Methanol Steam Reforming: Na Doping of Pt/YSZ Provides Fine Tuning of Selectivity
Abstract
:1. Introduction
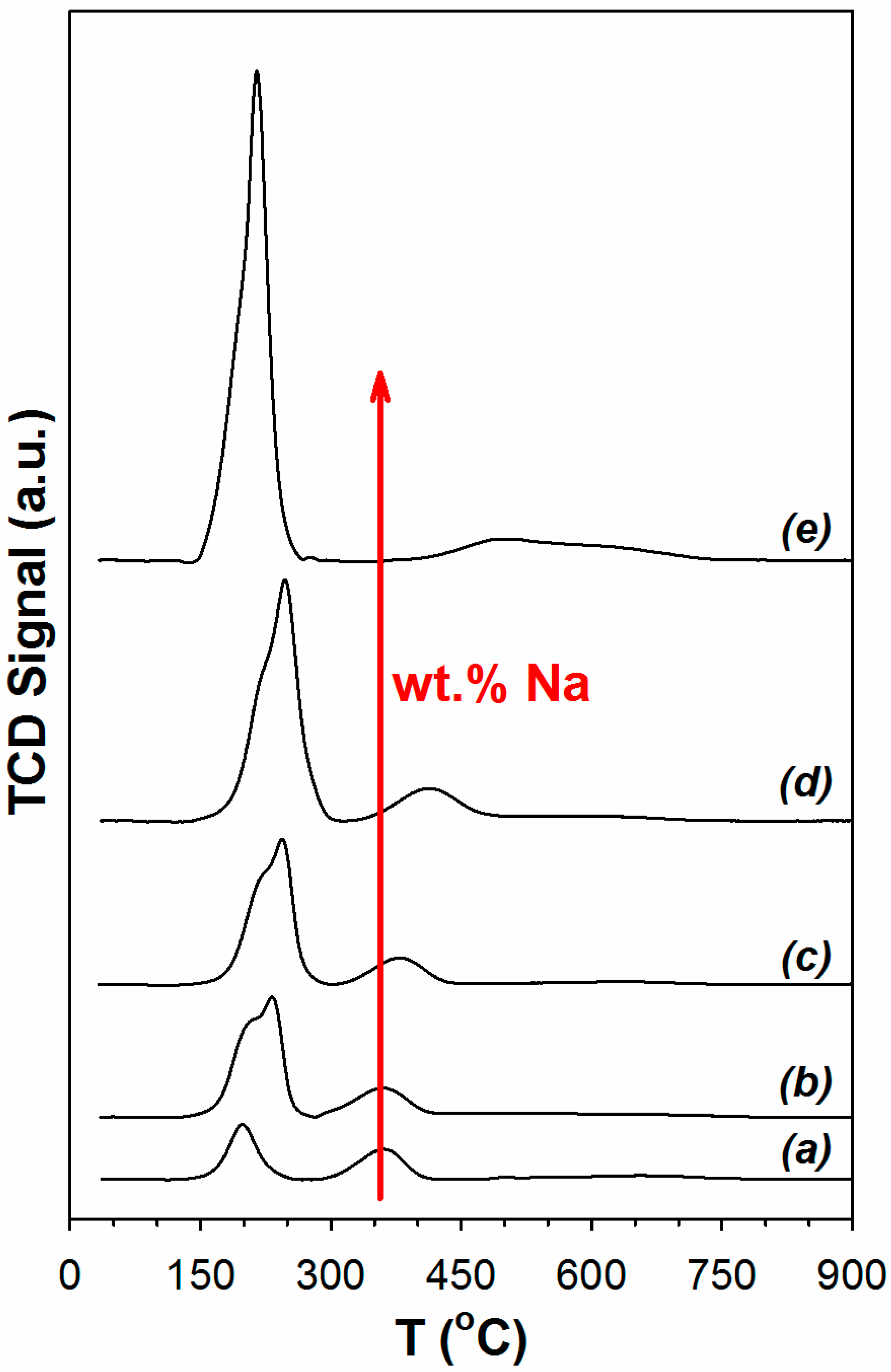
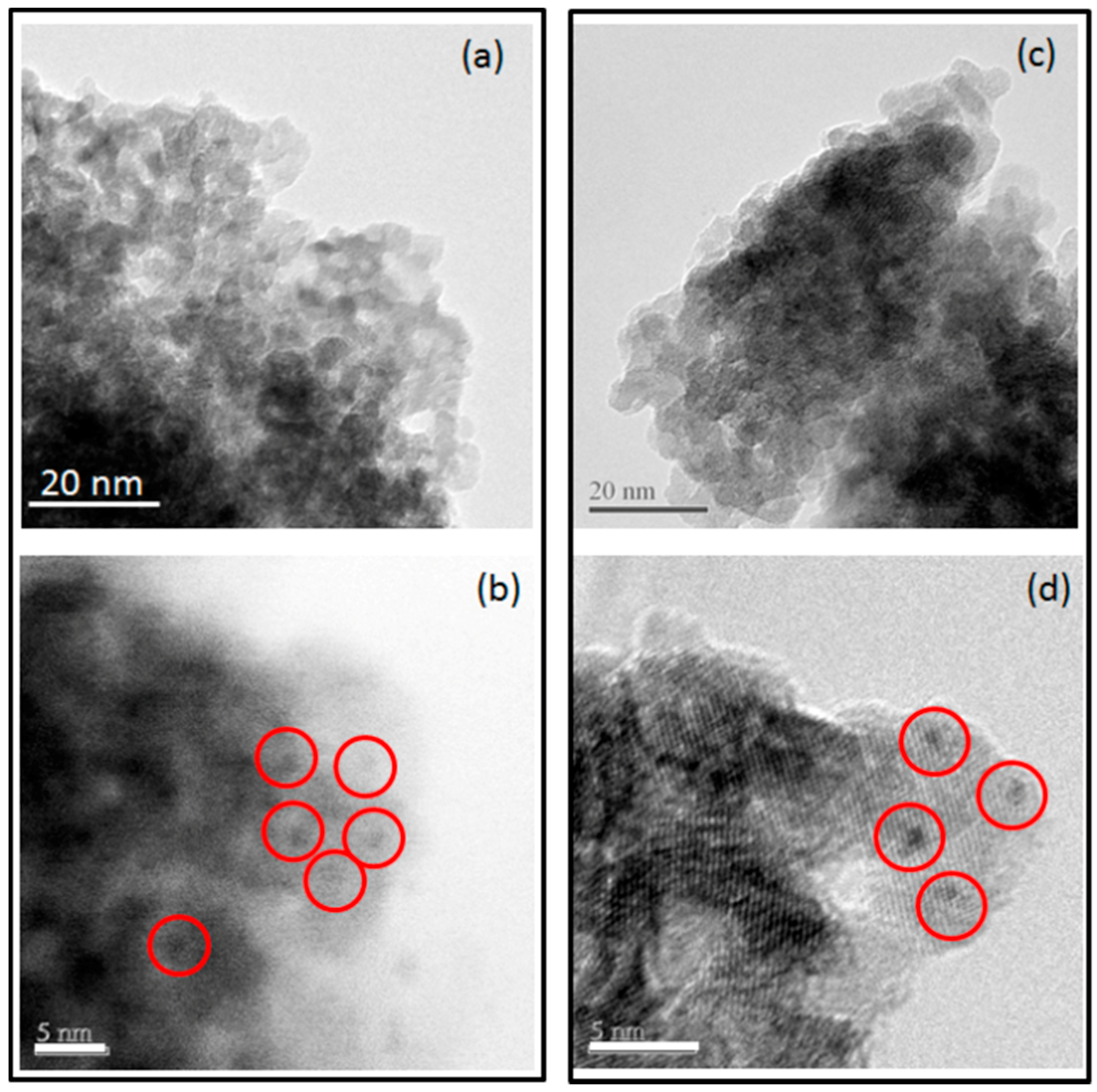
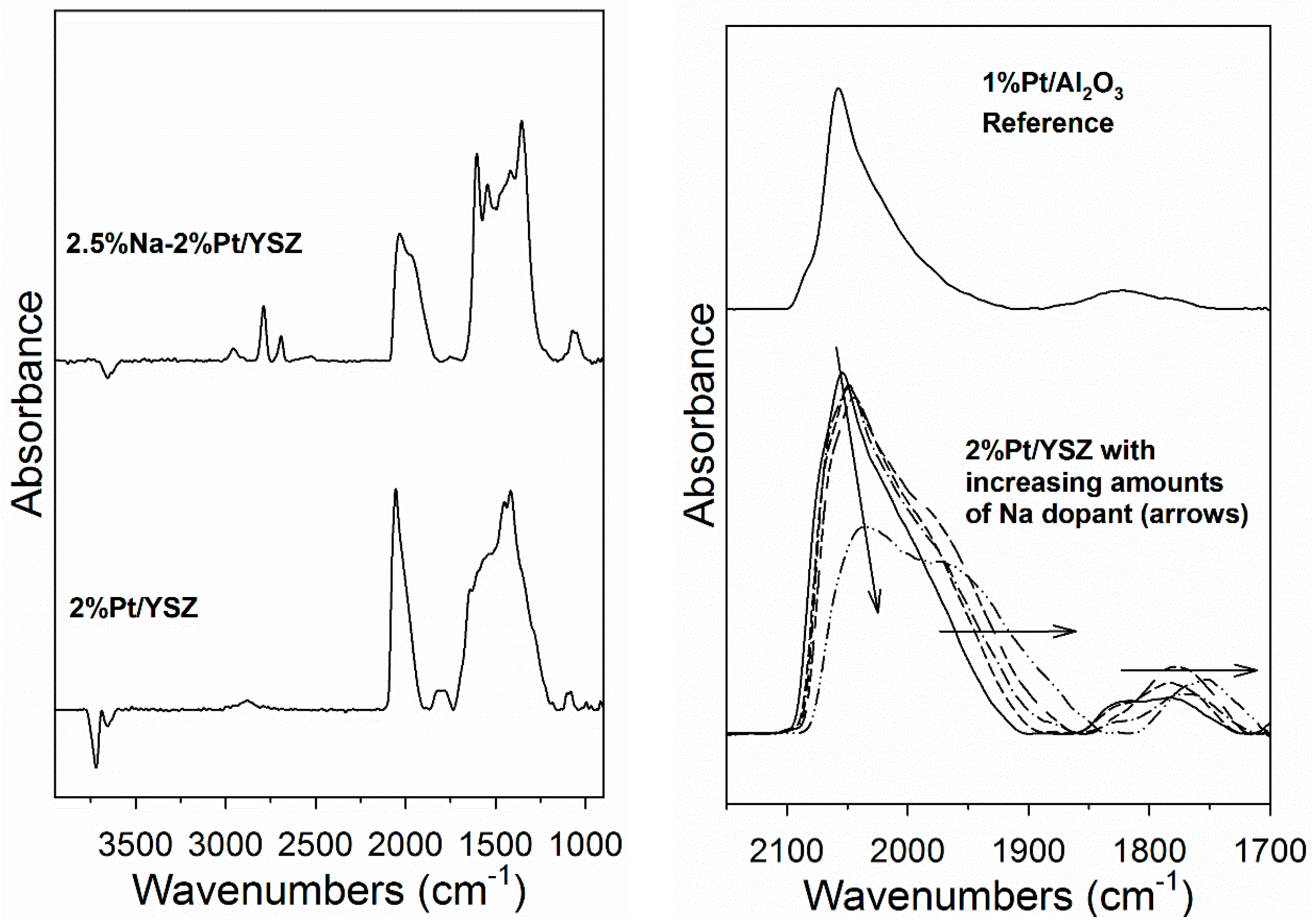
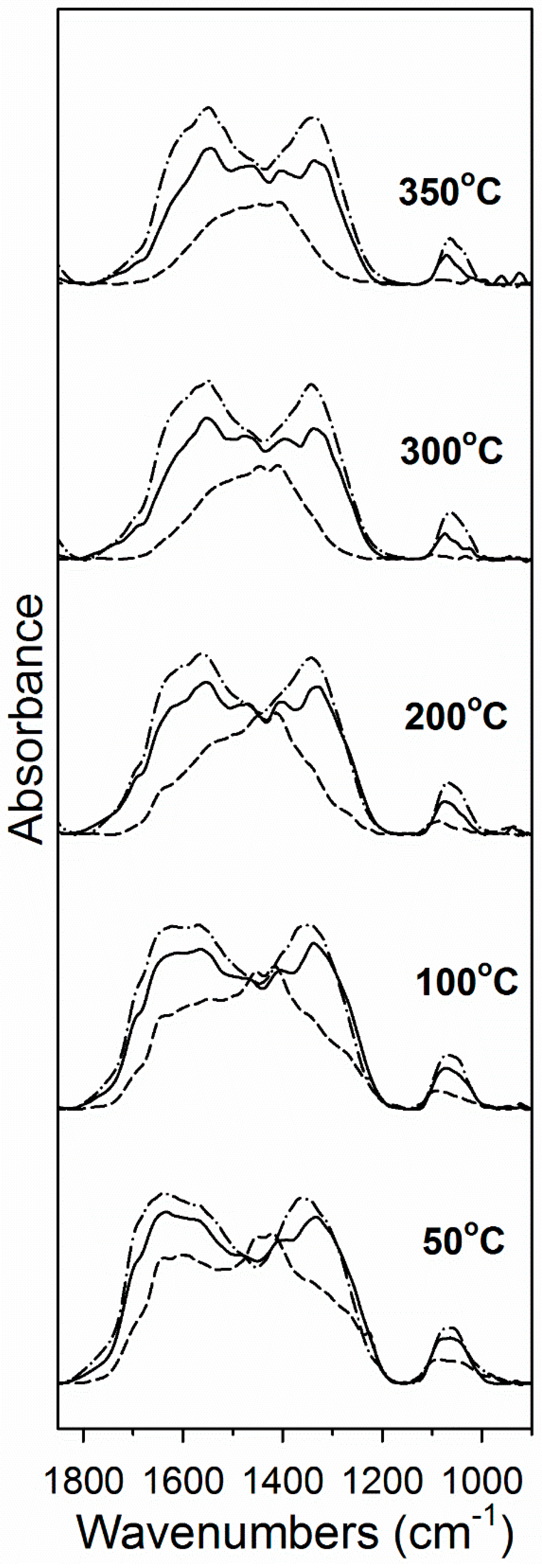
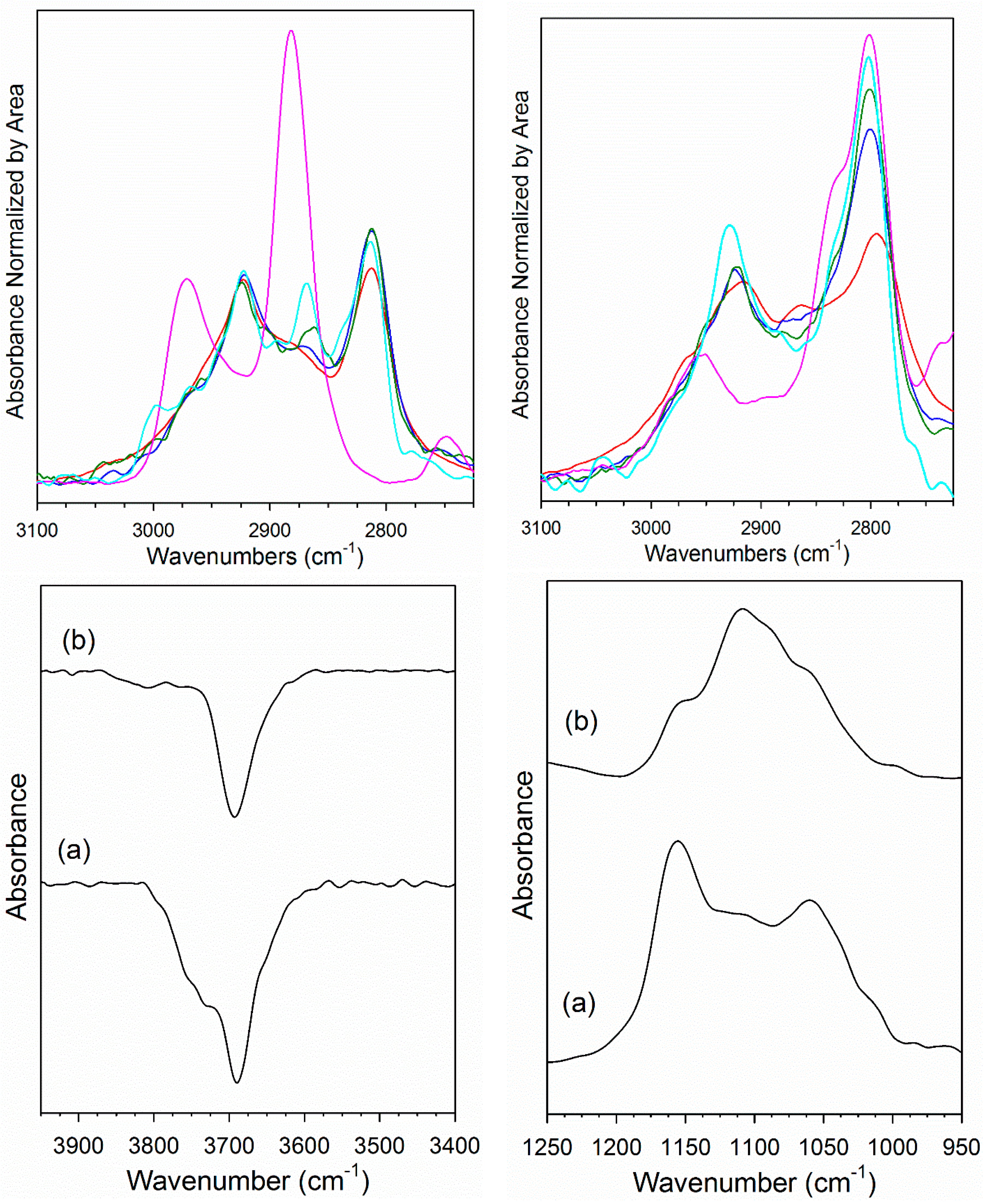
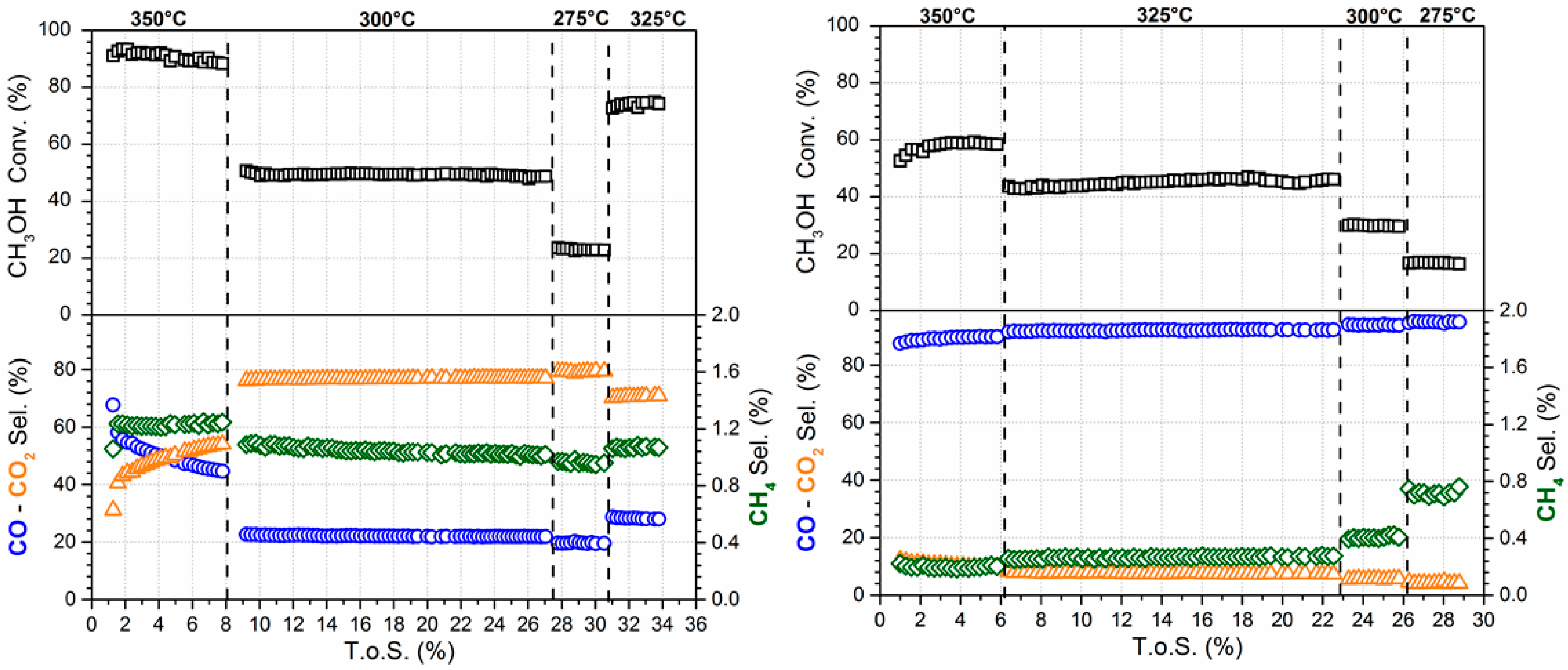
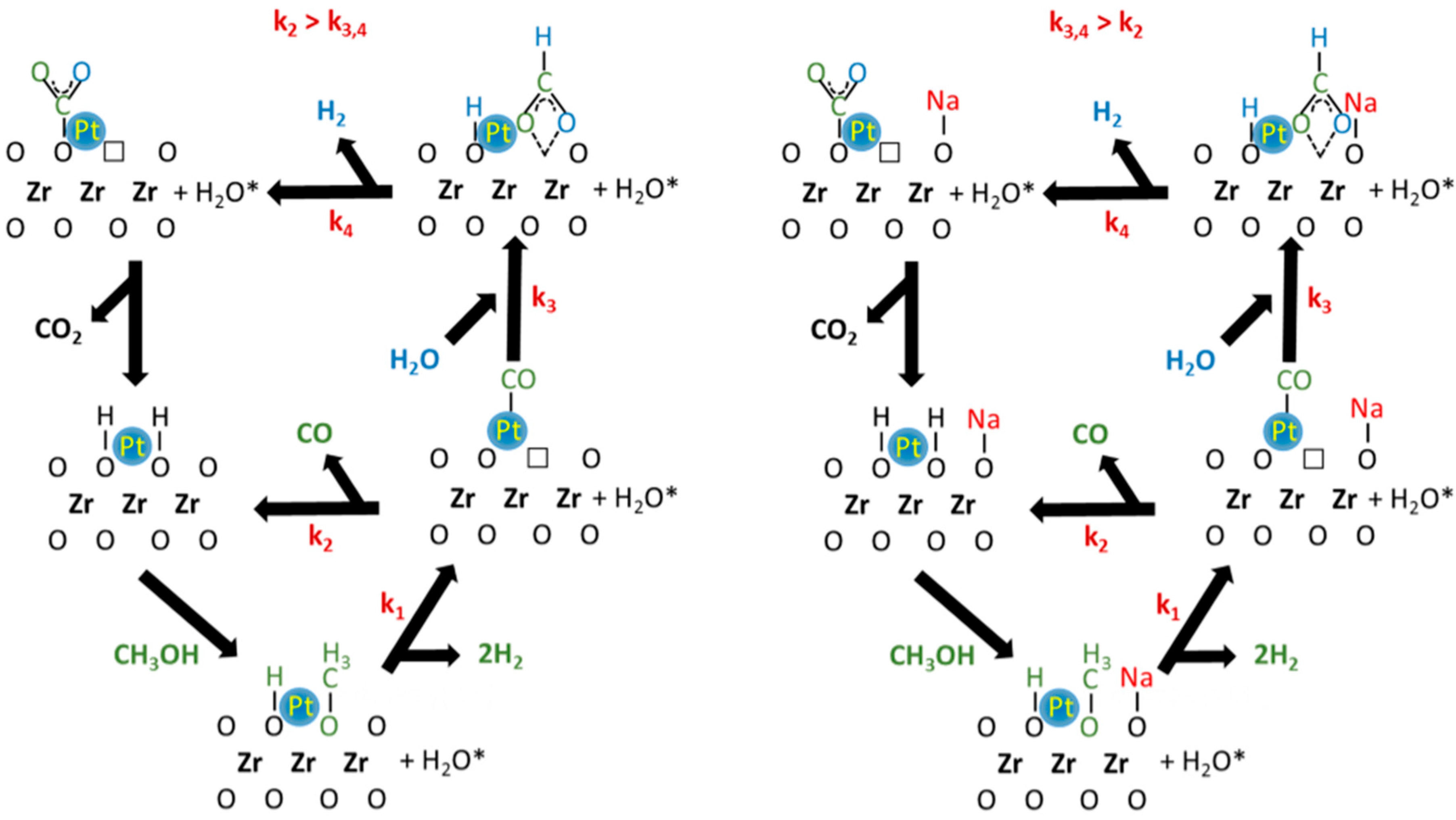
2. Results and Discussion
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Catalyst Characterization
3.2.1. BET Analysis
3.2.2. Temperature Programmed Reduction
3.2.3. Scanning Transmission Electron Microscopy (STEM)
3.2.4. Diffuse Reflectance Fourier Transform Infrared Spectroscopy
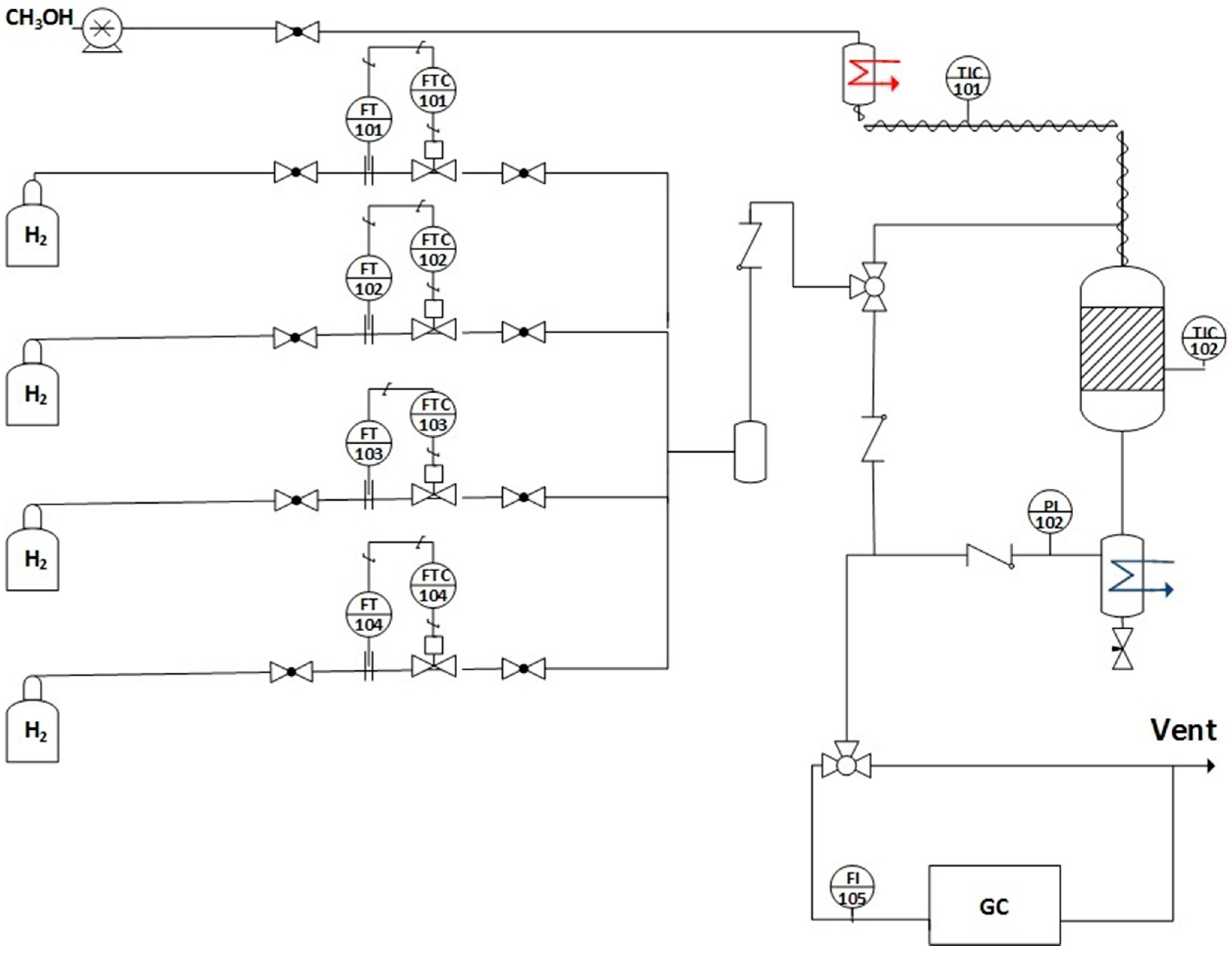
3.3. Reaction Testing
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ramirez, D.; Beites, L.F.; Blazquez, F.; Ballesteros, J.C. Distributed generation system with PEM fuel cell for electrical power quality improvement. Int. J. Hydrog. Energy 2008, 33, 4433–4443. [Google Scholar] [CrossRef]
- Palo, D.R.; Dagle, R.A.; Holladay, J.D. Methanol steam reforming for hydrogen production. Chem. Rev. 2007, 107, 3992–4021. [Google Scholar] [CrossRef] [PubMed]
- Olah, G.A. After oil and gas: Methanol economy. Catal. Lett. 2004, 93, 1–2. [Google Scholar] [CrossRef]
- Lindström, B.; Pettersson, L.J. Hydrogen generation by steam reforming of methanol over copper-based catalysts for fuel cell applications. Int. J. Hydrog. Energy 2001, 26, 923–933. [Google Scholar] [CrossRef]
