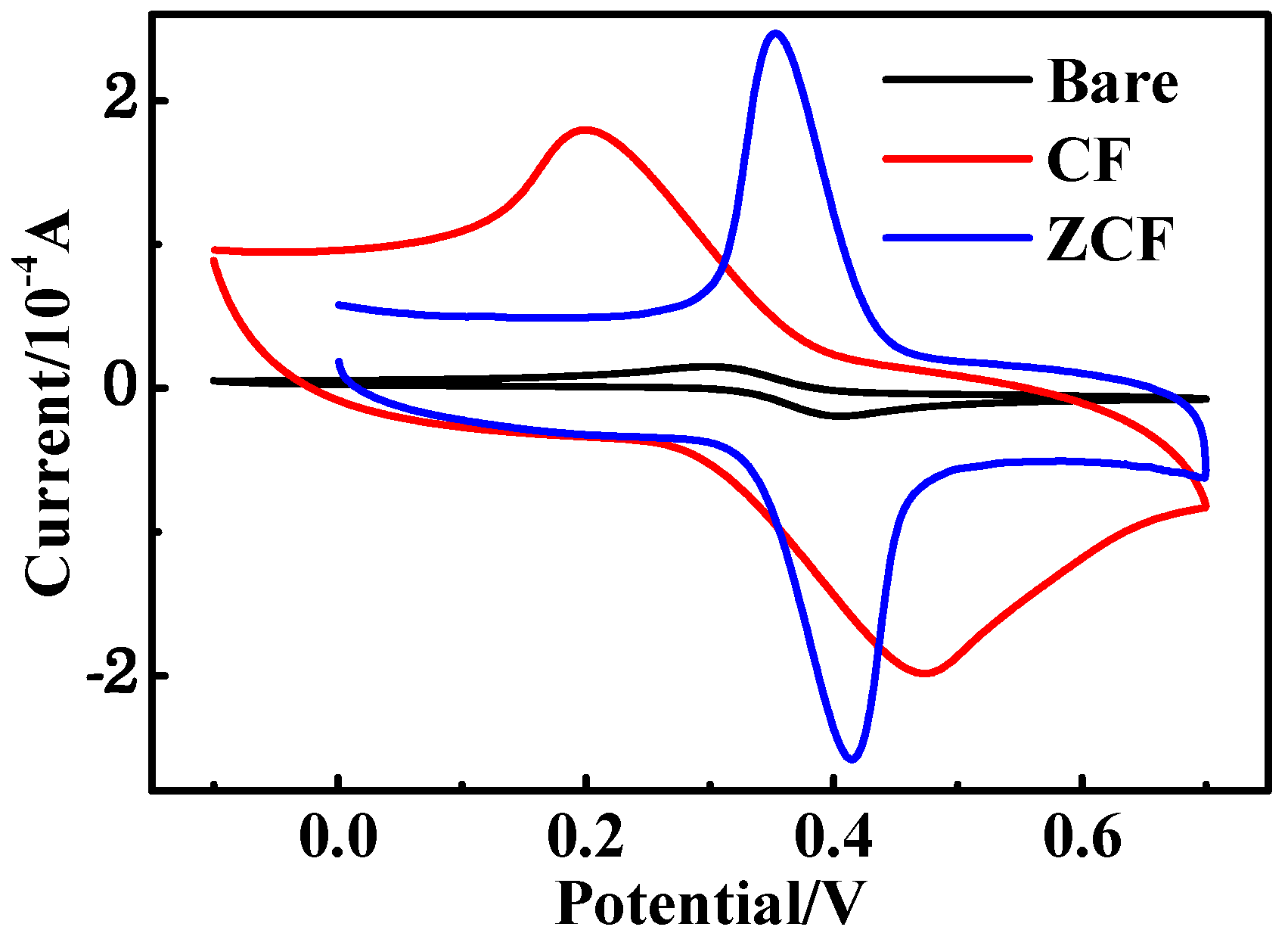
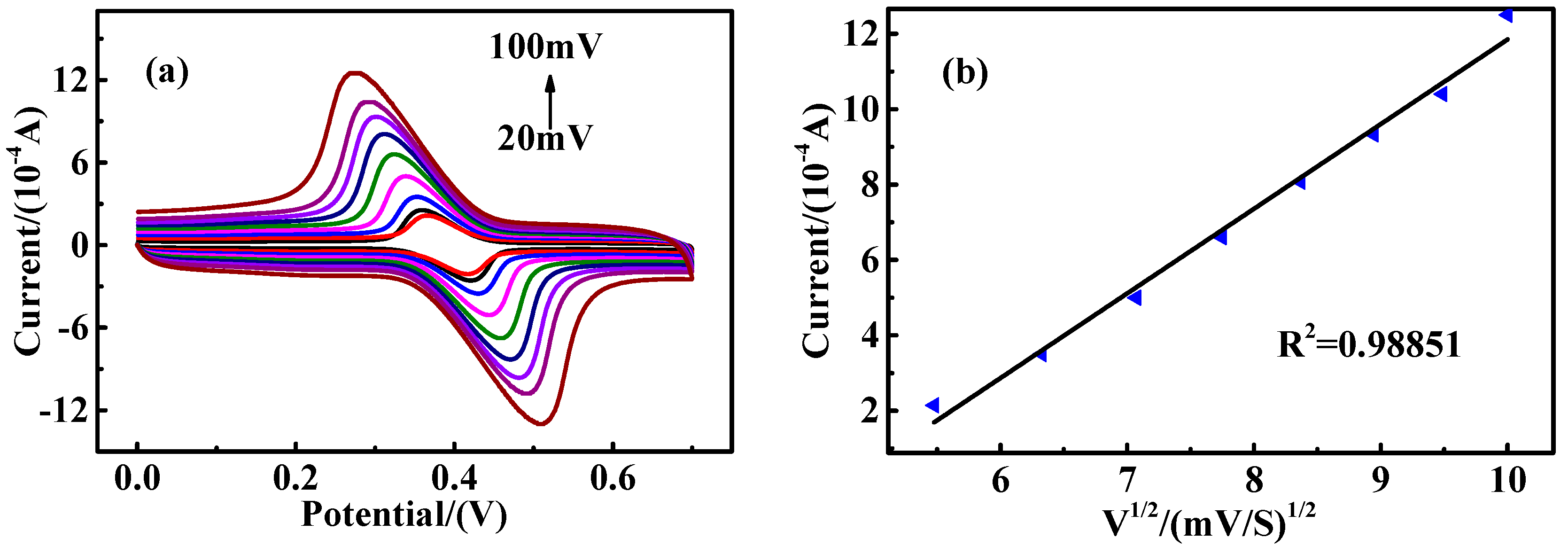
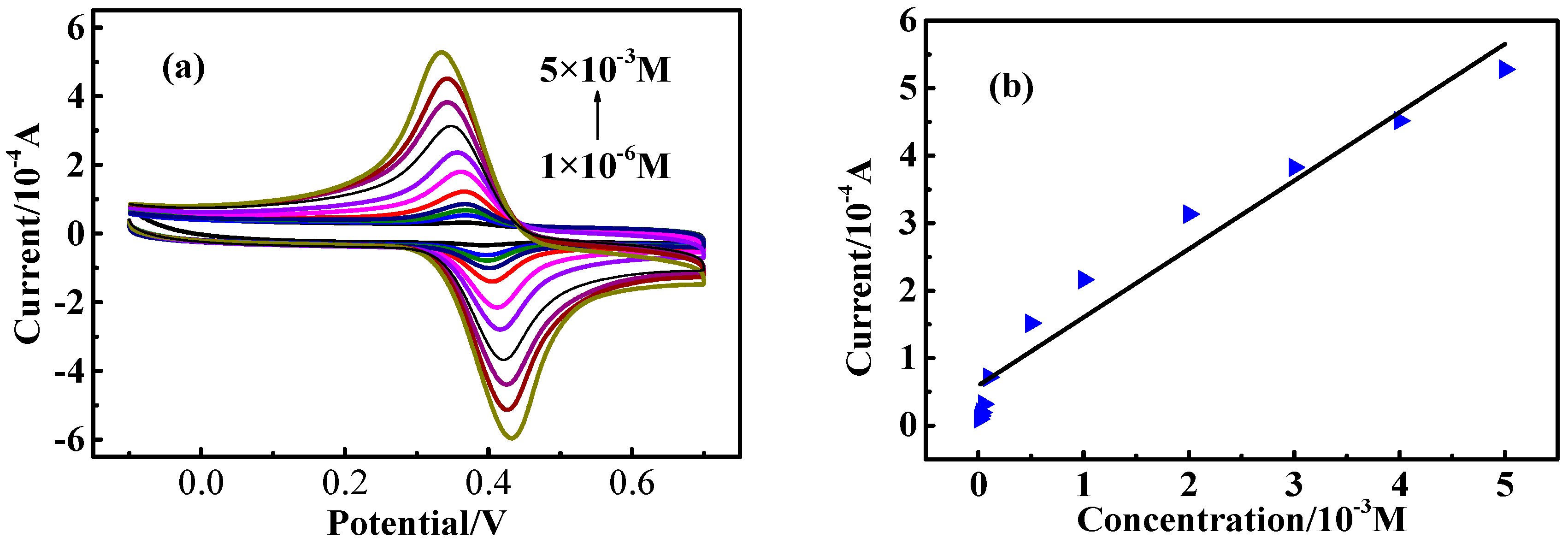
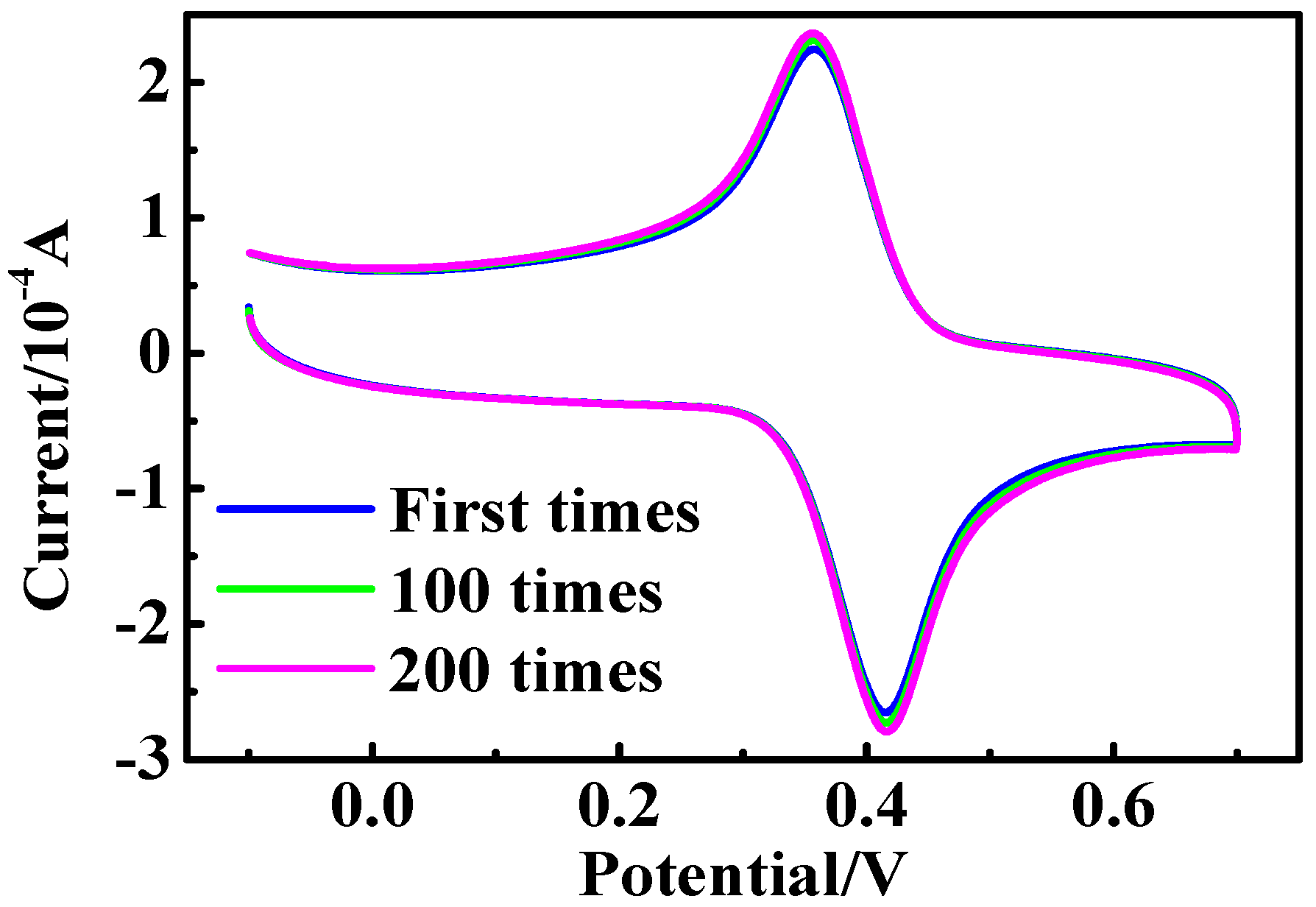
2.3. Electrochemical Measurements
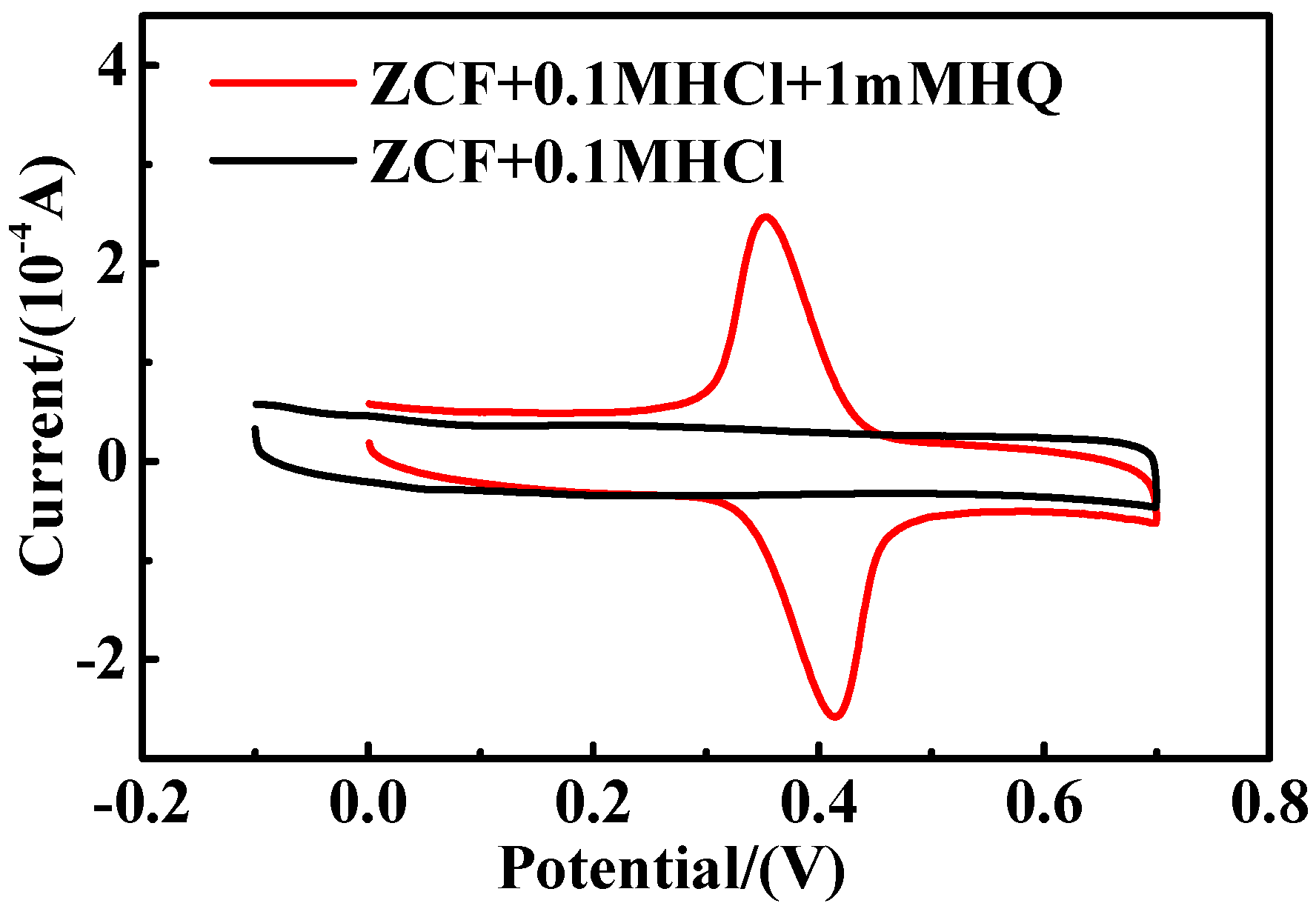
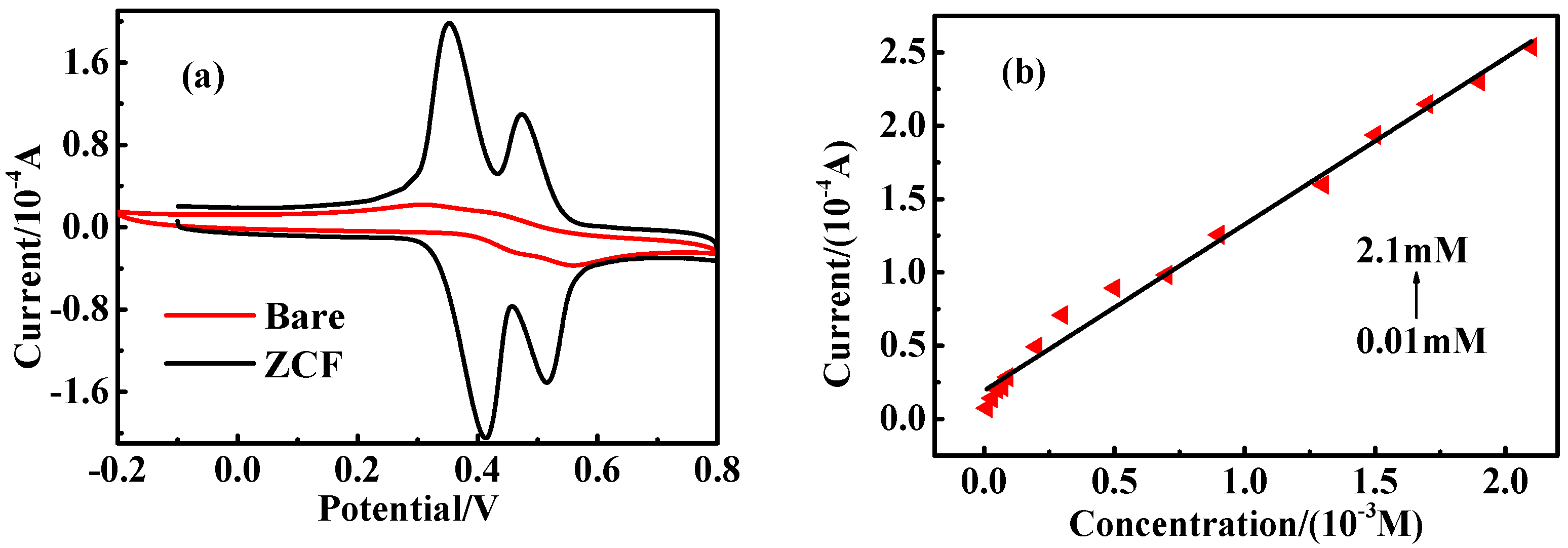
2.4. The Influence of the Coexistence of CC and RC
3. Materials and Methods
3.1. Materials
3.2. Preparation of Zinc Oxide-Loaded Carbon Fibers
3.3. Preparation of ZCF- and CF-Modified Electrodes
3.4. Characterization
3.5. Electrochemical Measurements
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kan, X.; Zhao, Q.; Zhang, Z.; Wang, Z.; Zhu, J.J. Molecularly imprinted polymers microsphere prepared by precipitation polymerization for hydroquinone recognition. Talanta 2008, 75, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.L.; Ong, H.Y.; Shi, C.Y.; Ong, C.N. Simultaneous determination of hydroquinone, catechol and phenol in urine using high-performance liquid chromatography with fluorimetric detection. J. Chromatogr. B 1993, 619, 259–266. [Google Scholar] [CrossRef]
- Wang, Z.; Tang, Y.; Hu, H.; Xing, L.; Zhang, G.; Gao, R. Chemiluminescence determination of hydroquinone with flow-injection analysis of luminol-hydrogen peroxide system catalyzed by Jacobsen’s catalyst. J. Lumin. 2014, 145, 818–823. [Google Scholar] [CrossRef]
