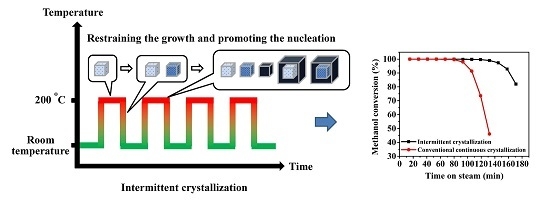
Synthesis of SAPO-34 Molecular Sieves via Novel Intermittent Hydrothermal Treatment and Its Effect on the Crystallization and Product Properties
Abstract
:1. Introduction
2. Results and Discussion
2.1. Influence of Total Crystallization Time on the Yields, Purity and Average Particle Size
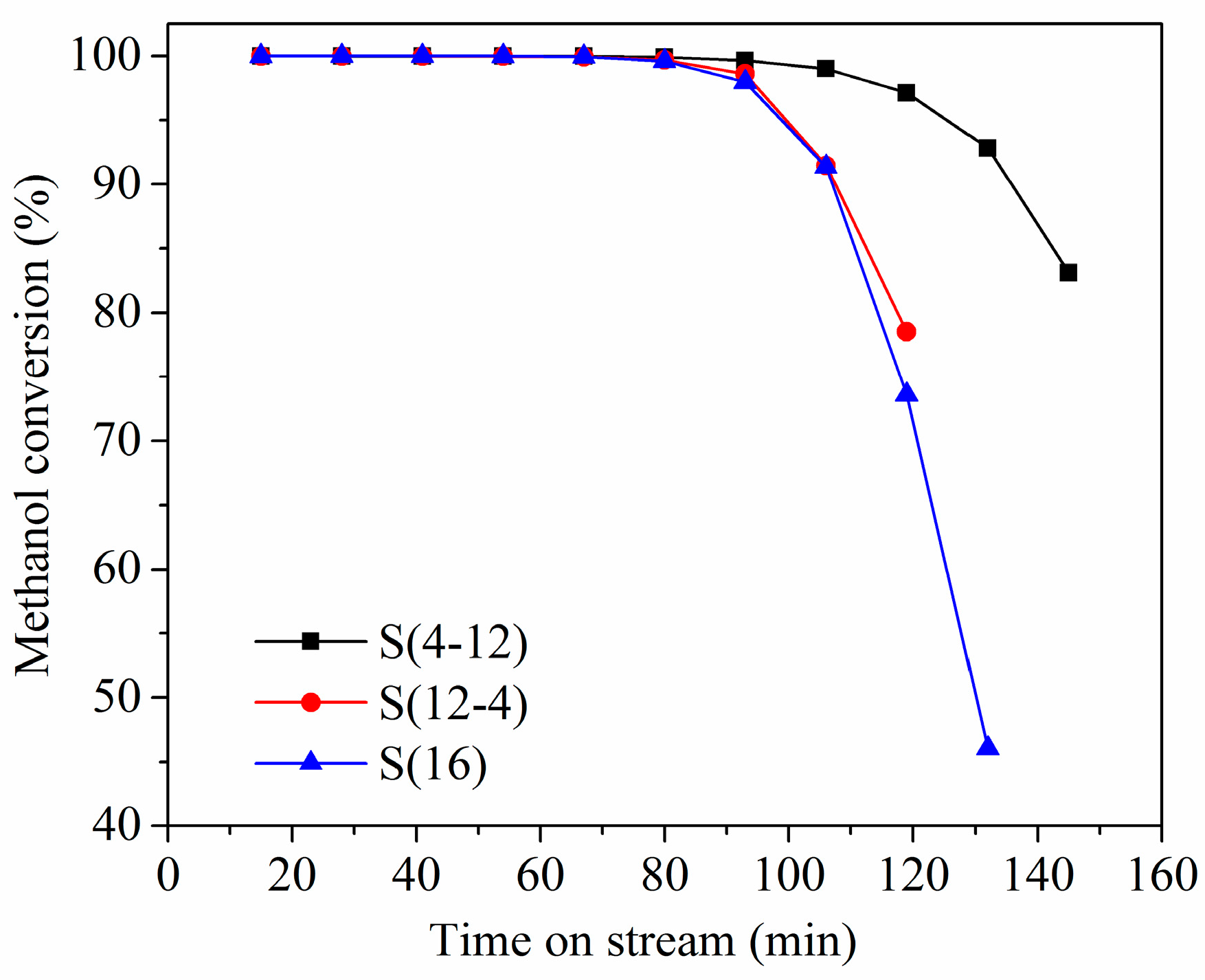
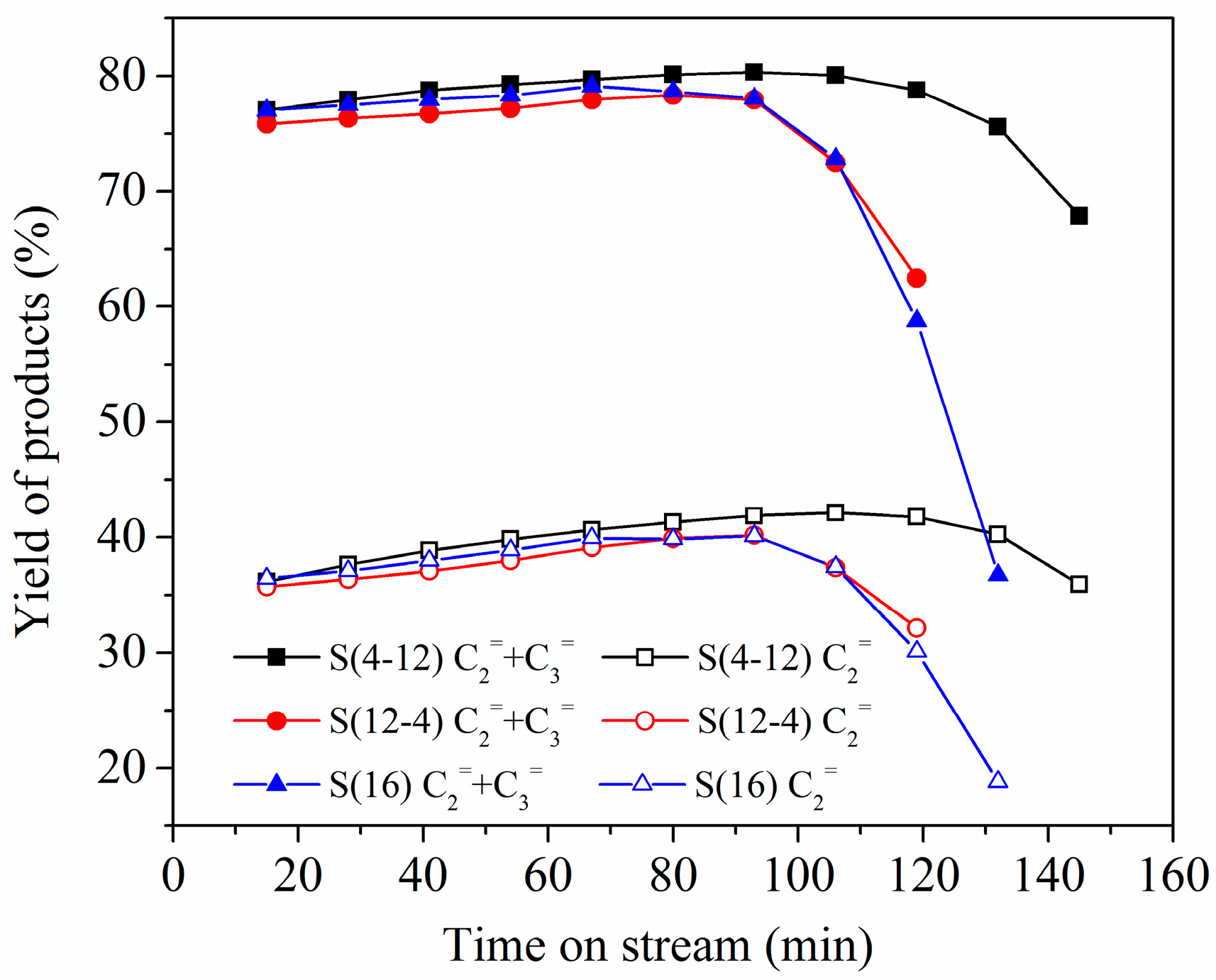
2.2. Two-Stage Crystallization
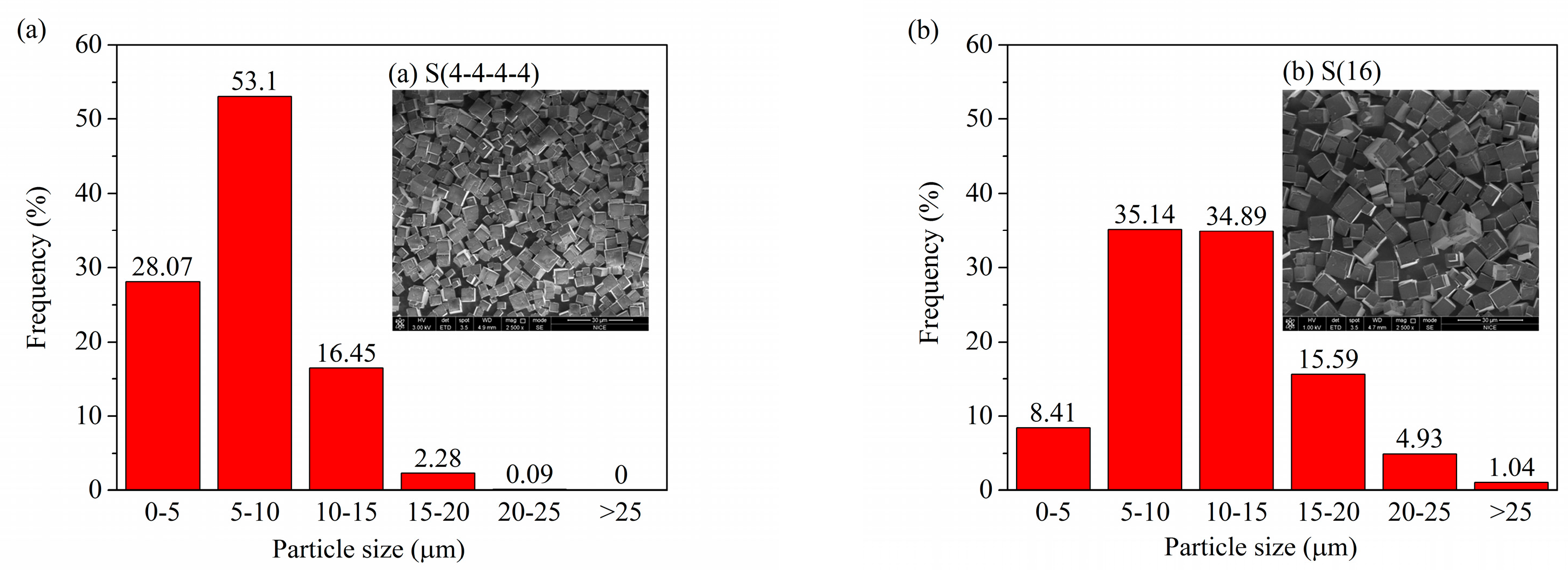
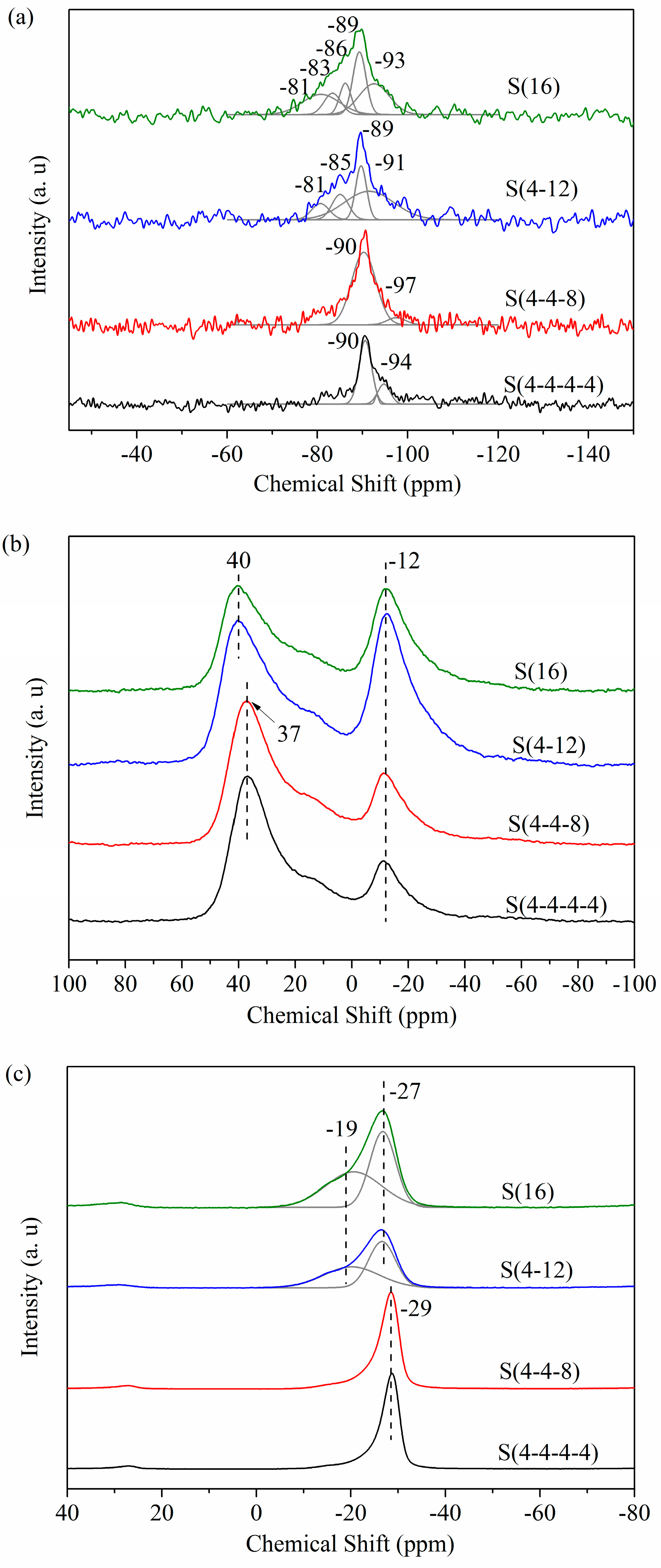
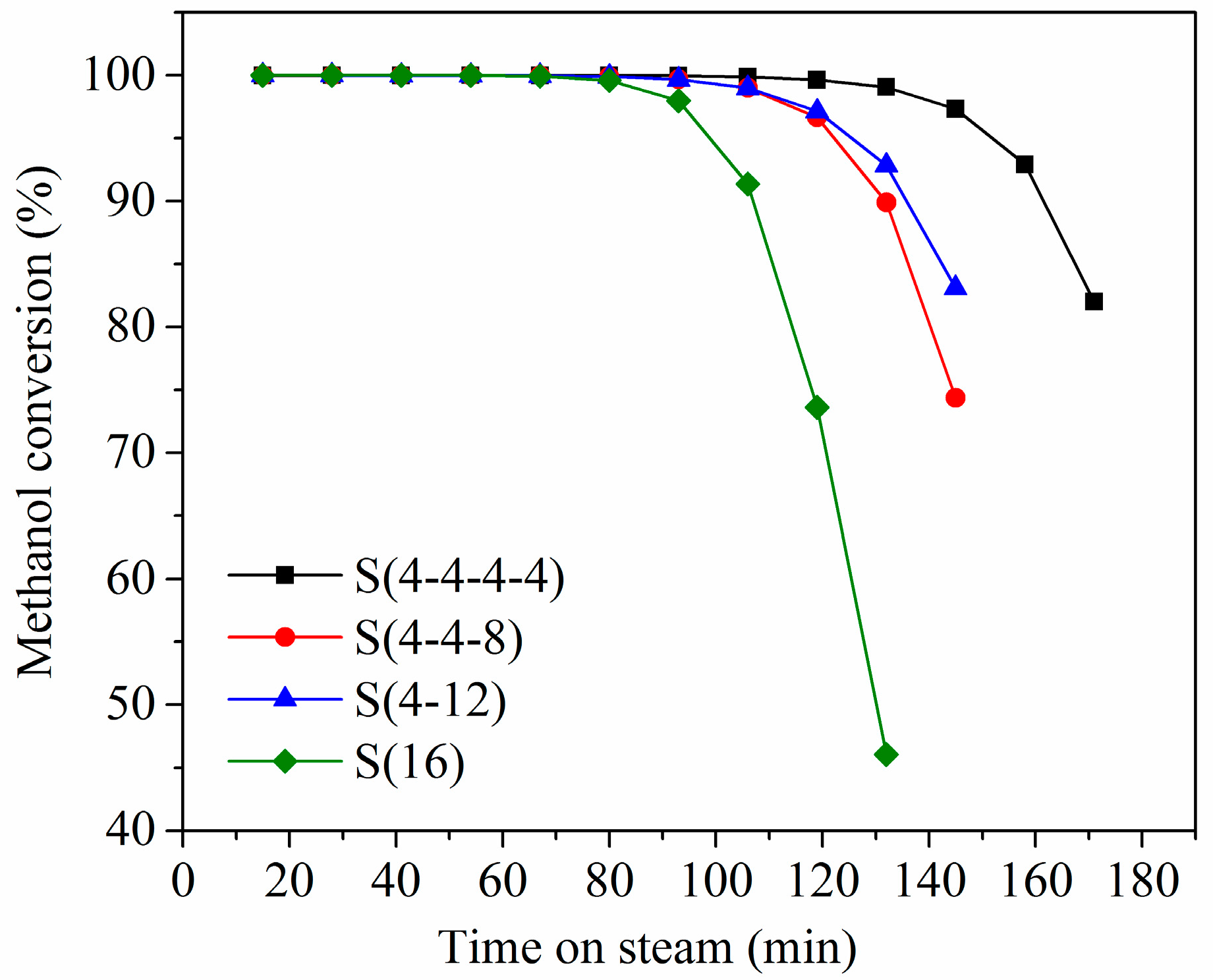
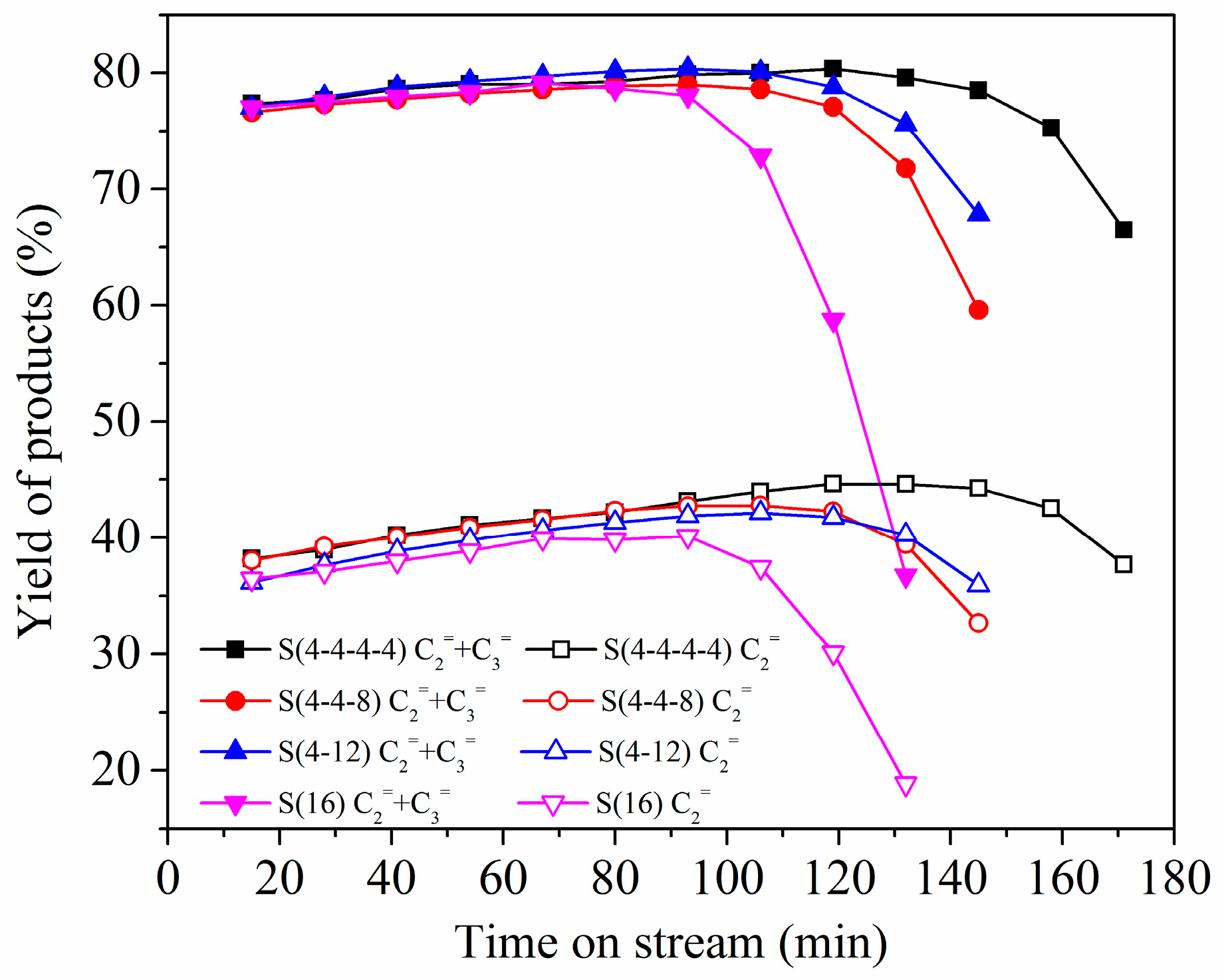
2.3. Multi-Stage Crystallization
3. Materials and Methods
3.1. Synthesis of SAPO-34 Molecular Sieves
3.2. Characterization
3.3. Catalytic Activity Evaluation
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tian, P.; Wei, Y.; Ye, M.; Liu, Z. Methanol to Olefins (MTO): From Fundamentals to Commercialization. ACS Catal. 2015, 5, 1922–1938. [Google Scholar] [CrossRef]
- Wang, P.; Yang, D.; Hu, J.; Xu, J.A.; Lu, G. Synthesis of SAPO-34 with small and tunable crystallite size by two-step hydrothermal crystallization and its catalytic performance for MTO reaction. Catal. Today 2013, 212, 62.e1–62.e8. [Google Scholar] [CrossRef]
- Aghaei, E.; Haghighi, M. Effect of crystallization time on properties and catalytic performance of nanostructured SAPO-34 molecular sieve synthesized at high temperatures for conversion of methanol to light olefins. Powder Technol. 2015, 269, 358–370. [Google Scholar] [CrossRef]