- Papavasiliou, J.; Avgouropoulos, G.; Ioannides, T. Steady-state isotopic transient kinetic analysis of steam reforming of methanol over Cu-based catalysts. Appl. Catal. B Environ. 2009, 88, 490–496. [Google Scholar] [CrossRef]
- Peppley, B.A.; Amphlett, J.C.; Kearns, L.M.; Mann, R.F. Methanol–steam reforming on Cu/ZnO/Al2O3. Part 1: The reaction network. Appl. Catal. A Gen. 1999, 179, 21–29. [Google Scholar] [CrossRef]
- Peppley, B.A.; Amphlett, J.C.; Kearns, L.M.; Mann, R.F. Methanol–steam reforming on Cu/ZnO/Al2O3 catalysts. Part 2. A comprehensive kinetic model. Appl. Catal. A Gen. 1999, 179, 31–49. [Google Scholar] [CrossRef]
- Qi, C.; Amphlett, J.C.; Peppley, B.A. K (Na)-promoted Ni, Al layered double hydroxide catalysts for the steam reforming of methanol. J. Power Source 2007, 171, 842–849. [Google Scholar] [CrossRef]
- Jiang, C.J.; Trimm, D.L.; Wainwright, M.S.; Cant, N.W. Kinetic study of steam reforming of methanol over copper-based catalysts. Appl. Catal. A Gen. 1993, 93, 245–255. [Google Scholar] [CrossRef]
- Yao, C.; Wang, L.; Liu, Y.; Wu, G.; Cao, Y.; Dai, W.; He, H.; Fan, K. Effect of preparation method on the hydrogen production from methanol steam reforming over binary Cu/ZrO2 catalysts. Appl. Catal. A Gen. 2006, 297, 151–158. [Google Scholar] [CrossRef]
- Karim, A.M.; Conant, T.; Datye, A.K. Controlling ZnO morphology for improved methanol steam reforming reactivity. Phys. Chem. Chem. Phys. 2008, 10, 5584–5590. [Google Scholar] [CrossRef] [PubMed]