- Oishi, M.; Matsuda, T.; Nojiri, S.; Saito, K. Simultaneous determination of five antioxidants in food by HPLC with fluorescence detection. J. Food Hyg. Soc. Jpn. 2002, 43, 104–109. [Google Scholar] [CrossRef]
- Wei, C.; Huang, Q.; Hu, S.; Zhang, H.; Zhang, W.; Wang, Z. Simultaneous electrochemical determination of hydroquinone, catechol and resorcinol at Nafion/multi-walled carbon nanotubes/carbon dots/multi-walled carbon nanotubes modified glassy carbon electrode. Electrochim. Acta 2014, 149, 237–244. [Google Scholar] [CrossRef]
- Hathoot, A.A. Studies on the electrocatalytic oxidation of hydroquinone at poly 1,8-diaminonaphtalene derivatives modified electrodes. Int. J. Electrochem. Sci. 2012, 7, 251–257. [Google Scholar]
- Guo, H.L.; Peng, S.; Xu, J.H.; Zhao, Y.Q.; Kang, X. Highly stable pyridinic nitrogen doped graphene modified electrode in simultaneous determination of hydroquinone and catechol. Sens. Actuators B 2014, 193, 623–629. [Google Scholar] [CrossRef]
- Wang, X.; Wu, M.; Li, H.; Wang, Q.; He, P.; Fang, Y. Simultaneous electrochemical determination of hydroquinone and catechol based on three-dimensional graphene/MWCNTs/BMIMPF6 nanocomposite modified electrode. Sens. Actuators B 2014, 192, 452–458. [Google Scholar] [CrossRef]