- Xing, A.; Wang, L.; Shi, Y. Evolution of coke deposit and its effect on product selectivity for methanol-to-olefin reaction in fluidized bed. Energy Fuels 2014, 28, 3339–3344. [Google Scholar]
- Wang, C.; Yang, M.; Zhang, W.; Su, X.; Xu, S.; Tian, P.; Liu, Z. Organophosphorous surfactant-assistant synthesis of SAPO-34 molecular sieve with special morphology and improved MTO performance. RSC Adv. 2016, 6, 47864–47872. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, N.; Guo, G.; Yu, J. Ultrafast synthesis of nano-sized zeolite SAPO-34 with excellent MTO catalytic performance. Chem. Commun. 2015, 51, 16397–16400. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Tian, P.; Xia, Q.; Liu, Z. An effective route to improve the catalytic performance of SAPO-34 in the methanol-to-olefin reaction. J. Nat. Gas Chem. 2012, 21, 431–434. [Google Scholar] [CrossRef]
- Charghand, M.; Haghighi, M.; Saedy, S.; Aghamohammadi, S. Efficient hydrothermal synthesis of nanostructured SAPO-34 using ultrasound energy: Physicochemical characterization and catalytic performance toward methanol conversion to light olefins. Adv. Powder Technol. 2014, 25, 1728–1736. [Google Scholar] [CrossRef]
- Shen, W.; Li, X.; Wei, Y.; Tian, P.; Deng, F.; Han, X.; Bao, X. A study of the acidity of SAPO-34 by solid-state NMR spectroscopy. Microporous Mesoporous Mater. 2012, 158, 19–25. [Google Scholar] [CrossRef]
