Old Yellow Enzyme-Catalysed Asymmetric Hydrogenation: Linking Family Roots with Improved Catalysis
Abstract
:1. Introduction
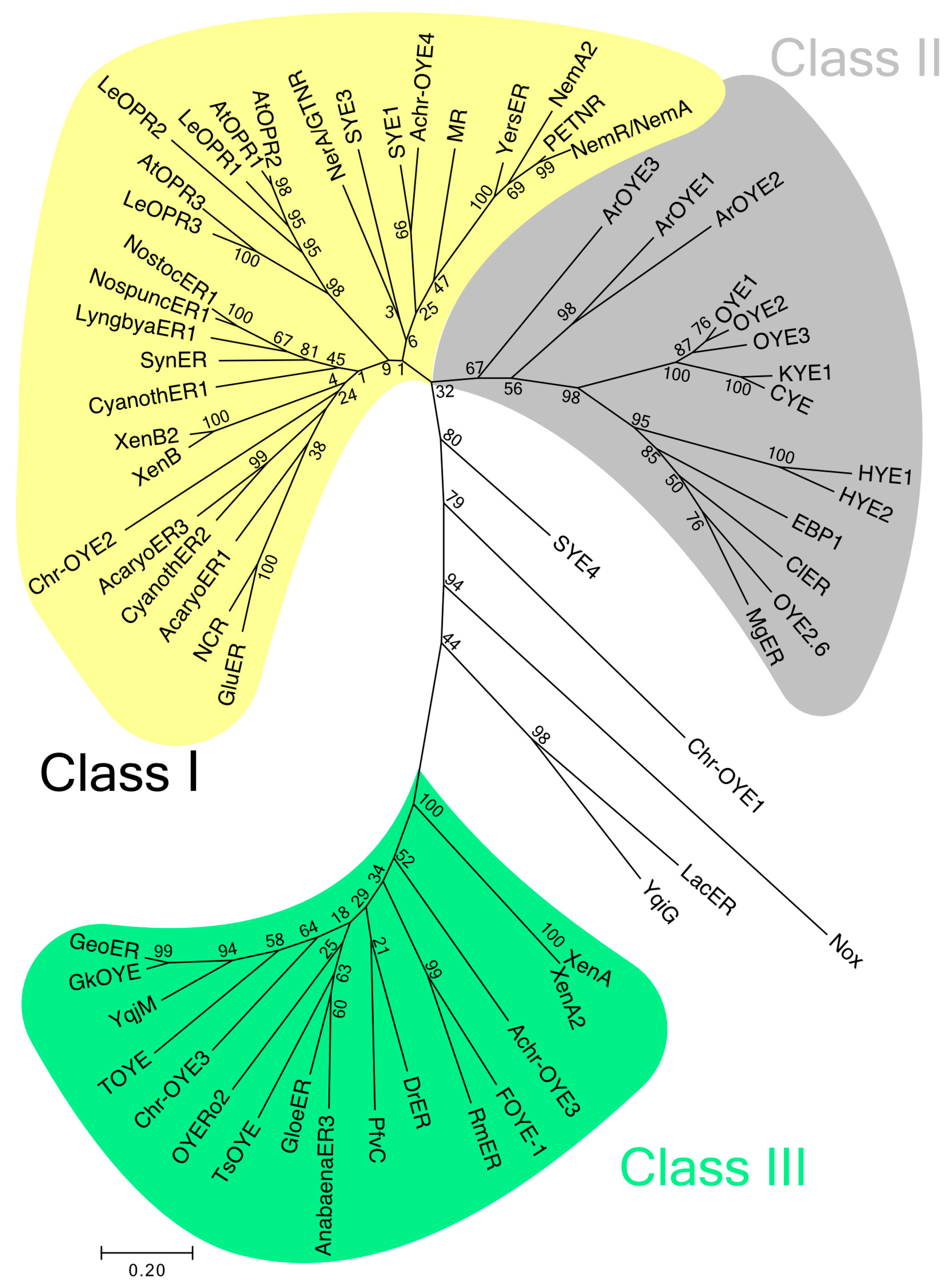
2. Phylogenetic Classification of OYEs
3. Structural Classification of OYEs: From Sequence to Structure
3.1. Multiple Sequence Alignment
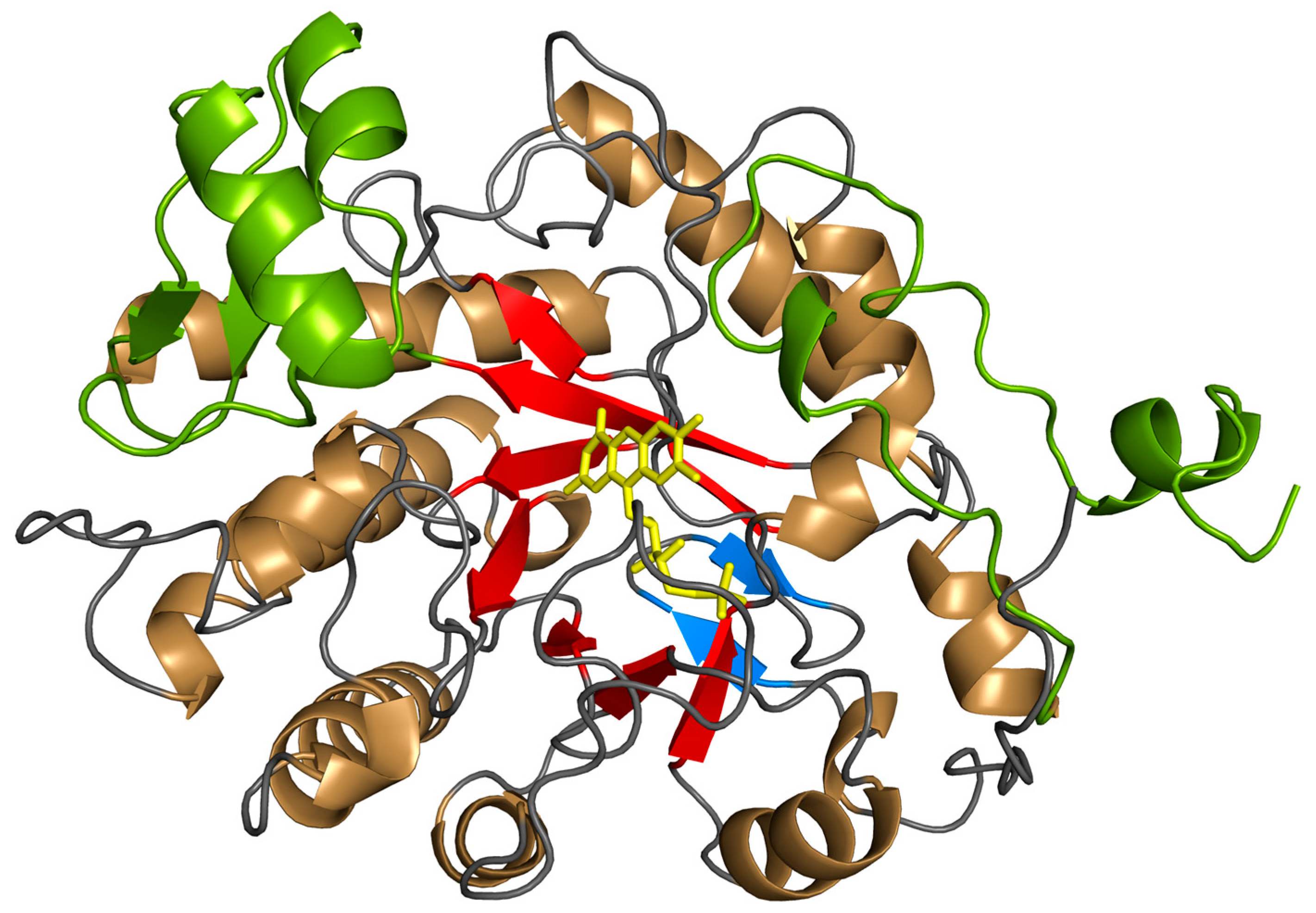
3.2. Monomeric Structure and Dimeric Interface
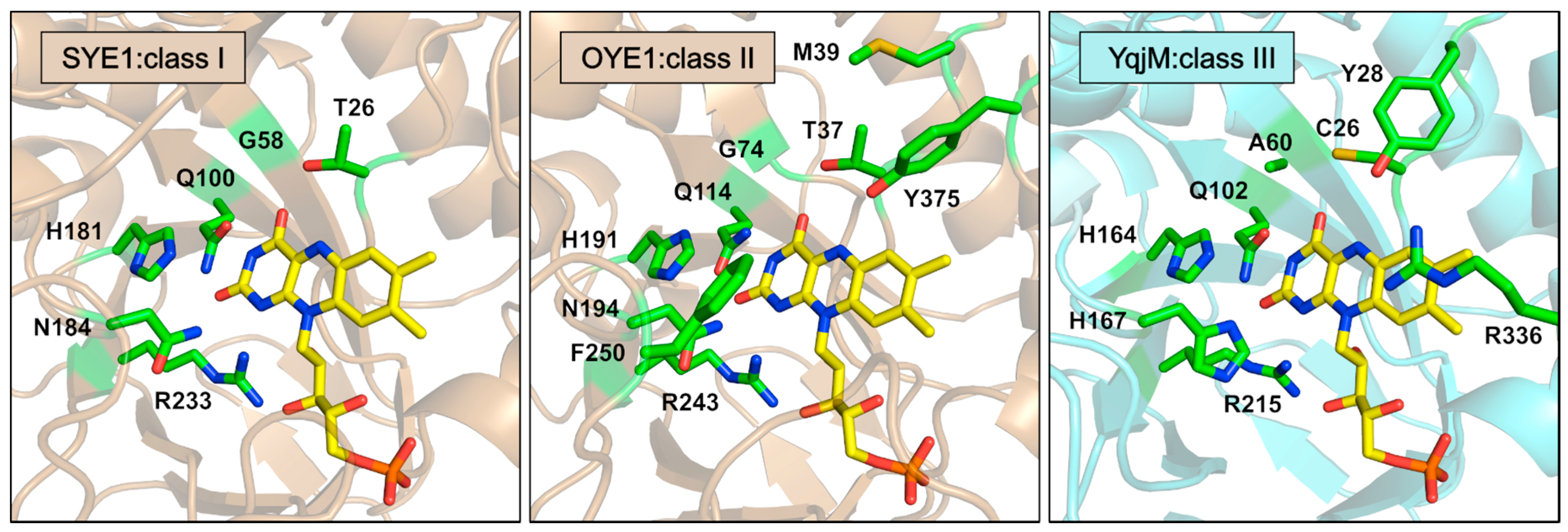
3.3. FMN Binding
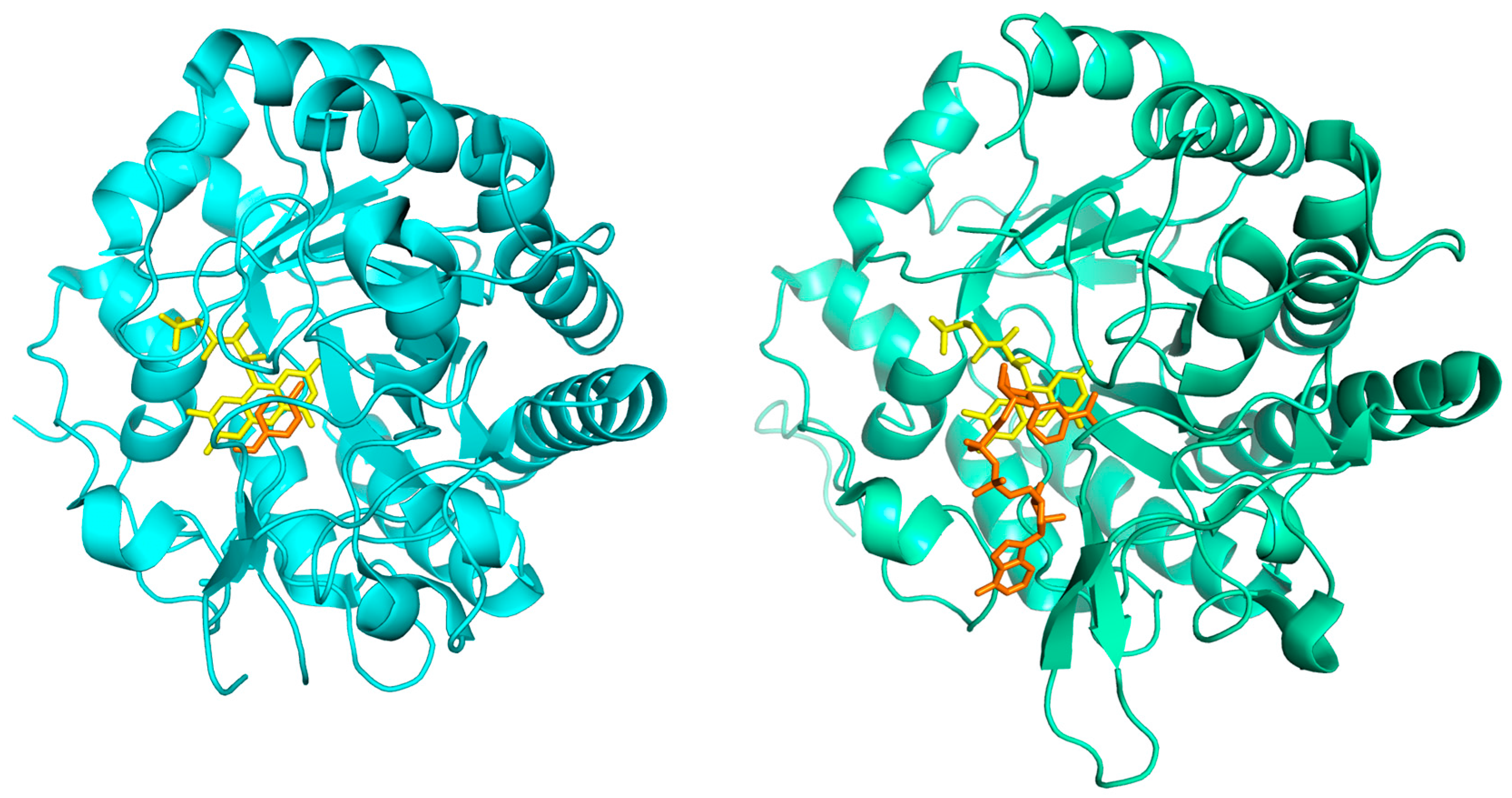
3.4. Coenzyme and Inhibitor Binding
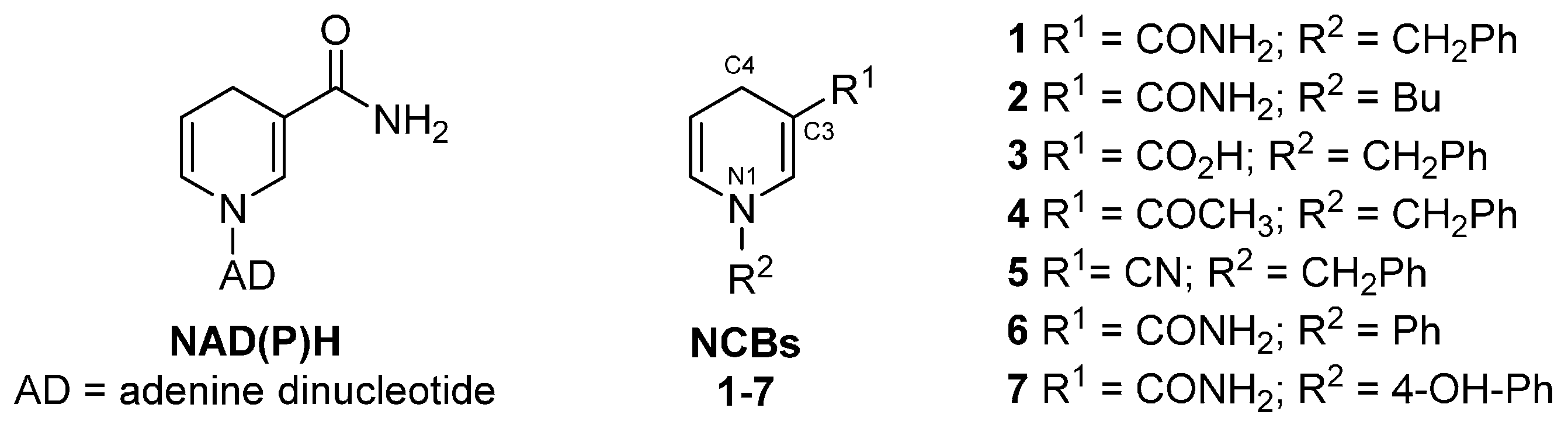
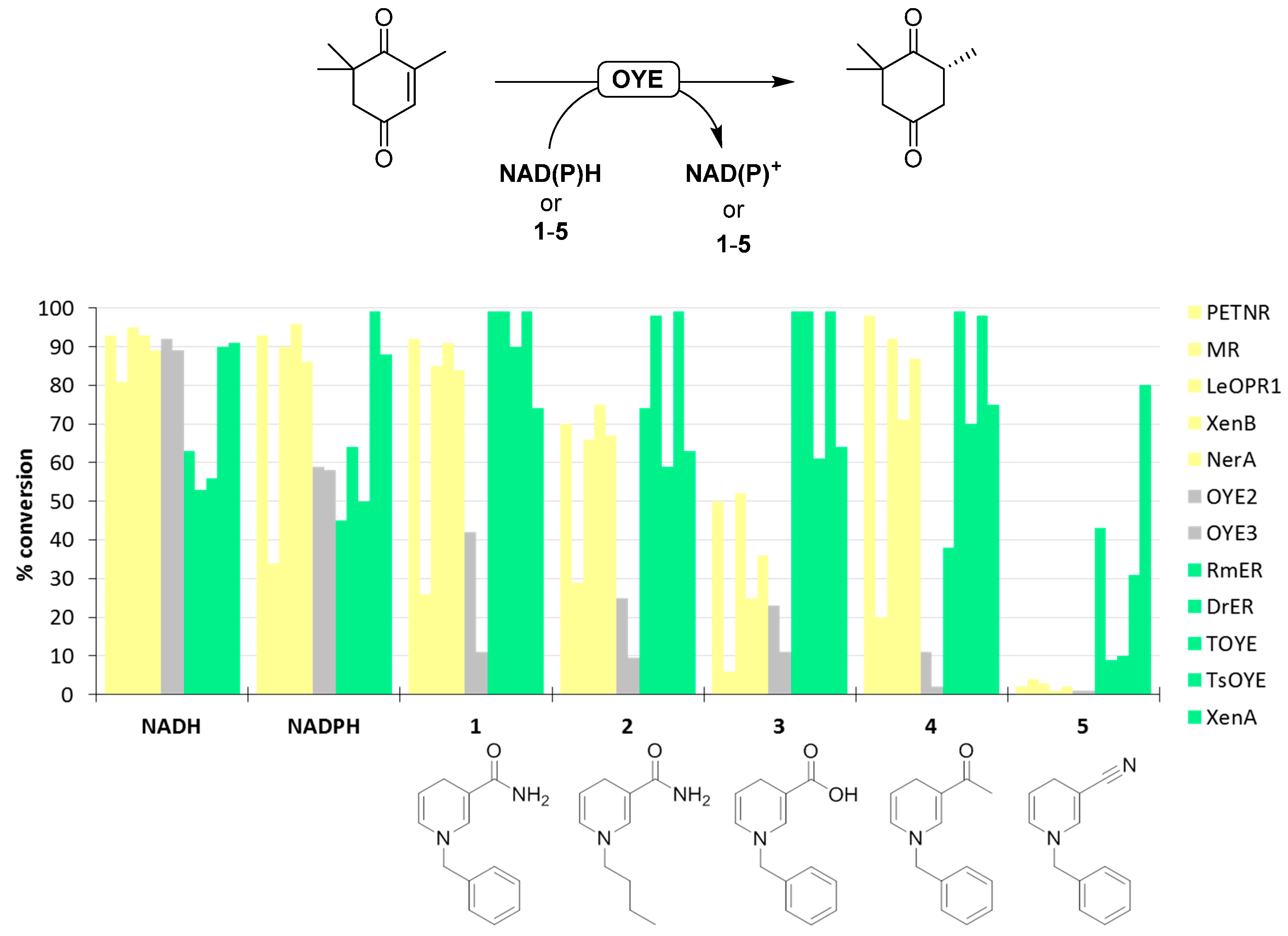
4. Reactivity with NCBs
4.1. Biocatalytic Conversions
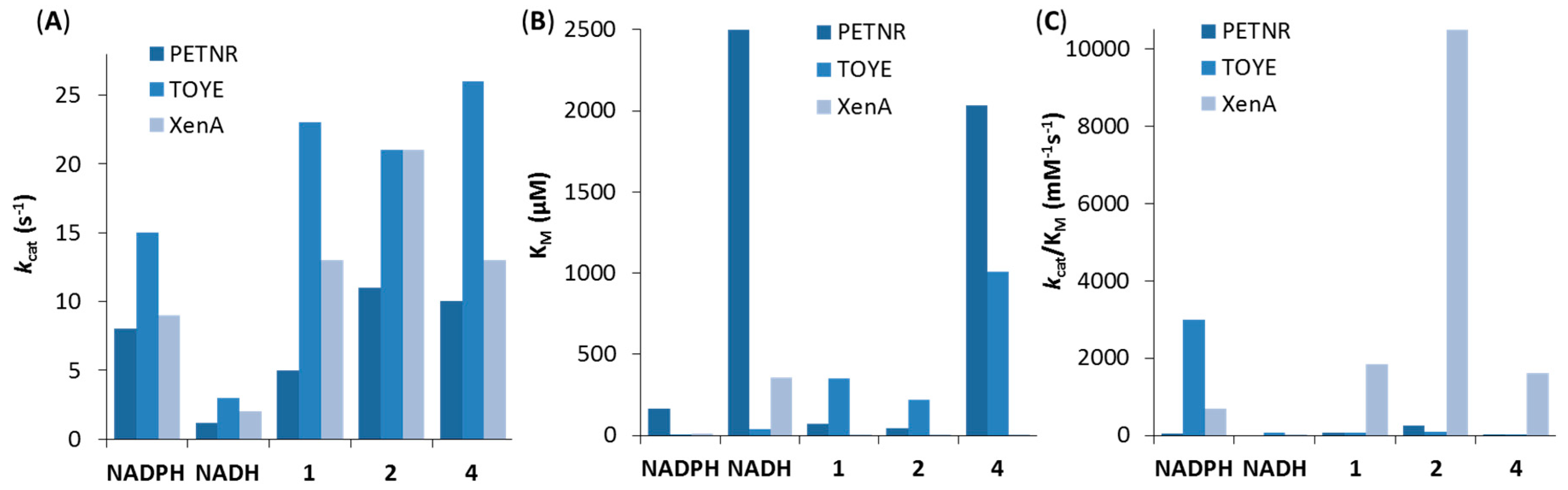
4.2. Kinetic Data: Steady State and Pre-Steady State
5. Classification of OYEs with Respect to Substrates
6. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Knowles, W.S.; Noyori, R. Pioneering perspectives on asymmetric hydrogenation. Acc. Chem. Res. 2007, 40, 1238–1239. [Google Scholar] [CrossRef] [PubMed]
- Clayden, J.; Greeves, N.; Warren, S.G. Organic Chemistry, 2nd ed.; Oxford University Press: Oxford, UK; New York, NY, USA, 2012. [Google Scholar]
- Atkins, P.W.; Shriver, D.F. Shriver and Atkins’ Inorganic Chemistry, 5th ed.; Oxford University Press: Oxford, UK; New York, NY, USA, 2010. [Google Scholar]
- Yang, J.W.; Fonseca, M.T.H.; Vignola, N.; List, B. Metal-free, organocatalytic asymmetric transfer hydrogenation of α,β-unsaturated aldehydes. Angew. Chem. Int. Ed. 2005, 44, 108–110. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.E.; Bruce, N.C. ’New uses for an old enzyme’-the old yellow enzyme family of flavoenzymes. Microbiology 2002, 148, 1607–1614. [Google Scholar] [CrossRef] [PubMed]
- Chaparro-Riggers, J.F.; Rogers, T.A.; Vazquez-Figueroa, E.; Polizzi, K.M.; Bommarius, A.S. Comparison of three enoate reductases and their potential use for biotransformations. Adv. Synth. Catal. 2007, 349, 1521–1531. [Google Scholar] [CrossRef]
- Stuermer, R.; Hauer, B.; Hall, M.; Faber, K. Asymmetric bioreduction of activated C=C bonds using enoate reductases from the old yellow enzyme family. Curr. Opin. Chem. Biol. 2007, 11, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Fryszkowska, A.; Toogood, H.; Sakuma, M.; Gardiner, J.M.; Stephens, G.M.; Scrutton, N.S. Asymmetric reduction of activated alkenes by pentaerythritol tetranitrate reductase: Specificity and control of stereochemical outcome by reaction optimisation. Adv. Synth. Catal. 2009, 351, 2976–2990. [Google Scholar] [CrossRef] [PubMed]
- Toogood, H.S.; Gardiner, J.M.; Scrutton, N.S. Biocatalytic reductions and chemical versatility of the old yellow enzyme family of flavoprotein oxidoreductases. ChemCatChem 2010, 2, 892–914. [Google Scholar] [CrossRef]
- Gao, X.Z.; Ren, J.; Wu, Q.Q.; Zhu, D.M. Biochemical characterization and substrate profiling of a new NADH-dependent enoate reductase from Lactobacillus casei. Enzyme Microb. Technol. 2012, 51, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Swiderska, M.A.; Stewart, J.D. Stereoselective enone reductions by Saccharomyces carlsbergensis old yellow enzyme. J. Mol. Catal. B Enzym. 2006, 42, 52–54. [Google Scholar] [CrossRef]
- Hall, M.; Stueckler, C.; Ehammer, H.; Pointner, E.; Oberdorfer, G.; Gruber, K.; Hauer, B.; Stuermer, R.; Kroutil, W.; Macheroux, P.; et al. Asymmetric bioreduction of C=C bonds using enoate reductases OPR1, OPR3 and YqjM: Enzyme-based stereocontrol. Adv. Synth. Catal. 2008, 350, 411–418. [Google Scholar] [CrossRef]
- Bertolotti, M.; Brenna, E.; Crotti, M.; Gatti, F.G.; Monti, D.; Parmeggiani, F.; Santangelo, S. Substrate scope evaluation of the enantioselective reduction of β-alkyl-β-arylnitroalkenes by old yellow enzymes 1–3 for organic synthesis applications. ChemCatChem 2016, 8, 577–583. [Google Scholar] [CrossRef]
- Nivinskas, H.; Sarlauskas, J.; Anusevicius, Z.; Toogood, H.S.; Scrutton, N.S.; Cenas, N. Reduction of aliphatic nitroesters and N-nitramines by Enterobacter cloacae PB2 pentaerythritol tetranitrate reductase. FEBS J. 2008, 275, 6192–6203. [Google Scholar] [CrossRef] [PubMed]