- Ranganathan, E.S.; Bej, S.K.; Thompson, L.T. Methanol steam reforming over Pd/ZnO and Pd/CeO2 catalysts. Appl. Catal. A Gen. 2005, 289, 153–162. [Google Scholar] [CrossRef]
- Conant, T.; Karim, A.M.; Lebarbier, V.; Wang, Y.; Girgsdies, F.; Schlögl, R.; Datye, A. Stability of bimetallic Pd–Zn catalysts for the steam reforming of methanol. J. Catal. 2008, 257, 64–70. [Google Scholar] [CrossRef]
- Suwa, Y.; Ito, S.-I.; Kameoka, S.; Tomishige, K.; Kunimori, K. Comparative study between Zn–Pd/C and Pd/ZnO catalysts for steam reforming of methanol. Appl. Catal. A Gen. 2004, 267, 9–16. [Google Scholar] [CrossRef]
- Iwasa, N.; Masuda, S.; Ogawa, N.; Takezawa, N. Steam reforming of methanol over Pd/ZnO: Effect of the formation of PdZn alloys upon the reaction. Appl. Catal. A Gen. 1995, 125, 145–157. [Google Scholar] [CrossRef]
- Jacobs, G.; Davis, B.H. In situ DRIFTS investigation of the steam reforming of methanol over Pt/ceria. Appl. Catal. A Gen. 2005, 285, 43–49. [Google Scholar] [CrossRef]
- Takezawa, N.; Iwasa, N. Steam reforming and dehydrogenation of methanol: Difference in the catalytic functions of copper and group VIII metals. Catal. Today 1997, 36, 45–56. [Google Scholar] [CrossRef]
- Iwasa, N.; Mayanagi, T.; Nomura, W.; Arai, M.; Takezawa, N. Effect of Zn addition to supported Pd catalysts in the steam reforming of methanol. Appl. Catal. A Gen. 2003, 248, 153–160. [Google Scholar] [CrossRef]