- Dahl, I.M.; Mostad, H.; Akporiaye, D.; Wendelbo, R. Structural and chemical influences on the MTO reaction: A comparison of chabazite and SAPO-34 as MTO catalysts. Microporous Mesoporous Mater. 1999, 29, 185–190. [Google Scholar] [CrossRef]
- Nishiyama, N.; Kawaguchi, M.; Hirota, Y.; Van Vu, D.; Egashira, Y.; Ueyama, K. Size control of SAPO-34 crystals and their catalyst lifetime in the methanol-to-olefin reaction. Appl. Catal. A Gen. 2009, 362, 193–199. [Google Scholar] [CrossRef]
- Álvaro-Muñoz, T.; Márquez-Álvarez, C.; Sastre, E. Use of different templates on SAPO-34 synthesis: Effect on the acidity and catalytic activity in the MTO reaction. Catal. Today 2012, 179, 27–34. [Google Scholar] [CrossRef]
- Rimaz, S.; Halladj, R.; Askari, S. Synthesis of hierarchal SAPO-34 nano catalyst with dry gel conversion method in the presence of carbon nanotubes as a hard template. J. Colloid Interface Sci. 2016, 464, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Aghamohammadi, S.; Haghighi, M. Dual-template synthesis of nanostructured CoAPSO-34 used in methanol to olefins: Effect of template combinations on catalytic performance and coke formation. Chem. Eng. J. 2015, 264, 359–375. [Google Scholar] [CrossRef]