- Qi, H.; Zhang, C. Simultaneous determination of hydroquinone and catechol at a glassy carbon electrode modified with multiwall carbon nanotubes. Electroanalysis 2005, 17, 832–838. [Google Scholar] [CrossRef]
- Liu, Y.; Liao, H.; Zhou, Y.; Du, Y.; Wei, C.; Zhao, J. Fe2O3/Nanoparticle/SWCNT composite electrode for sensitive electrocatalytic oxidation of hydroquinone. Electrochim. Acta 2015, 180, 1059–1067. [Google Scholar] [CrossRef]
- Alshahrani, L.A.; Li, X.; Luo, H.; Yang, L.; Wang, M.; Yan, S. The simultaneous electrochemical determination of catechol and hydroquinone with [Cu(Sal-β-Ala)(3,5-DMPz)2]/SWCNTs/GCE. Sensors 2014, 14, 22274–22284. [Google Scholar] [CrossRef] [PubMed]
- Banks, C.E.; Compton, R.G. Exploring the electrocatalytic sites of carbon nanotubes for NADH determination: An edge plane pyrolytic graphite electrode study. Analyst 2005, 130, 1232–1239. [Google Scholar] [CrossRef] [PubMed]
- Weeks, M.L.; Rahman, T.; Frymier, P.D.; Islam, S.K.; Mcknight, T.E. A reagentless enzymatic amperometric biosensor using vertically aligned carbon nanofibers (VACNF). Sens. Actuators B 2008, 133, 53–59. [Google Scholar] [CrossRef]