- Iwasa, N.; Yoshikawa, M.; Nomura, W.; Arai, M. Transformation of methanol in the presence of steam and oxygen over ZnO-supported transition metal catalysts under stream reforming conditions. Appl. Catal. A Gen. 2005, 292, 215–222. [Google Scholar] [CrossRef]
- Liu, D.; Men, Y.; Wang, J.; Kolb, G.; Liu, X.; Wang, Y.; Sun, Q. Highly active and durable Pt/In2O3/Al2O3 catalysts in methanol steam reforming. Int. J. Hydrog. Energy 2016, 41, 21990–21999. [Google Scholar] [CrossRef]
- Kaftan, A.; Kusche, M.; Laurin, M.; Wasserscheid, P.; Libuda, J. KOH-promoted Pt/Al2O3 catalysts for water gas shift and methanol steam reforming: An operando DRIFTS-MS study. Appl. Catal. B Environ. 2017, 201, 169–181. [Google Scholar] [CrossRef]
- Wichert, M.; Zapf, R.; Ziogas, A.; Kolb, G.; Klemm, E. Kinetic investigations of the steam reforming of methanol over a Pt/In2O3/Al2O3 catalyst in microchannels. Chem. Eng. Sci. 2016, 155, 201–209. [Google Scholar] [CrossRef]
- Martinelli, M.; Jacobs, G.; Shafer, W.D.; Davis, B.H. Effect of alkali on CH bond scission over Pt/YSZ catalyst during water-gas-shift, steam-assisted formic acid decomposition and methanol steam reforming. Catal. Today 2016. [Google Scholar] [CrossRef]
- Iwasa, N.; Mayanagi, T.; Ogawa, N.; Sakata, K.; Takezawa, N. New catalytic functions of Pd–Zn, Pd–Ga, Pd–In, Pt–Zn, Pt–Ga and Pt–In alloys in the conversions of methanol. Catal. Lett. 1998, 54, 119–123. [Google Scholar] [CrossRef]