- Toogood, H.S.; Fryszkowska, A.; Hare, V.; Fisher, K.; Roujeinikova, A.; Leys, D.; Gardiner, J.M.; Stephens, G.M.; Scrutton, N.S. Structure-based insight into the asymmetric bioreduction of the C=C double bond of α,β-unsaturated nitroalkenes by pentaerythritol tetranitrate reductase. Adv. Synth. Catal. 2008, 350, 2789–2803. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.J.; Pei, X.Q.; Lin, H.; Gai, P.; Liu, Y.C.; Wu, Z.L. Asymmetric bioreduction of activated alkenes by a novel isolate of Achromobacter species producing enoate reductase. Appl. Microbiol. Biotechnol. 2012, 95, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.B.; Pei, X.Q.; Wu, Z.L. An enoate reductase Achr-OYE4 from Achromobacter sp. JA81: Characterization and application in asymmetric bioreduction of C=C bonds. Appl. Microbiol. Biotechnol. 2014, 98, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Stueckler, C.; Hall, M.; Ehammer, H.; Pointner, E.; Kroutil, W.; Macheroux, P.; Faber, K. Stereocomplementary bioreduction of α,β-unsaturated dicarboxylic acids and dimethyl esters using enoate reductases: Enzyme- and substrate-based stereocontrol. Org. Lett. 2007, 9, 5409–5411. [Google Scholar] [CrossRef] [PubMed]
- Tasnádi, G.; Winkler, C.K.; Clay, D.; Sultana, N.; Fabian, W.M.F.; Hall, M.; Ditrich, K.; Faber, K. A substrate-driven approach to determine reactivities of α,β-unsaturated carboxylic esters towards asymmetric bioreduction. Chem. Eur. J. 2012, 18, 10362–10367. [Google Scholar] [CrossRef] [PubMed]
- Tasnádi, G.; Winkler, C.K.; Clay, D.; Hall, M.; Faber, K. Reductive dehalogenation of β-haloacrylic ester derivatives mediated by ene-reductases. Catal. Sci. Technol. 2012, 2, 1548–1552. [Google Scholar] [CrossRef]
- Fu, Y.L.; Hoelsch, K.; Weuster-Botz, D. A novel ene-reductase from Synechococcus sp. PCC 7942 for the asymmetric reduction of alkenes. Process. Biochem. 2012, 47, 1988–1997. [Google Scholar] [CrossRef]
- Riedel, A.; Mehnert, M.; Paul, C.E.; Westphal, A.H.; van Berkel, W.J.H.; Tischler, D. Functional characterization and stability improvement of a ‘thermophilic-like’ ene-reductase from Rhodococcus opacus 1CP. Front. Microbiol. 2015, 6, 1073. [Google Scholar] [CrossRef] [PubMed]
- Scholtissek, A.; Ullrich, S.R.; Mühling, M.; Schlömann, M.; Paul, C.E.; Tischler, D. A thermophilic-like ene-reductase originating from an acidophilic iron oxidizer. Appl. Microbiol. Biotechnol. 2017, 101, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Winkler, C.K.; Clay, D.; Turrini, N.G.; Lechner, H.; Kroutil, W.; Davies, S.; Debarge, S.; O’Neill, P.; Steflik, J.; Karmilowicz, M.; et al. Nitrile as activating group in the asymmetric bioreduction of β-cyanoacrylic acids catalyzed by ene-reductases. Adv. Synth. Catal. 2014, 356, 1878–1882. [Google Scholar] [CrossRef] [PubMed]
- Brenna, E.; Crotti, M.; Gatti, F.G.; Monti, D.; Parmeggiani, F.; Powell, R.W.; Santangelo, S.; Stewart, J.D. Opposite enantioselectivity in the bioreduction of (Z)-β-aryl-β-cyanoacrylates mediated by the tryptophan 116 mutants of old yellow enzyme 1: Synthetic approach to (R)-and (S)-β-aryl-γ-lactams. Adv. Synth. Catal. 2015, 357, 1849–1860. [Google Scholar] [CrossRef]
- Swiderska, M.A.; Stewart, J.D. Asymmetric bioreductions of β-nitro acrylates as a route to chiral β2-amino acids. Org. Lett. 2006, 8, 6131–6133. [Google Scholar] [CrossRef] [PubMed]
- Durchschein, K.; Hall, M.; Faber, K. Unusual reactions mediated by FMN-dependent ene- and nitro-reductases. Green Chem. 2013, 15, 1764–1772. [Google Scholar] [CrossRef]
- Wohlgemuth, R. Biocatalysis-key to sustainable industrial chemistry. Curr. Opin. Biotechnol. 2010, 21, 713–724. [Google Scholar] [CrossRef] [PubMed]
- Bougioukou, D.J.; Walton, A.Z.; Stewart, J.D. Towards preparative-scale, biocatalytic alkene reductions. Chem. Commun. 2010, 46, 8558–8560. [Google Scholar] [CrossRef] [PubMed]
- Huisman, G.W.; Collier, S.J. On the development of new biocatalytic processes for practical pharmaceutical synthesis. Curr. Opin. Chem. Biol. 2013, 17, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Ress, T.; Hummel, W.; Hanlon, S.P.; Iding, H.; Gröger, H. The organic-synthetic potential of recombinant ene reductases: Substrate-scope evaluation and process optimization. ChemCatChem 2015, 7, 1302–1311. [Google Scholar] [CrossRef]
- Pietruszka, J.; Scholzel, M. Ene reductase-catalysed synthesis of (R)-profen derivatives. Adv. Synth. Catal. 2012, 354, 751–756. [Google Scholar] [CrossRef]
- Winkler, C.K.; Clay, D.; Davies, S.; O’Neill, P.; McDaid, P.; Debarge, S.; Steflik, J.; Karmilowicz, M.; Wong, J.W.; Faber, K. Chemoenzymatic asymmetric synthesis of pregabalin precursors via asymmetric bioreduction of β-cyanoacrylate esters using ene-reductases. J. Org. Chem. 2013, 78, 1525–1533. [Google Scholar] [CrossRef] [PubMed]
- Turrini, N.G.; Hall, M.; Faber, K. Enzymatic synthesis of optically active lactones via asymmetric bioreduction using ene-reductases from the old yellow enzyme family. Adv. Synth. Catal. 2015, 357, 1861–1871. [Google Scholar] [CrossRef]
- Collins, I. Saturated and unsaturated lactones. J. Chem. Soc. Perkin Trans. 1999, 1, 1377–1395. [Google Scholar] [CrossRef]