- Najafi, N.; Askari, S.; Halladj, R. Hydrothermal synthesis of nanosized SAPO-34 molecular sieves by different combinations of multi templates. Powder Technol. 2014, 254, 324–330. [Google Scholar] [CrossRef]
- Sadeghpour, P.; Haghighi, M. DEA/TEAOH templated synthesis and characterization of nanostructured NiAPSO-34 particles: Effect of single and mixed templates on catalyst properties and performance in the methanol to olefin reaction. Particuology 2015, 19, 69–81. [Google Scholar] [CrossRef]
- Xu, L.; Du, A.; Wei, Y.; Wang, Y.; Yu, Z.; He, Y.; Zhang, X.; Liu, Z. Synthesis of SAPO-34 with only Si(4Al) species: Effect of Si contents on Si incorporation mechanism and Si coordination environment of SAPO-34. Microporous Mesoporous Mater. 2008, 115, 332–337. [Google Scholar] [CrossRef]
- Liu, G.; Tian, P.; Zhang, Y.; Li, J.; Xu, L.; Meng, S.; Liu, Z. Synthesis of SAPO-34 templated by diethylamine: Crystallization process and Si distribution in the crystals. Microporous Mesoporous Mater. 2008, 114, 416–423. [Google Scholar] [CrossRef]
- Wu, X.C.; Anthony, R.G. Effect of feed composition on methanol conversion to light olefins over SAPO-34. Appl. Catal. A Gen. 2001, 218, 241–250. [Google Scholar] [CrossRef]
- Dargahi, M.; Kazemian, H.; Soltanieh, M.; Rohani, S.; Hosseinpour, M. Rapid high-temperature synthesis of SAPO-34 nanoparticles. Particuology 2011, 9, 452–457. [Google Scholar] [CrossRef]