- Bulushev, D.A.; Zacharska, M.; Guo, Y.; Beloshapkin, S.; Simakov, A. CO-free hydrogen production from decomposition of formic acid over Au/Al2O3 catalysts doped with potassium ions. Catal. Commun. 2017, 92, 86–89. [Google Scholar] [CrossRef]
- Iwasawa, Y. Surface catalytic reactions assisted by gas phase molecules. Acc. Chem. Res. 1997, 30, 103–109. [Google Scholar] [CrossRef]
- Brooks, A.H.C.J.; Yaccato, K.; Carhart, R.; Herrman, M.; Lesik, A.; Strasser, P.; Volpe, A.; Turner, H.; Weinberg, H. Combinatorial methods for the discovery of novel catalysts for the WGS reaction. In Proceedings of the 19th Meeting of the North American Catalysis Society, Philadelphia, PA, USA, 22–27 May 2005. [Google Scholar]
- Pigos, J.M.; Brooks, C.J.; Jacobs, G.; Davis, B.H. Low temperature water-gas shift: Characterization of Pt-based ZrO2 catalyst promoted with Na discovered by combinatorial methods. Appl. Catal. A Gen. 2007, 319, 47–57. [Google Scholar] [CrossRef]
- Davis, B.H.; Occelli, M.L. Advances in Fischer-Tropsch Synthesis, Catalysts, and Catalysis; CRC Press: Boca Raton, FL, USA, 2010; pp. 365–394. [Google Scholar]
- Martinelli, M.; Jacobs, G.; Graham, U.M.; Shafer, W.D.; Cronauer, D.C.; Kropf, A.J.; Marshall, C.L.; Khalid, S.; Visconti, C.G.; Lietti, L.; et al. Water-gas shift: Characterization and testing of nanoscale YSZ supported Pt catalysts. Appl. Catal. A Gen. 2015, 497, 184–197. [Google Scholar] [CrossRef]