- Toogood, H.S.; Scrutton, N.S. New developments in ‘ene’-reductase catalysed biological hydrogenations. Curr. Opin. Chem. Biol. 2014, 19, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Breithaupt, C.; Strassner, J.; Breitinger, U.; Huber, R.; Macheroux, P.; Schaller, A.; Clausen, T. X-ray structure of 12-oxophytodienoate reductase 1 provides structural insight into substrate binding and specificity within the family of OYE. Structure 2001, 9, 419–429. [Google Scholar] [CrossRef]
- Kohli, R.M.; Massey, V. The oxidative half-reaction of old yellow enzyme-The role of tyrosine 196. J. Biol. Chem. 1998, 273, 32763–32770. [Google Scholar] [CrossRef] [PubMed]
- Lonsdale, R.; Reetz, M.T. Reduction of α,β-unsaturated ketones by old yellow enzymes: Mechanistic insights from quantum mechanics/molecular mechanics calculations. J. Am. Chem. Soc. 2015, 137, 14733–14742. [Google Scholar] [CrossRef] [PubMed]
- Karplus, P.A.; Fox, K.M.; Massey, V. Flavoprotein structure and mechanism. 8. Structure-function relations for old yellow enzyme. FASEB J. 1995, 9, 1518–1526. [Google Scholar] [PubMed]
- Brown, B.J.; Hyun, J.W.; Duvvuri, S.; Karplus, P.A.; Massey, V. The role of glutamine 114 in old yellow enzyme. J. Biol. Chem. 2002, 277, 2138–2145. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.; Stueckler, C.; Hauer, B.; Stuermer, R.; Friedrich, T.; Breuer, M.; Kroutil, W.; Faber, K. Asymmetric bioreduction of activated C=C bonds using Zymomonas mobilis NCR enoate reductase and old yellow enzymes OYE 1–3 from yeasts. Eur. J. Org. Chem. 2008, 1511–1516. [Google Scholar] [CrossRef]
- Hall, M.; Stueckler, C.; Kroutil, W.; Macheroux, P.; Faber, K. Asymmetric bioreduction of activated alkenes using cloned 12-oxophytodienoate reductase isoenzymes OPR-1 and OPR-3 from Lycopersicon esculentum (tomato): A striking change of stereoselectivity. Angew. Chem. Int. Ed. 2007, 46, 3934–3937. [Google Scholar] [CrossRef] [PubMed]
- Mueller, N.J.; Stueckler, C.; Hauer, B.; Baudendistel, N.; Housden, H.; Bruce, N.C.; Faber, K. The substrate spectra of pentaerythritol tetranitrate reductase, morphinone reductase, N-ethylmaleimide reductase and estrogen-binding protein in the asymmetric bioreduction of activated alkenes. Adv. Synth. Catal. 2010, 352, 387–394. [Google Scholar] [CrossRef]
- Tauber, K.; Hall, M.; Kroutil, W.; Fabian, W.M.F.; Faber, K.; Glueck, S.M. A highly efficient ADH-coupled NADH-recycling system for the asymmetric bioreduction of carbon-carbon double bonds using enoate reductases. Biotechnol. Bioeng. 2011, 108, 1462–1467. [Google Scholar] [CrossRef] [PubMed]
- Toogood, H.S.; Knaus, T.; Scrutton, N.S. Alternative hydride sources for ene-reductases: Current trends. ChemCatChem 2014, 6, 951–954. [Google Scholar] [CrossRef]
- Peers, M.K.; Toogood, H.S.; Heyes, D.J.; Mansell, D.; Coe, B.J.; Scrutton, N.S. Light-driven biocatalytic reduction of α,β-unsaturated compounds by ene reductases employing transition metal complexes as photosensitizers. Catal. Sci. Technol. 2016, 6, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Winkler, C.K.; Clay, D.; Entner, M.; Plank, M.; Faber, K. NAD(P)H-independent asymmetric C=C bond reduction catalyzed by ene reductases by using artificial co-substrates as the hydrogen donor. Chem. Eur. J. 2014, 20, 1403–1409. [Google Scholar] [CrossRef] [PubMed]
- Winkler, C.K.; Clay, D.; van Heerden, E.; Faber, K. Overcoming co-product inhibition in the nicotinamide independent asymmetric bioreduction of activated C=C-bonds using flavin-dependent ene-reductases. Biotechnol. Bioeng. 2013, 110, 3085–3092. [Google Scholar] [CrossRef] [PubMed]
- Durchschein, K.; Wallner, S.; Macheroux, P.; Schwab, W.; Winkler, T.; Kreis, W.; Faber, K. Nicotinamide-dependent ene reductases as alternative biocatalysts for the reduction of activated alkenes. Eur. J. Org. Chem. 2012, 4963–4968. [Google Scholar] [CrossRef]
- Stueckler, C.; Reiter, T.C.; Baudendistel, N.; Faber, K. Nicotinamide-independent asymmetric bioreduction of C=C-bonds via disproportionation of enones catalyzed by enoate reductases. Tetrahedron 2010, 66, 663–667. [Google Scholar] [CrossRef] [PubMed]
- Paul, C.E.; Gargiulo, S.; Opperman, D.J.; Lavandera, I.; Gotor-Fernández, V.; Gotor, V.; Taglieber, A.; Arends, I.W.C.E.; Hollmann, F. Mimicking nature: Synthetic nicotinamide cofactors for C=C bioreduction using enoate reductases. Org. Lett. 2013, 15, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Paul, C.E.; Arends, I.W.C.E.; Hollmann, F. Is simpler better? Synthetic nicotinamide cofactor analogues for redox chemistry. ACS Catal. 2014, 4, 788–797. [Google Scholar] [CrossRef]
- Hollmann, F.; Paul, C.E. Synthetische nikotinamide in der biokatalyse. BIOspektrum 2015, 21, 376–378. [Google Scholar] [CrossRef]