- Werner, P.; Verdejo, R.; Wöllecke, F.; Altstädt, V.; Sandler, J.; Shaffer, M. Carbon nanofibers allow foaming of semicrystalline poly(ether ether ketone). Adv. Mater. 2005, 17, 2864–2869. [Google Scholar] [CrossRef]
- Guo, Q.; Huang, J.; Chen, P.; Liu, Y.; Hou, H.; You, T. Simultaneous determination of catechol and hydroquinone using electrospun carbon nanofibers modified electrode. Sens. Actuators B 2012, 163, 179–185. [Google Scholar] [CrossRef]
- Liu, L.; Ma, Z.; Zhu, X.; Zeng, R.; Tie, S.; Nan, J. Electrochemical behavior and simultaneous determination of catechol, resorcinol, and hydroquinone using thermally reduced carbon nano-fragment modified glassy carbon electrode. Anal. Methods 2016, 8, 605–613. [Google Scholar] [CrossRef]
- Banks, C.E.; Compton, R.G. New electrodes for old: From carbon nanotubes to edge plane pyrolytic graphite. Analyst 2006, 131, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhang, X.; Ju, H. Determination of NADH and ethanol based on catalytic activity of soluble carbon nanofiber with low overpotential. Anal. Chem. 2007, 79, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Huo, Z.; Zhou, Y.; Liu, Q.; He, X.; Liang, Y.; Xu, M. Sensitive simultaneous determination of catechol and hydroquinone using a gold electrode modified with carbon nanofibers and gold nanoparticles. Microchim. Acta 2011, 173, 119–125. [Google Scholar] [CrossRef]