- Davis, B.H.; Occelli, M.L. Fischer-Tropsch Synthesis, Catalysts and Catalysis: Advances and Applications; CRC Press: Boca Raton, FL, USA, 2016; pp. 309–326. [Google Scholar]
- Lavalley, J.C. Infrared spectrometric studies of the surface basicity of metal oxides and zeolites using adsorbed probe molecules. Catal. Today 1996, 27, 377–401. [Google Scholar] [CrossRef]
- Binet, C.; Daturi, M.; Lavalley, J.-C. IR study of polycrystalline ceria properties in oxidised and reduced states. Catal. Today 1999, 50, 207–225. [Google Scholar] [CrossRef]
- Pigos, J.M.; Brooks, C.J.; Jacobs, G.; Davis, B.H. Low temperature water–Gas shift: The effect of alkali doping on the CH bond of formate over Pt/ZrO2 catalysts. Appl. Catal. A Gen. 2007, 328, 14–26. [Google Scholar] [CrossRef]
- Jacobs, G.; Williams, L.; Graham, U.; Thomas, G.A.; Sparks, D.E.; Davis, B.H. Low temperature water–Gas shift: In situ DRIFTS-reaction study of ceria surface area on the evolution of formates on Pt/CeO2 fuel processing catalysts for fuel cell applications. Appl. Catal. A Gen. 2003, 252, 107–118. [Google Scholar] [CrossRef]
- Rogemond, E.; Essayem, N.; Frety, R.; Perrichon, V.; Primet, M.; Mathis, F. Characterization of model three-way catalysts. J. Catal. 1997, 166, 229–235. [Google Scholar] [CrossRef]
- Pantu, P.; Gavalas, G.R. Methane partial oxidation on Pt/CeO2 and Pt/Al2O3 catalysts. Appl. Catal. A Gen. 2002, 223, 253–260. [Google Scholar] [CrossRef]