- Askari, S.; Halladj, R.; Askari, R.; Bosari, S.S. Catalytic performance of SAPO-34 catalysts of different crystal sizes in methanol-to-olefins reactions: Effects of synthetic parameters. Prog. React. Kinet. Mech. 2015, 40, 143–153. [Google Scholar]
- Askari, S.; Siahmard, A.B.; Halladj, R.; Alipour, S.M. Different techniques and their effective parameters in nano SAPO-34 synthesis: A review. Powder Technol. 2016, 301, 268–287. [Google Scholar] [CrossRef]
- Valizadeh, B.; Askari, S.; Halladj, R.; Haghmoradi, A. Effect of Synthesis Conditions on Selective Formation of SAPO-5 and SAPO-34. Synth. React. Inorg. Metal-Org. Nano-Metal Chem. 2014, 44, 79–83. [Google Scholar] [CrossRef]
- Xu, R.; Pang, W.; Huo, Q. Chemistry-Zeolite and Porous Materials; Science Press Ltd.: Beijing, China, 2015. (In Chinese) [Google Scholar]
- Niwa, M.; Katada, N. Measurements of acidic property of zeolites by temperature programmed desorption of ammonia. Catal. Surv. Asia 1997, 1, 215–226. [Google Scholar] [CrossRef]
- Prakash, A.M.; Unnikrishnan, S. Synthesis of SAPO-34: High silicon incorporation in the presence of morpholine as template. J. Chem. Soc. Faraday Trans. 1994, 90, 2291–2296. [Google Scholar] [CrossRef]
- Zhang, L.; Bates, J.; Chen, D.; Nie, H.Y.; Huang, Y. Investigations of Formation of Molecular Sieve SAPO-34. J. Phys. Chem. C 2011, 115, 22309–22319. [Google Scholar] [CrossRef]