- Li, M.; Ni, F.; Wang, Y.; Xu, S.; Zhang, D.; Chen, S. Sensitive and facile determination of catechol and hydroquinone simultaneously under coexistence of resorcinol with a Zn/Al layered double hydroxide film modified glassy carbon electrode. Electroanalysis 2009, 21, 1521–1526. [Google Scholar] [CrossRef]
- Bai, P.; Fan, G.; Li, F. Novel Zn–Al layered double hydroxide/carbon nanotube nanocomposite for electrochemical determination of catechol and hydroquinone. Mater. Lett. 2011, 65, 2330–2332. [Google Scholar] [CrossRef]
- Fan, L.F.; Wu, X.Q.; Guo, M.D.; Gao, Y.T. Cobalt hydroxide film deposited on glassy carbon electrode for electrocatalytic oxidation of hydroquinone. Electrochim. Acta 2007, 52, 3654–3659. [Google Scholar] [CrossRef]
- Huang, J.; Liu, Y.; Hou, H.; You, T. Simultaneous electrochemical determination of dopamine, uric acid and ascorbic acid using palladium nanoparticle-loaded carbon nanofibers modified electrode. Biosens. Bioelectron. 2008, 24, 632–637. [Google Scholar] [CrossRef] [PubMed]
- Gan, T.; Sun, J.; Huang, K. A graphene oxide-mesoporous MnO2, nanocomposite modified glassy carbon electrode as a novel and efficient voltammetric sensor for simultaneous determination of hydroquinone and catechol. Sens. Actuators B 2013, 177, 412–418. [Google Scholar] [CrossRef]
- Meng, Z.; Zhang, H.; Zheng, J. An electrochemical sensor based on titanium oxide–carbon nanotubes nanocomposite for simultaneous determination of hydroquinone and catechol. Res. Chem. Intermed. 2015, 41, 3135–3146. [Google Scholar] [CrossRef]
- Huang, C.; Chen, S.; Reneker, D.H.; Lai, C.; Hou, H. High-strength mats from electrospun poly (p-phenylene biphenyltetracarboximide) nanofibers. Adv. Mater. 2006, 18, 668–671. [Google Scholar] [CrossRef]
- Hou, H.; Reneker, D.H. Carbon nanotubes on carbon nanofibers: A novel structure based on electrospun polymer nanofibers. Adv. Mater. 2004, 16, 69–73. [Google Scholar] [CrossRef]
- Britto, P.J.; Santhanam, K.S.V.; Ajayan, P.M. Carbon nanotube electrode for oxidation of dopamine. Bioelectrochem. Bioenerg. 1996, 41, 121–125. [Google Scholar] [CrossRef]
- Shigemitsu, T.; Nagata, T.; Matsumoto, G.; Tsukahara, S. Electrical properties of the carbon fibre electrode and its application. Med. Biol. Eng. Comput. 1980, 18, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Xue, K.H.; Xu, W.; Yin, S.Y. Electrochemical determination of dihydroxybenzene isomers at the carbon-atom-wire-modified electrode. J. Electrochem. Soc. 2007, 154, 147–151. [Google Scholar] [CrossRef]