| Sample Description | Catalyst Composition | BET SA (m2/g) | Single Point Average Pore Volume (cm3/g) | Single Point Average Pore Diameter (nm) |
|---|---|---|---|---|
| YSZ | Zr0.9Y0.1O1.95 | 149.2 | 0.19 | 3.6 |
| 2% Pt/YSZ | 2% Pt/Zr0.9Y0.1O1.95 | 143.6 | 0.18 | 3.6 |
| 2% Pt–0.25% Na/YSZ | 2% Pt–0.25% Na/Zr0.9Y0.1O1.95 | 163.6 | 0.16 | 4.0 |
| 2% Pt–0.5% Na /YSZ | 2% Pt–0.5% Na/Zr0.9Y0.1O1.95 | 150.5 | 0.15 | 4.0 |
| 2% Pt–1% Na/YSZ | 2% Pt–1% Na/Zr0.9Y0.1O1.95 | 145.4 | 0.14 | 4.0 |
| 2% Pt–2.5% Na /YSZ | 2% Pt–2.5% Na/Zr0.9Y0.1O1.95 | 94.9 | 0.13 | 3.4 |
| TPD T (°C) | 2% Pt/YSZ (% of Band at 50 °C) | 2% Pt–1%Na/YSZ (% of Band at 50 °C) | 2% Pt–2.5% Na/YSZ (% of Band at 50 °C) |
|---|---|---|---|
| 50 | 100 | 100 | 100 |
| 100 | 85 | 93 | 95 |
| 200 | 58 | 80 | 84 |
| 300 | 41 | 68 | 80 |
| 350 | 37 | 62 | 76 |
| Catalyst | T (°C) | % CH3OH Conv. | % CO Select. | % CO2 Select. | % CH4 Select. |
|---|---|---|---|---|---|
| 2% Pt/YSZ | 275 | 21.6 ± 0.1 | 79.3 ± 0.1 | 19.7 ± 0.1 | 1.0 ± 0.005 |
| 300 | 49.4 ± 0.1 | 76.8 ± 0.05 | 22.1 ± 0.05 | 1.1 ± 0.005 | |
| 325 | 74.1 ± 0.3 | 70.5 ± 0.1 | 28.5 ± 0.1 | 1.1 ± 0.003 | |
| 350 | 89.1 ± 0.5 | 53.2 ± 0.6 | 45.6 ± 0.5 | 1.2 ± 0.02 | |
| 2% Pt–0.25% Na/YSZ | 275 | 21.5 ± 0.1 | 76.2 ± 0.1 | 23.1 ± 0.1 | 0.8 ± 0.02 |
| 300 | 44.2 ± 0.2 | 74.3 ± 0.1 | 24.9 ± 0.1 | 0.8 ± 0.01 | |
| 325 | 68.7 ± 0.6 | 68.2 ± 0.1 | 30.9 ± 0.1 | 0.9 ± 0.02 | |
| 350 | 87.8 ± 0.3 | 55.9 ± 0.9 | 43.2 ± 0.9 | 1.0 ± 0.01 | |