- Paul, C.E.; Hollmann, F. A survey of synthetic nicotinamide cofactors in enzymatic processes. Appl. Microbiol. Biotechnol. 2016, 100, 4773–4778. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, Y.; Köhler, V.; Paul, C.E.; Hollmann, F.; Ward, T.R. Efficient in situ regeneration of NADH mimics by an artificial metalloenzyme. ACS Catal. 2016, 6, 3553–3557. [Google Scholar] [CrossRef]
- Geddes, A.; Paul, C.E.; Hay, S.; Hollmann, F.; Scrutton, N.S. Donor-acceptor distance sampling enhances the performance of “better than Nature” nicotinamide coenzyme biomimetics. J. Am. Chem. Soc. 2016, 138, 11089–11092. [Google Scholar] [CrossRef] [PubMed]
- Löw, S.A.; Löw, I.M.; Weissenborn, M.J.; Hauer, B. Enhanced ene-reductase activity through alteration of artificial nicotinamide cofactor substituents. ChemCatChem 2016, 8, 911–915. [Google Scholar] [CrossRef]
- Knaus, T.; Paul, C.E.; Levy, C.W.; de Vries, S.; Mutti, F.G.; Hollmann, F.; Scrutton, N.S. Better than Nature: Nicotinamide biomimetics that outperform natural coenzymes. J. Am. Chem. Soc. 2016, 138, 1033–1039. [Google Scholar] [CrossRef] [PubMed]
- Winkler, C.K.; Tasnádi, G.; Clay, D.; Hall, M.; Faber, K. Asymmetric bioreduction of activated alkenes to industrially relevant optically active compounds. J. Biotechnol. 2012, 162, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Thiele, D.J.; Davio, M.; Lockridge, O.; Massey, V. The cloning and expression of a gene encoding old yellow enzyme from Saccharomyces carlsbergensis. J. Biol. Chem. 1991, 266, 20720–20724. [Google Scholar] [PubMed]
- Kataoka, M.; Kotaka, A.; Hasegawa, A.; Wada, M.; Yoshizumi, A.; Nakamori, S.; Shimizu, S. Old yellow enzyme from Candida macedoniensis catalyzes the stereospecific reduction of the C=C bond of ketoisophorone. Biosci. Biotechnol. Biochem. 2002, 66, 2651–2657. [Google Scholar] [CrossRef] [PubMed]
- Nizam, S.; Verma, S.; Borah, N.N.; Gazara, R.K.; Verma, P.K. Comprehensive genome-wide analysis reveals different classes of enigmatic old yellow enzyme in fungi. Sci. Rep. 2014, 4, 4013. [Google Scholar] [CrossRef] [PubMed]
- Nizam, S.; Gazara, R.K.; Verma, S.; Singh, K.; Verma, P.K. Comparative structural modeling of six old yellow enzymes (OYEs) from the necrotrophic fungus Ascochyta rabiei: Insight into novel OYE classes with differences in cofactor binding, organization of active site residues and stereopreferences. PLoS ONE 2014, 9, e95989. [Google Scholar] [CrossRef] [PubMed]
- Schaller, F.; Weiler, E.W. Molecular cloning and characterization of 12-oxophytodienoate reductase, an enzyme of the octadecanoid signaling pathway from Arabidopsis thaliana-Structural and functional relationship to yeast old yellow enzyme. J. Biol. Chem. 1997, 272, 28066–28072. [Google Scholar] [CrossRef] [PubMed]
- Müssig, C.; Biesgen, C.; Lisso, J.; Uwer, U.; Weiler, E.W.; Altmann, T. A novel stress-inducible 12-oxophytodienoate reductase from Arabidopsis thaliana provides a potential link between Brassinosteroid-action and Jasmonic-acid synthesis. J. Plant Physiol. 2000, 157, 143–152. [Google Scholar] [CrossRef]
- Strassner, J.; Schaller, F.; Frick, U.B.; Howe, G.A.; Weiler, E.W.; Amrhein, N.; Macheroux, P.; Schaller, A. Characterization and cDNA-microarray expression analysis of 12-oxophytodienoate reductases reveals differential roles for octadecanoid biosynthesis in the local versus the systemic wound response. Plant J. 2002, 32, 585–601. [Google Scholar] [CrossRef] [PubMed]
- Strassner, J.; Furholz, A.; Macheroux, P.; Amrhein, N.; Schaller, A. A homolog of old yellow enzyme in tomato-Spectral properties and substrate specificity of the recombinant protein. J. Biol. Chem. 1999, 274, 35067–35073. [Google Scholar] [CrossRef] [PubMed]
- French, C.E.; Nicklin, S.; Bruce, N.C. Sequence and properties of pentaerythritol tetranitrate reductase from Enterobacter cloacae PB2. J. Bacteriol. 1996, 178, 6623–6627. [Google Scholar] [CrossRef] [PubMed]
- Blehert, D.S.; Knoke, K.L.; Fox, B.G.; Chambliss, G.H. Regioselectivity of nitroglycerin denitration by flavoprotein nitroester reductases purified from two Pseudomonas species. J. Bacteriol. 1997, 179, 6912–6920. [Google Scholar] [CrossRef] [PubMed]
- Husserl, J.; Hughes, J.B.; Spain, J.C. Key enzymes enabling the growth of Arthrobacter sp. strain JBH1 with nitroglycerin as the sole source of carbon and nitrogen. Appl. Environ. Microbiol. 2012, 78, 3649–3655. [Google Scholar] [CrossRef] [PubMed]
- Khairy, H.; Wübbeler, J.H.; Steinbüchel, A. The NADH: Flavin oxidoreductase Nox from Rhodococcus erythropolis MI2 is the key enzyme of 4,4’-dithiodibutyric acid degradation. Lett. Appl. Microbiol. 2016, 63, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Pei, X.Q.; Xu, M.Y.; Wu, Z.L. Two “classical” old yellow enzymes from Chryseobacterium sp. CA49: Broad substrate specificity of Chr-OYE1 and limited activity of Chr-OYE2. J. Mol. Catal. B Enzym. 2016, 123, 91–99. [Google Scholar] [CrossRef]