- Yang, M.; Tian, P.; Wang, C.; Yuan, Y.; Yang, Y.; Xu, S.; He, Y.; Liu, Z. A top-down approach to prepare silicoaluminophosphate molecular sieve nanocrystals with improved catalytic activity. Chem. Commun. 2014, 50, 1845–1847. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Martinez-Triguero, J.; Concepcion, P.; Yu, J.; Corma, A. Methanol to olefins: Activity and stability of nanosized SAPO-34 molecular sieves and control of selectivity by silicon distribution. Phys. Chem. Chem. Phys. 2013, 15, 14670–14680. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Deng, Z.; Zhu, K.; Zhou, X.G. Insights into the growth of small-sized SAPO-34 crystals synthesized by a vapor-phase transport method. Crystengcomm 2015, 17, 3214–3218. [Google Scholar] [CrossRef]
- Fjermestad, T.; Svelle, S.; Swang, O. Mechanism of Si Island Formation in SAPO-34. J. Phys. Chem. C 2015, 119, 2086–2095. [Google Scholar] [CrossRef]
- Dai, W.; Wu, G.; Li, L.; Guan, N.; Hunger, M. Mechanisms of the Deactivation of SAPO-34 Materials with Different Crystal Sizes Applied as MTO Catalysts. ACS Catal. 2013, 3, 588–596. [Google Scholar] [CrossRef]
- Li, Z.; Martínez-Triguero, J.; Yu, J.; Corma, A. Conversion of methanol to olefins: Stabilization of nanosized SAPO-34 by hydrothermal treatment. J. Catal. 2015, 329, 379–388. [Google Scholar] [CrossRef]
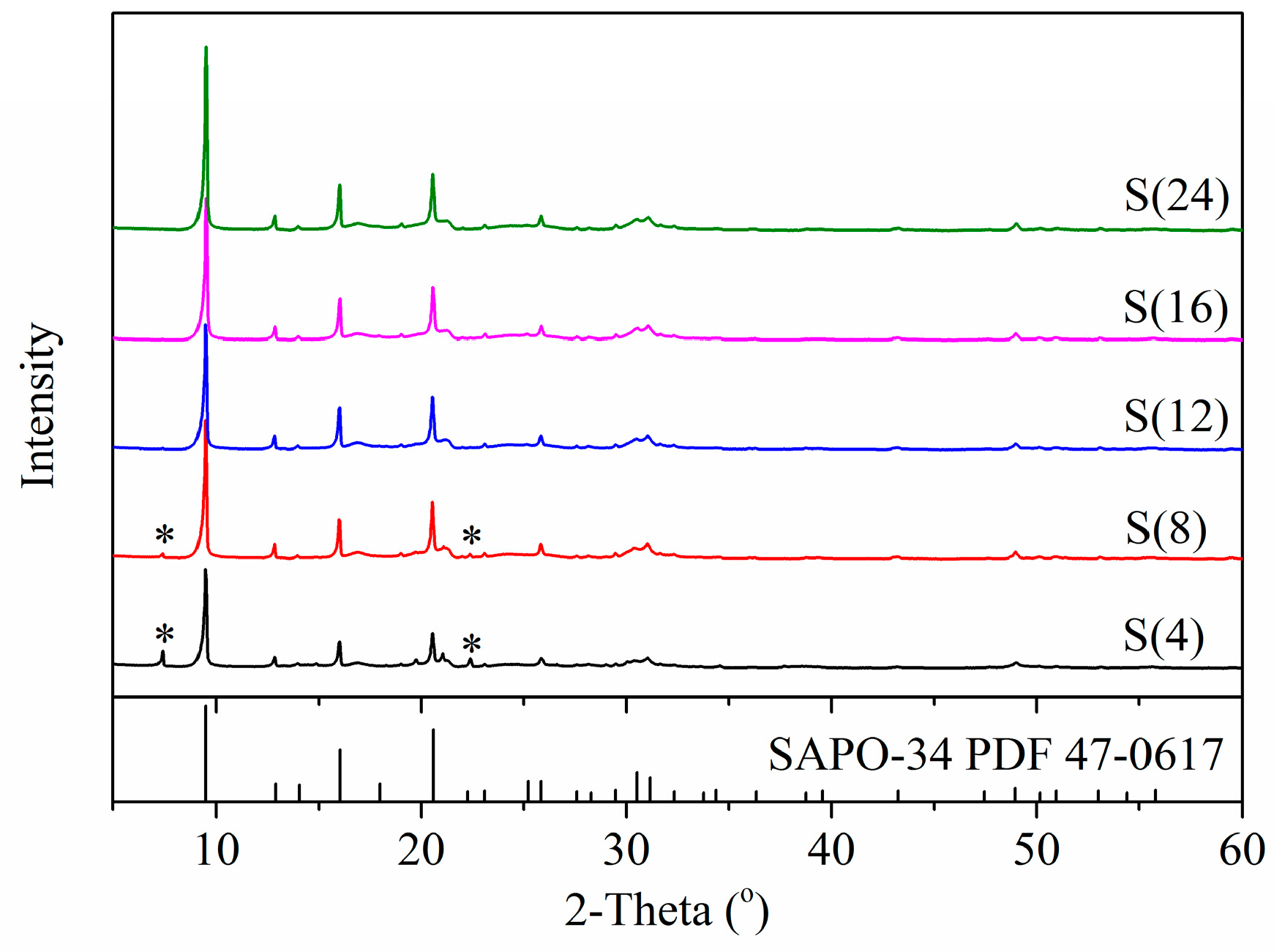

| Sample | Yield (%) | The Relative Content of SAPO-34 in the Crystals (%) | Particle Size (μm) | |
|---|---|---|---|---|
| D(50) | SEM | |||