| 2% Pt–0.5% Na /YSZ | 275 | 20.6 ± 0.1 | 71.0 ± 0.1 | 28.2 ± 0.1 | 0.7 ± 0.01 |
| 300 | 41.1 ± 0.1 | 67.9 ± 0.05 | 31.2 ± 0.05 | 0.8 ± 0.01 | |
| 325 | 63.3 ± 0.5 | 59.2 ± 0.05 | 40.0 ± 0.05 | 0.9 ± 0.01 | |
| 350 | 79.9 ± 0.6 | 38.9 ± 0.4 | 60.2 ± 0.4 | 2.8 ± 0.02 | |
| 2% Pt–1% Na/YSZ | 275 | 14.4 ± 0.1 | 60.0 ± 0.2 | 38.0 ± 0.1 | 0.5 ± 0.06 |
| 300 | 31.9 ± 0.10 | 59.7 ± 0.1 | 39.3 ± 0.1 | 0.6 ± 0.01 | |
| 325 | 53.6 ± 0.4 | 55.6 ± 0.05 | 43.3 ± 0.06 | 0.7 ± 0.02 | |
| 350 | 80.9 ± 0.3 | 43.2 ± 0.5 | 60.6 ± 0.5 | 0.8 ± 0.02 | |
| 2% Pt–2.5% Na /YSZ | 275 | 16.7 ± 0.1 | 4.3 ± 0.1 | 95.0 ± 0.1 | 0.7 ± 0.01 |
| 300 | 29.9 ± 0.1 | 5.7 ± 0.1 | 94.0 ± 0.1 | 0.4 ± 0.004 | |
| 325 | 45.2 ± 0.2 | 7.6 ± 0.1 | 92.2 ± 0.1 | 0.3 ± 0.002 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinelli, M.; Jacobs, G.; Graham, U.M.; Davis, B.H. Methanol Steam Reforming: Na Doping of Pt/YSZ Provides Fine Tuning of Selectivity. Catalysts 2017, 7, 148. https://doi.org/10.3390/catal7050148
Martinelli M, Jacobs G, Graham UM, Davis BH. Methanol Steam Reforming: Na Doping of Pt/YSZ Provides Fine Tuning of Selectivity. Catalysts. 2017; 7(5):148. https://doi.org/10.3390/catal7050148
Chicago/Turabian StyleMartinelli, Michela, Gary Jacobs, Uschi M. Graham, and Burtron H. Davis. 2017. "Methanol Steam Reforming: Na Doping of Pt/YSZ Provides Fine Tuning of Selectivity" Catalysts 7, no. 5: 148. https://doi.org/10.3390/catal7050148