- Xu, M.Y.; Pei, X.Q.; Wu, Z.L. Identification and characterization of a novel “thermophilic-like” old yellow enzyme from the genome of Chryseobacterium sp. CA49. J. Mol. Catal. B Enzym. 2014, 108, 64–71. [Google Scholar] [CrossRef]
- Kitzing, K.; Fitzpatrick, T.B.; Wilken, C.; Sawa, J.; Bourenkov, G.P.; Macheroux, P.; Clausen, T. The 1.3 Å crystal structure of the flavoprotein YqjM reveals a novel class of old yellow enzymes. J. Biol. Chem. 2005, 280, 27904–27913. [Google Scholar] [CrossRef] [PubMed]
- Schittmayer, M.; Glieder, A.; Uhl, M.K.; Winkler, A.; Zach, S.; Schrittwieser, J.H.; Kroutil, W.; Macheroux, P.; Gruber, K.; Kambourakis, S.; et al. Old yellow enzyme-catalyzed dehydrogenation of saturated ketones. Adv. Synth. Catal. 2011, 353, 268–274. [Google Scholar] [CrossRef]
- Adalbjörnsson, B.V.; Toogood, H.S.; Fryszkowska, A.; Pudney, C.R.; Jowitt, T.A.; Leys, D.; Scrutton, N.S. Biocatalysis with thermostable enzymes: Structure and properties of a thermophilic ‘ene’-reductase related to old yellow enzyme. ChemBioChem 2010, 11, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Opperman, D.J.; Sewell, B.T.; Litthauer, D.; Isupov, M.N.; Littlechild, J.A.; van Heerden, E. Crystal structure of a thermostable old yellow enzyme from Thermus scotoductus SA-01. Biochem. Biophys. Res. Commun. 2010, 393, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Opperman, D.J.; Piater, L.A.; van Heerden, E. A novel chromate reductase from Thermus scotoductus SA-01 related to old yellow enzyme. J. Bacteriol. 2008, 190, 3076–3082. [Google Scholar] [CrossRef] [PubMed]
- Litthauer, S.; Gargiulo, S.; van Heerden, E.; Hollmann, F.; Opperman, D.J. Heterologous expression and characterization of the ene-reductases from Deinococcus radiodurans and Ralstonia metallidurans. J. Mol. Catal. B Enzym. 2014, 99, 89–95. [Google Scholar] [CrossRef]
- Fu, Y.L.; Castiglione, K.; Weuster-Botz, D. Comparative characterization of novel ene-reductases from cyanobacteria. Biotechnol. Bioeng. 2013, 110, 1293–1301. [Google Scholar] [CrossRef] [PubMed]
- Warburg, O.; Christian, W. Yellow enzyme and its effects. Biochem. Z. 1933, 266, 377–411. [Google Scholar]
- Fox, K.M.; Karplus, P.A. Old yellow enzyme at 2 Å resolution: Overall structure, ligand-binding, and comparison with related flavoproteins. Structure 1994, 2, 1089–1105. [Google Scholar] [CrossRef]
- Müller, A.; Hauer, B.; Rosche, B. Asymmetric alkene reduction by yeast old yellow enzymes and by a novel Zymomonas mobilis reductase. Biotechnol. Bioeng. 2007, 98, 22–29. [Google Scholar] [CrossRef] [PubMed]
- French, C.E.; Bruce, N.C. Purification and characterization of morphinone reductase from Pseudomonas putida M10. Biochem. J. 1994, 301, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Haas, E. Isolation of a new yellow enzyme. Biochem. Z. 1938, 298, 378–390. [Google Scholar]
- Stott, K.; Saito, K.; Thiele, D.J.; Massey, V. Old yellow enzyme-the discovery of multiple isozymes and a family of related proteins. J. Biol. Chem. 1993, 268, 6097–6106. [Google Scholar] [PubMed]
- Niino, Y.S.; Chakraborty, S.; Brown, B.J.; Massey, V. A new old yellow enzyme of Saccharomyces cerevisiae. J. Biol. Chem. 1995, 270, 1983–1991. [Google Scholar] [PubMed]
- Madani, N.D.; Malloy, P.J.; Rodriguezpombo, P.; Krishnan, A.V.; Feldman, D. Candida albicans estrogen-binding protein gene encodes an oxidoreductase that is inhibited by estradiol. Proc. Natl. Acad. Sci. USA 1994, 91, 922–926. [Google Scholar] [CrossRef] [PubMed]
- Komduur, J.A.; Leao, A.N.; Monastyrska, I.; Veenhuis, M.; Kiel, J.A.K.W. Old yellow enzyme confers resistance of Hansenula polymorpha towards allyl alcohol. Curr. Genet. 2002, 41, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, M.; Kotaka, A.; Thiwthong, R.; Wada, M.; Nakamori, S.; Shimizu, S. Cloning and overexpression of the old yellow enzyme gene of Candida macedoniensis, and its application to the production of a chiral compound. J. Biotechnol. 2004, 114, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.; Ramirez, J.; Guevara, S.; Ongaylarios, L.; Pena, A.; Coria, R. Nucleotide-sequence and chromosomal localization of the gene encoding the old yellow enzyme from Kluyveromyces lactis. Yeast 1995, 11, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Padhi, S.K.; Bougioukou, D.J.; Stewart, J.D. Site-saturation mutagenesis of tryptophan 116 of Saccharomyces pastorianus old yellow enzyme uncovers stereocomplementary variants. J. Am. Chem. Soc. 2009, 131, 3271–3280. [Google Scholar] [CrossRef] [PubMed]