| S(4) | 0.11 | 81.4 | - | - |
| S(8) | 2.88 | 99.9 | - | - |
| S(12) | 12.50 | 100 | 8.92 | 8.10 |
| S(16) | 23.07 | 100 | 10.70 | 9.38 |
| S(24) | 28.05 | 100 | 12.70 | 11.76 |
| Sample | Yield (%) | Particle Size (μm) | Elemental Composition (mol %) | Total Acidity (mmol NH3/g) | Strong Acid 1 (mmol NH3/g) | Weak Acid 2 (mmol NH3/g) | |
|---|---|---|---|---|---|---|---|
| D(50) | SEM | ||||||
| S(4-12) | 24.04 | 9.70 | 8.38 | Si0.085Al0.474P0.441 | 1.48 | 0.86 | 0.62 |
| S(12-4) | 23.83 | 10.20 | 8.80 | Si0.083Al0.475P0.442 | 1.28 | 0.76 | 0.52 |
| S(16) | 23.07 | 10.70 | 9.38 | Si0.086Al0.472P0.442 | 1.24 | 0.73 | 0.51 |
| Sample | Yield (%) | Particle Size (μm) | Elemental Composition (mol %) | Surface Area (m2/g) | Pore Volume (cm3/g) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| D(50) | SEM | Smicro | Sext | SBET | Vmicro | Vmeso | Vtotal | |||
| S(4-4-4-4) | 22.36 | 6.75 | 5.28 | Si0.081Al0.475P0.444 | 890 | 15 | 905 | 0.32 | 0.01 | 0.33 |
| S(4-4-8) | 22.61 | 7.53 | 6.87 | Si0.085Al0.474P0.441 | 758 | 18 | 777 | 0.28 | 0.01 | 0.29 |
| S(4-12) | 24.04 | 9.70 | 8.38 | Si0.085Al0.474P0.441 | 755 | 6 | 761 | 0.27 | 0.00 | 0.27 |
| S(16) | 23.07 | 10.70 | 9.38 | Si0.086Al0.472P0.442 | 773 | 14 | 787 | 0.28 | 0.01 | 0.29 |
| Sample | Serial Number | Yield (%) | Particle Size (μm) | |
|---|---|---|---|---|
| D(50) | SEM | |||
| S(4-4-4-4) | 1 | 22.36 | 6.75 | 5.28 |
| 2 | 26.99 | 3.53 | 2.30 | |
