Caprolactam-Based Brønsted Acidic Ionic Liquids for Biodiesel Production from Jatropha Oil
Abstract
:1. Introduction
2. Results and Discussion
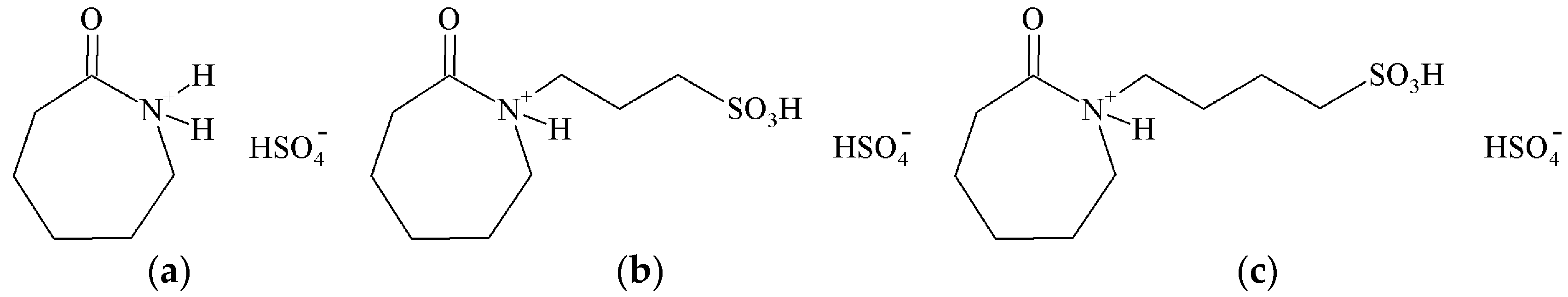