- Patterson-Orazem, A.; Sullivan, B.; Stewart, J.D. Pichia stipitis OYE 2.6 variants with improved catalytic efficiencies from site-saturation mutagenesis libraries. Bioorg. Med. Chem. 2014, 22, 5628–5632. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Yu, H.L.; Lin, G.Q.; Xu, J.H. An ene reductase from Clavispora lusitaniae for asymmetric reduction of activated alkenes. Enzyme Microb. Technol. 2014, 56, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.Q.; Zheng, L.D.; Lin, J.P.; Wei, D.Z. Characterization of an ene-reductase from Meyerozyma guilliermondii for asymmetric bioreduction of α,β-unsaturated compounds. Biotechnol. Lett. 2016, 38, 1527–1534. [Google Scholar] [CrossRef] [PubMed]
- Biesgen, C.; Weiler, E.W. Structure and regulation of OPR1 and OPR2, two closely related genes encoding 12-oxophytodienoic acid-10,11-reductases from Arabidopsis thaliana. Planta 1999, 208, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Snape, J.R.; Walkley, N.A.; Morby, A.P.; Nicklin, S.; White, G.F. Purification, properties, and sequence of glycerol trinitrate reductase from Agrobacterium radiobacter. J. Bacteriol. 1997, 179, 7796–7802. [Google Scholar] [CrossRef] [PubMed]
- Richter, N.; Gröger, H.; Hummel, W. Asymmetric reduction of activated alkenes using an enoate reductase from Gluconobacter oxydans. Appl. Microbiol. Biotechnol. 2011, 89, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Miura, K.; Tomioka, Y.; Suzuki, H.; Yonezawa, M.; Hishinuma, T.; Mizugaki, M. Molecular cloning of the nemA gene encoding N-ethylmaleimide reductase from Escherichia coli. Biol. Pharm. Bull. 1997, 20, 110–112. [Google Scholar] [CrossRef] [PubMed]
- Peters, C.; Kolzsch, R.; Kadow, M.; Skalden, L.; Rudroff, F.; Mihovilovic, M.D.; Bornscheuer, U.T. Identification, characterization, and application of three enoate reductases from Pseudomonas putida in in vitro enzyme cascade reactions. ChemCatChem 2014, 6, 1021–1027. [Google Scholar] [CrossRef]
- Blehert, D.S.; Fox, B.G.; Chambliss, G.H. Cloning and sequence analysis of two Pseudomonas flavoprotein xenobiotic reductases. J. Bacteriol. 1999, 181, 6254–6263. [Google Scholar] [PubMed]
- Brigé, A.; van den Hemel, D.; Carpentier, W.; De Smet, L.; Van Beeumen, J.J. Comparative characterization and expression analysis of the four old yellow enzyme homologues from Shewanella oneidensis indicate differences in physiological function. Biochem. J. 2006, 394, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Sheng, X.Q.; Yan, M.; Xu, L.; Wei, M. Identification and characterization of a novel old yellow enzyme from Bacillus subtilis str.168. J. Mol. Catal. B Enzym. 2016, 130, 18–24. [Google Scholar] [CrossRef]
- Tsuji, N.; Honda, K.; Wada, M.; Okano, K.; Ohtake, H. Isolation and characterization of a thermotolerant ene reductase from Geobacillus sp. 30 and its heterologous expression in Rhodococcus opacus. Appl. Microbiol. Biotechnol. 2014, 98, 5925–5935. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, T.B.; Amrhein, N.; Macheroux, P. Characterization of YqjM, an old yellow enzyme homolog from Bacillus subtilis involved in the oxidative stress response. J. Biol. Chem. 2003, 278, 19891–19897. [Google Scholar] [CrossRef] [PubMed]
- Brooks, D.J.; Fresco, J.R.; Lesk, A.M.; Singh, M. Evolution of amino acid frequencies in proteins over deep time: Inferred order of introduction of amino acids into the genetic code. Mol. Biol. Evol. 2002, 19, 1645–1655. [Google Scholar] [CrossRef] [PubMed]
- Longo, L.M.; Blaber, M. Protein design at the interface of the pre-biotic and biotic worlds. Arch. Biochem. Biophys. 2012, 526, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Barna, T.; Messiha, H.L.; Petosa, C.; Bruce, N.C.; Scrutton, N.S.; Moody, P.C.E. Crystal structure of bacterial morphinone reductase and properties of the C191A mutant enzyme. J. Biol. Chem. 2002, 277, 30976–30983. [Google Scholar] [CrossRef] [PubMed]
- Wierenga, R.K. The TIM-barrel fold: A versatile framework for efficient enzymes. FEBS Lett. 2001, 492, 193–198. [Google Scholar] [CrossRef]
- Barna, T.M.; Khan, H.; Bruce, N.C.; Barsukov, I.; Scrutton, N.S.; Moody, P.C.E. Crystal structure of pentaerythritol tetranitrate reductase: “Flipped” binding geometries for steroid substrates in different redox states of the enzyme. J. Mol. Biol. 2001, 310, 433–447. [Google Scholar] [CrossRef] [PubMed]
- Spiegelhauer, O.; Werther, T.; Mende, S.; Knauer, S.H.; Dobbek, H. Determinants of substrate binding and protonation in the flavoenzyme Xenobiotic reductase A. J. Mol. Biol. 2010, 403, 286–298. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Kohli, R.M.; Massey, V. The role of threonine 37 in flavin reactivity of the old yellow enzyme. Proc. Natl. Acad. Sci. USA 1999, 96, 3556–3561. [Google Scholar] [CrossRef] [PubMed]