| 3 | 20.91 | 5.36 | 4.01 | |
| Std. Dev. | 3.18 | 1.62 | 1.50 | |
| S(16) | 1 | 23.07 | 10.70 | 9.38 |
| 2 | 22.99 | 13.20 | 11.96 | |
| 3 | 26.83 | 12.30 | 10.88 | |
| Std. Dev. | 2.19 | 1.27 | 1.30 | |
| Sample | Total Acidity (mmol NH3/g) | Strong Acid 1 (mmol NH3/g) | Weak Acid 2 (mmol NH3/g) |
|---|---|---|---|
| S(4-4-4-4) | 1.75 | 1.03 | 0.72 |
| S(4-4-8) | 1.73 | 0.99 | 0.74 |
| S(4-12) | 1.48 | 0.86 | 0.62 |
| S(16) | 1.24 | 0.73 | 0.51 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Z.; Miao, P.; Zhu, W.; Guo, L.; Li, F.; Xue, Y.; Yin, Q.; Yuan, R.; Xu, L. Synthesis of SAPO-34 Molecular Sieves via Novel Intermittent Hydrothermal Treatment and Its Effect on the Crystallization and Product Properties. Catalysts 2017, 7, 150. https://doi.org/10.3390/catal7050150
Guo Z, Miao P, Zhu W, Guo L, Li F, Xue Y, Yin Q, Yuan R, Xu L. Synthesis of SAPO-34 Molecular Sieves via Novel Intermittent Hydrothermal Treatment and Its Effect on the Crystallization and Product Properties. Catalysts. 2017; 7(5):150. https://doi.org/10.3390/catal7050150
Chicago/Turabian StyleGuo, Zhihui, Ping Miao, Weiping Zhu, Lei Guo, Fei Li, Yunpeng Xue, Qi Yin, Ruixue Yuan, and Lianbin Xu. 2017. "Synthesis of SAPO-34 Molecular Sieves via Novel Intermittent Hydrothermal Treatment and Its Effect on the Crystallization and Product Properties" Catalysts 7, no. 5: 150. https://doi.org/10.3390/catal7050150