- Li, S.J.; Qian, C.; Wang, K.; Hua, B.Y.; Wang, F.B.; Sheng, Z.H. Application of thermally reduced graphene oxide modified electrode in simultaneous determination of dihydroxybenzene isomers. Sens. Actuators B 2012, 174, 441–448. [Google Scholar] [CrossRef]
- Liu, Y.T.; Lin, L.W.; Chen, C.Y.; Wang, C.P.; Liu, H.P.; Houng, J.Y. Polymorphism of angiotensin I-converting enzyme gene is related to oral cancer and lymph node metastasis in male betel quid chewers. Oral Oncol. 2012, 48, 1257–1262. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.P.; Liu, W.L.; Wu, Q.S.; Wang, X.G. Direct simultaneous determination of dihydroxybenzene isomers at C-nanotube-modified electrodes by derivative voltammetry. J. Electroanal. Chem. 2005, 575, 275–280. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, J.; Hou, H.; You, T. Simultaneous determination of dopamine, ascorbic acid and uric acid with electrospun carbon nanofibers modified electrode. Electrochem. Commun. 2008, 10, 1431–1434. [Google Scholar] [CrossRef]


| Sample | ZCF | CF |
|---|---|---|
| SBET (m2/g) | 486.02 | 554.90 |
| Vt (cm3/g) | 0.222 | 0.363 |