- Messiha, H.L.; Bruce, N.C.; Sattelle, B.M.; Sutcliffe, M.J.; Munro, A.W.; Scrutton, N.S. Role of active site residues and solvent in proton transfer and the modulation of flavin reduction potential in bacterial morphinone reductase. J. Biol. Chem. 2005, 280, 27103–27110. [Google Scholar] [CrossRef] [PubMed]
- Spiegelhauer, O.; Dickert, F.; Mende, S.; Niks, D.; Hille, R.; Ullmann, M.; Dobbek, H. Kinetic characterization of Xenobiotic reductase A from Pseudomonas putida 86. Biochemistry 2009, 48, 11412–11420. [Google Scholar] [CrossRef] [PubMed]
- Pudney, C.R.; Hay, S.; Pang, J.Y.; Costello, C.; Leys, D.; Sutcliffe, M.J.; Scrutton, N.S. Mutagenesis of morphinone reductase induces multiple reactive configurations and identifies potential ambiguity in kinetic analysis of enzyme tunneling mechanisms. J. Am. Chem. Soc. 2007, 129, 13949–13956. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.E.; Rathbone, D.A.; Scrutton, N.S.; Bruce, N.C. Biotransformation of explosives by the old yellow enzyme family of flavoproteins. Appl. Environ. Microbiol. 2004, 70, 3566–3574. [Google Scholar] [CrossRef] [PubMed]
- Reich, S.; Nestl, B.M.; Hauer, B. Loop-grafted old yellow enzymes in the bienzymatic cascade reduction of allylic alcohols. ChemBioChem 2016, 17, 561–565. [Google Scholar] [CrossRef] [PubMed]
- Nestl, B.M.; Hauer, B. Engineering of flexible loops in enzymes. ACS Catal. 2014, 4, 3201–3211. [Google Scholar] [CrossRef]
- Reich, S.; Kress, N.; Nestl, B.M.; Hauer, B. Variations in the stability of NCR ene reductase by rational enzyme loop modulation. J. Struct. Biol. 2014, 185, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Basran, J.; Harris, R.J.; Sutcliffe, M.J.; Scrutton, N.S. H-tunneling in the multiple H-transfers of the catalytic cycle of morphinone reductase and in the reductive half-reaction of the homologous pentaerythritol tetranitrate reductase. J. Biol. Chem. 2003, 278, 43973–43982. [Google Scholar] [CrossRef] [PubMed]
- Pudney, C.R.; Hay, S.; Sutcliffe, M.J.; Scrutton, N.S. α-Secondary isotope effects as probes of “tunneling-ready” configurations in enzymatic H-tunneling: Insight from environmentally coupled tunneling models. J. Am. Chem. Soc. 2006, 128, 14053–14058. [Google Scholar] [CrossRef] [PubMed]
- Pudney, C.R.; Hay, S.; Levy, C.; Pang, J.Y.; Sutcliffe, M.J.; Leys, D.; Scrutton, N.S. Evidence to support the hypothesis that promoting vibrations enhance the rate of an enzyme catalyzed H-tunneling reaction. J. Am. Chem. Soc. 2009, 131, 17072–17073. [Google Scholar] [CrossRef] [PubMed]
- Brenna, E.; Gatti, F.G.; Monti, D.; Parmeggiani, F.; Serra, S. Stereochemical outcome of the biocatalysed reduction of activated tetrasubstituted olefins by old yellow enzymes 1–3. Adv. Synth. Catal. 2012, 354, 105–112. [Google Scholar] [CrossRef]
- Oberdorfer, G.; Steinkellner, G.; Stueckler, C.; Faber, K.; Gruber, K. Stereopreferences of old yellow enzymes: Structure correlations and sequence patterns in enoate reductases. ChemCatChem 2011, 3, 1562–1566. [Google Scholar] [CrossRef]
- Classen, T.; Pietruszka, J.; Schuback, S.M. Revisiting the enantioselective sequence patterns in enoate reductases. ChemCatChem 2013, 5, 711–713. [Google Scholar] [CrossRef]
- Pompeu, Y.A.; Sullivan, B.; Stewart, J.D. X-ray crystallography reveals how subtle changes control the orientation of substrate binding in an alkene reductase. ACS Catal. 2013, 3, 2376–2390. [Google Scholar] [CrossRef]
- Walton, A.Z.; Sullivan, B.; Patterson-Orazem, A.C.; Stewart, J.D. Residues controlling facial selectivity in an alkene reductase and semirational alterations to create stereocomplementary variants. ACS Catal. 2014, 4, 2307–2318. [Google Scholar] [CrossRef] [PubMed]
- Walton, A.Z.; Conerly, W.C.; Pompeu, Y.; Sullivan, B.; Stewart, J.D. Biocatalytic reductions of Baylis-Hillman adducts. ACS Catal. 2011, 1, 989–993. [Google Scholar] [CrossRef]
- Amato, E.D.; Stewart, J.D. Applications of protein engineering to members of the old yellow enzyme family. Biotechnol. Adv. 2015, 33, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Bougioukou, D.J.; Kille, S.; Taglieber, A.; Reetz, M.T. Directed evolution of an enantioselective enoate-reductase: Testing the utility of iterative saturation mutagenesis. Adv. Synth. Catal. 2009, 351, 3287–3305. [Google Scholar] [CrossRef]


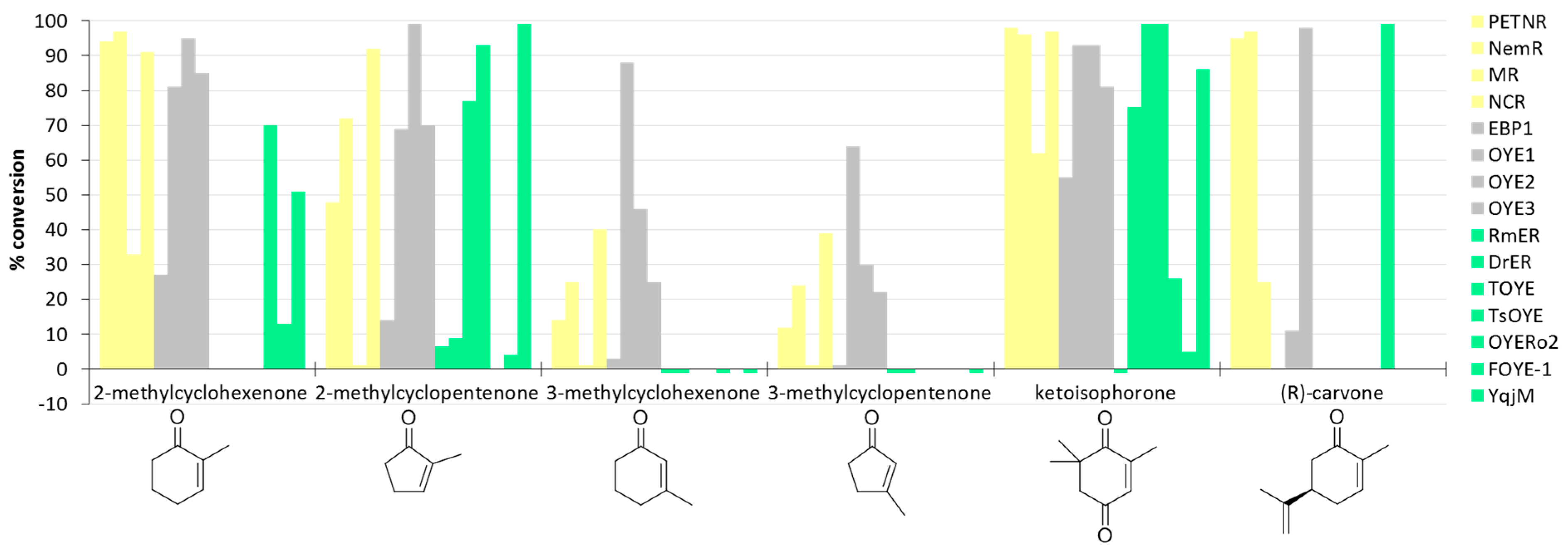
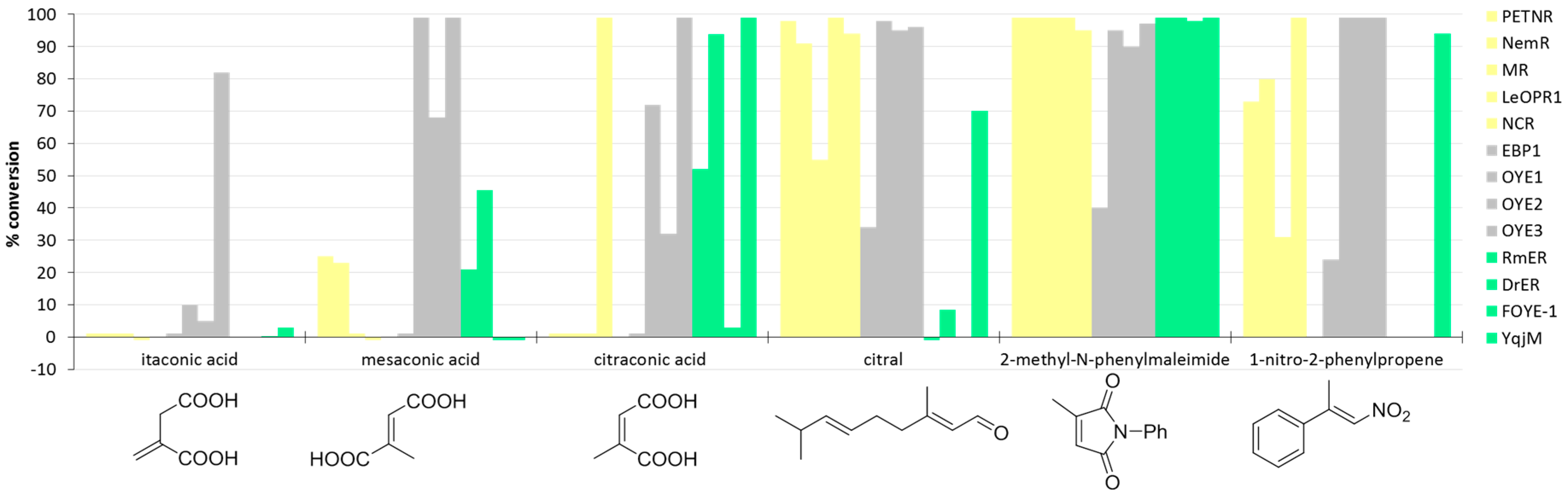
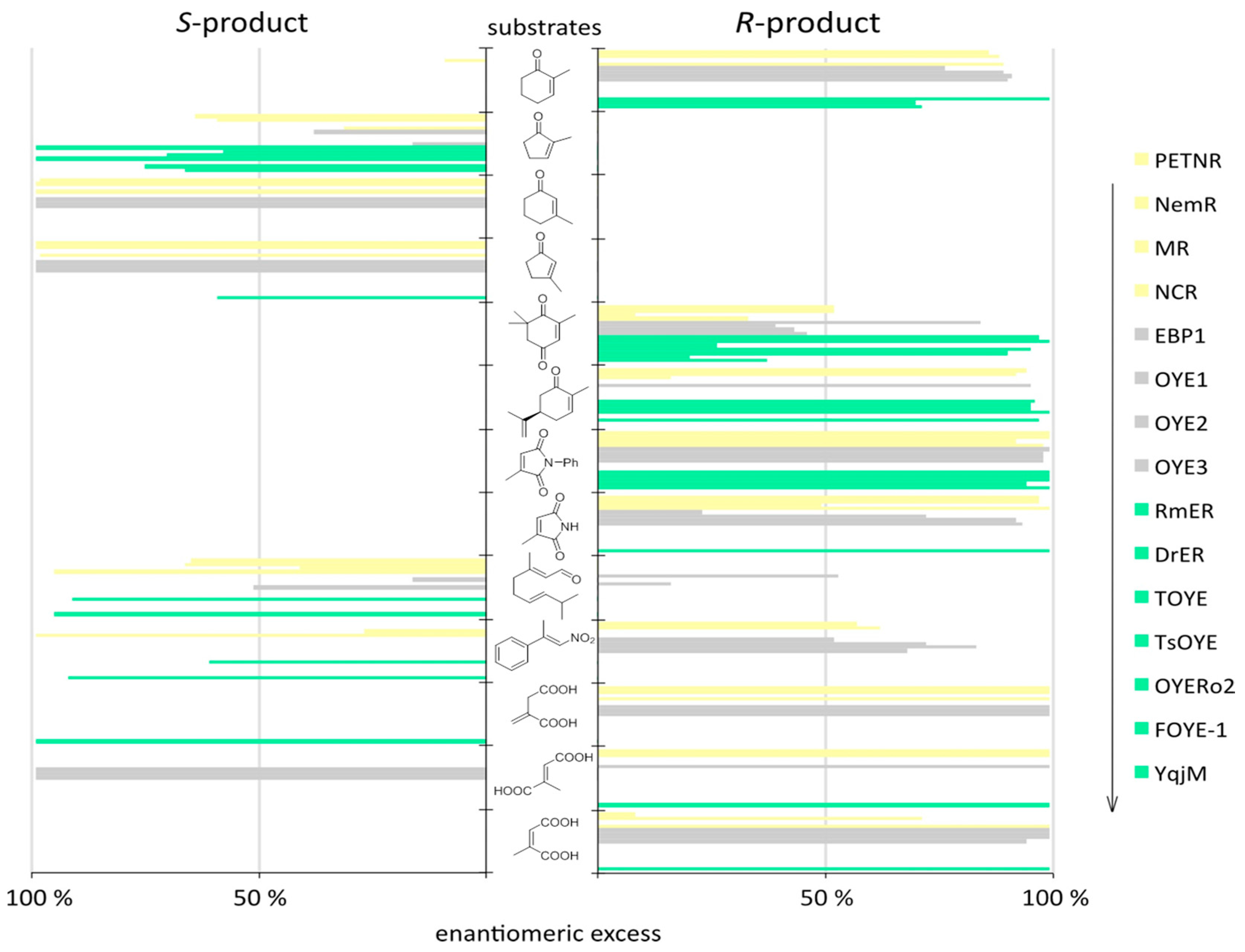
| Kingdom | Enzyme (Accession Number) | Source | Reference(s) |
|---|---|---|---|
| Fungi | OYE1 (CAA37666) | Saccharomyces pastorianus | [61] |
| OYE2 (AAA83386) | Saccharomyces cerevisiae | [87] | |
| OYE3 (AAA64522) | Saccharomyces cerevisiae | [88] | |
| EBP1 (AAA18013) | Candida albicans | [89] | |
| HYE1 (AAN09952) | Ogataea angusta | [90] | |
| HYE2 (AAN09953) | Ogataea angusta | [90] | |
| CYE (BAD24850) | Kluyveromyces marxianus | [62,91] | |
| KYE1 (AAA98815) | Kluyveromyces lactis | [6,92] | |
| OYE2.6 (ABN66026) | Scheffersomyces stipitis CBS 6054 | [93,94] | |
| ArOYE1–3 (AHL17019, AHL1720, AHL17021) | Ascochyta rabiei | [64] | |
| ClER (EEQ40235) | Clavispora lusitaniae ATCC 42720 | [95] | |
| MgER (EDK41665) | Meyerozyma guilliermondii ATCC 6260 | [96] | |
| Plants | LeOPR1 (NP_001234781) | Solanum lycopersicum | [68] |
| LeOPR2 (NP_001233868) | Solanum lycopersicum | [67] | |
| LeOPR3 (NP_001233873) | Solanum lycopersicum | [67] | |
| AtOPR1 (NP_177794) | Arabidopsis thaliana | [65] | |
| AtOPR2 (NP_177795) | Arabidopsis thaliana | [97] | |
| AtOPR3 (NP_001077884) | Arabidopsis thaliana | [66] |
| Group/Order | Enzyme (Ncbi Accession) | Source | Reference(s) |
|---|---|---|---|
| Proteobacteria/α-Proteobacteria | NerA/GTNR (CAA74280) | Agrobacterium radiobacter | [98] |
| NCR (AAV90509) | Zymomonas mobilis | [84] | |
| GluER (AAW60280) | Gluconobacter oxidans DSM 2343 | [99] | |
| Proteobacteria/β-Proteobacteria | FOYE-1 (KRH78075) | Ferrovum sp. JA12 | [23] |
| RmER (ABF11721) | Cupriavidus metallidurans CH34 | [80] | |
| Achr-OYE3 (AFK73187) | Achromobacter sp. JA81 | [16] | |
| Achr-OYE4 (AFK73188) | Achromobacter sp. JA81 | [16,17] | |
| Proteobacteria/γ-Proteobacteria | MR (AAC43569) | Pseudomonas putida M10 | [85] |
| PETNR (AAB38683) | Enterobacter cloacae PB2 | [69] | |
| NemR/NemA (BAA13186) | Escherichia coli | [100] | |
| NemA2 (AHC69715) | Pseudomonas putida ATCC 17453 | [101] | |
| XenA (AAF02538) | Pseudomonas putida II-B | [102] | |
| XenA2 (AHH54488) | Pseudomonas putida ATCC 17453 | [101] | |
| XenB (AAF02539) | Pseudomonas fluorescens I-C | [102] | |
| XenB2 (AGS77941) | Pseudomonas putida ATCC 17453 | [101] | |
| YersER (WP_032896199) | Yersinia bercovieri | [6] | |
| SYE1 (AAN55488) | Shewanella oneidensis | [103] | |
| SYE3 (AAN57126) | Shewanella oneidensis | [103] | |
| SYE4 (AAN56390) | Shewanella oneidensis | [103] | |
| Actinobacteria | OYERo2 (ALL54975) | Rhodococcus opacus 1CP | [22] |
| Nox (ALG03744) | Rhodococcus erythropolis | [72] | |
| PfvC (AFF18622) | Arthrobacter sp. JBH1 | [71] | |
| Bacteroidetes/Flavobacteria | Chr-OYE1 (ALE60336) | Chryseobacterium sp. CA49 | [73] |
| Chr-OYE2 (ALE60337) | Chryseobacterium sp. CA49 | [73] | |
| Chr-OYE3 (AHV90721) | Chryseobacterium sp. CA49 | [74] | |
| Firmicutes/(Bacilli, Clostridia) | YqjM (BAA12619) | Bacillus subtilis strain 168 | [75] |
| YqiG (BAA12582) | Bacillus subtilis strain 168 | [104] | |
| GkOYE (BAD76617) | Geobacillus kaustophilus DSM7263 | [76] | |
| GeoER (BAO37313) | Geobacillus sp. 30 | [105] | |
| LacER (ADK19581) | Lactobacillus casei str. Zhang | [10] | |
| TOYE (ABY93685) | Thermoanaerobacter pseudethanolicus E39 | [77] | |
| Deinococcus-Thermus | TsOYE (CAP16804) | Thermus scotoductus SA-01 | [79] |
| DrER (AAF11740) | Deinococcus radiodurans R1 | [80] | |
| Cyanobacteria/(Gloebacteria, Oscillatoriophycidea, Nostocales) | GloeoER (BAC91769) | Gloeobacter violaceus PCC7421 | [81] |
| CyanothER1 (ACK64210) | Cyanothece sp. PCC 8801 | [81] | |
| CyanothER2 (ACK65723) | Cyanothece sp. PCC 8801 | [81] | |
| LyngbyaER1 (EAW37813) | Lyngbya sp. PCC 8106 | [81] | |
| AcaryoER1 (ABW29811) | Acaryochloris marina MBIC11017 | [81] | |
| AcaryoER3 (ABW32756) | Acaryochloris marina MBIC11017 | [81] | |
| SynER (ABB56505) | Synechococcus elongatus PCC 7942 | [21] | |
| NospuncER1 (ACC84535) | Nostoc punctiforme PCC 73102 | [81] | |
| NostocER1 (BAB73564) | Nostoc sp. PCC 7120 | [81] | |
| AnabaenaER3 (ABA25236) | Anabaena variabilis ATCC 29413 | [81] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scholtissek, A.; Tischler, D.; Westphal, A.H.; Van Berkel, W.J.H.; Paul, C.E. Old Yellow Enzyme-Catalysed Asymmetric Hydrogenation: Linking Family Roots with Improved Catalysis. Catalysts 2017, 7, 130. https://doi.org/10.3390/catal7050130
Scholtissek A, Tischler D, Westphal AH, Van Berkel WJH, Paul CE. Old Yellow Enzyme-Catalysed Asymmetric Hydrogenation: Linking Family Roots with Improved Catalysis. Catalysts. 2017; 7(5):130. https://doi.org/10.3390/catal7050130
Chicago/Turabian StyleScholtissek, Anika, Dirk Tischler, Adrie H. Westphal, Willem J. H. Van Berkel, and Caroline E. Paul. 2017. "Old Yellow Enzyme-Catalysed Asymmetric Hydrogenation: Linking Family Roots with Improved Catalysis" Catalysts 7, no. 5: 130. https://doi.org/10.3390/catal7050130