| Modified Electrode | Determination Limit | Linear Range | Reference |
|---|---|---|---|
| Carbon atom wires | 6 × 10−7 M | 10~10,000 μM | [31] |
| Nafion/multi-walled carbon nanotubes/carbon dots/multi-walled carbon nanotubes | 0.7 × 10−7 M | 1~200 μM | [5] |
| Pyridinic nitrogen doped graphene | 3.8 × 10−7 M | 5~200 μM | [7] |
| Cobalt hydroxide film | 5 × 10−7 M | 5~125 μM | [22] |
| Thermally reduced carbon nano-fragment | 4 × 10−7 M | 10~120 μM | [32] |
| Zinc nanoparticles-loaded carbon fiber | 2.5 × 10−7 M | 1~300 μM | This work |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Y.; Song, M.; Yu, L.; Tang, X. Preparation of ZnO-Loaded Lignin-Based Carbon Fiber for the Electrocatalytic Oxidation of Hydroquinone. Catalysts 2017, 7, 180. https://doi.org/10.3390/catal7060180
Wei Y, Song M, Yu L, Tang X. Preparation of ZnO-Loaded Lignin-Based Carbon Fiber for the Electrocatalytic Oxidation of Hydroquinone. Catalysts. 2017; 7(6):180. https://doi.org/10.3390/catal7060180
Chicago/Turabian StyleWei, Yuexing, Min Song, Lei Yu, and Xinhong Tang. 2017. "Preparation of ZnO-Loaded Lignin-Based Carbon Fiber for the Electrocatalytic Oxidation of Hydroquinone" Catalysts 7, no. 6: 180. https://doi.org/10.3390/catal7060180