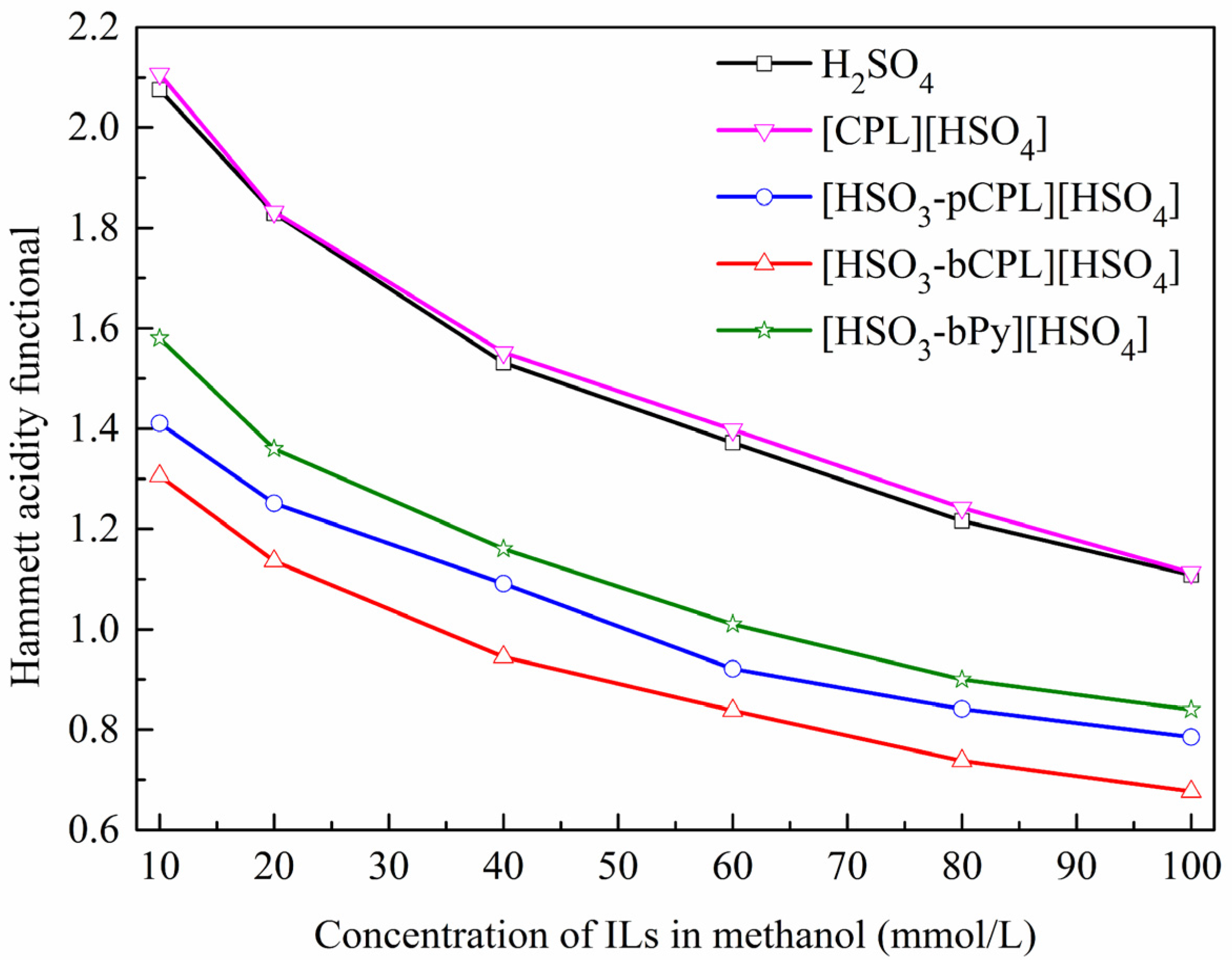
2.1. Acidity Determination of the ILs
2.2. Catalytic Test of the ILs
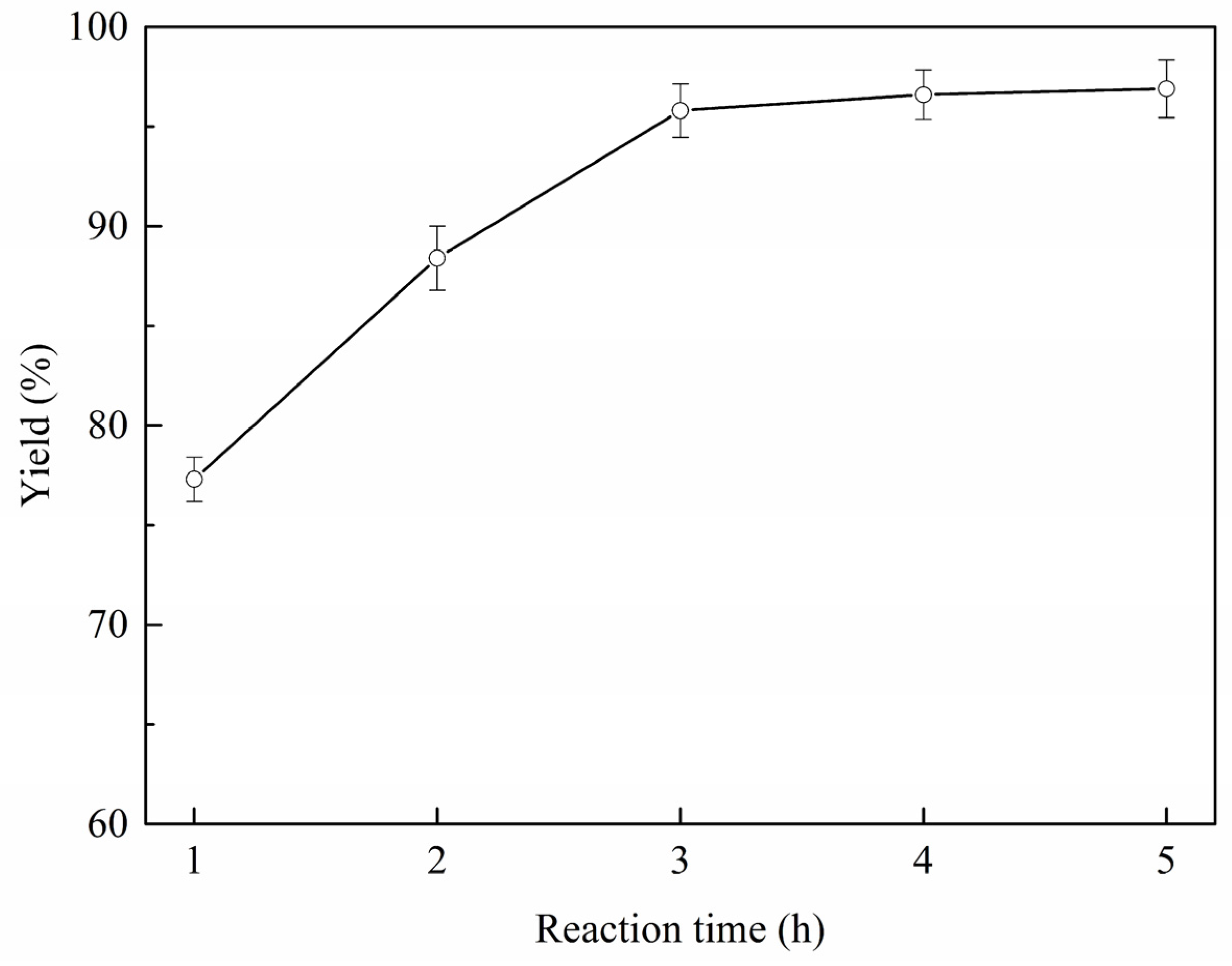
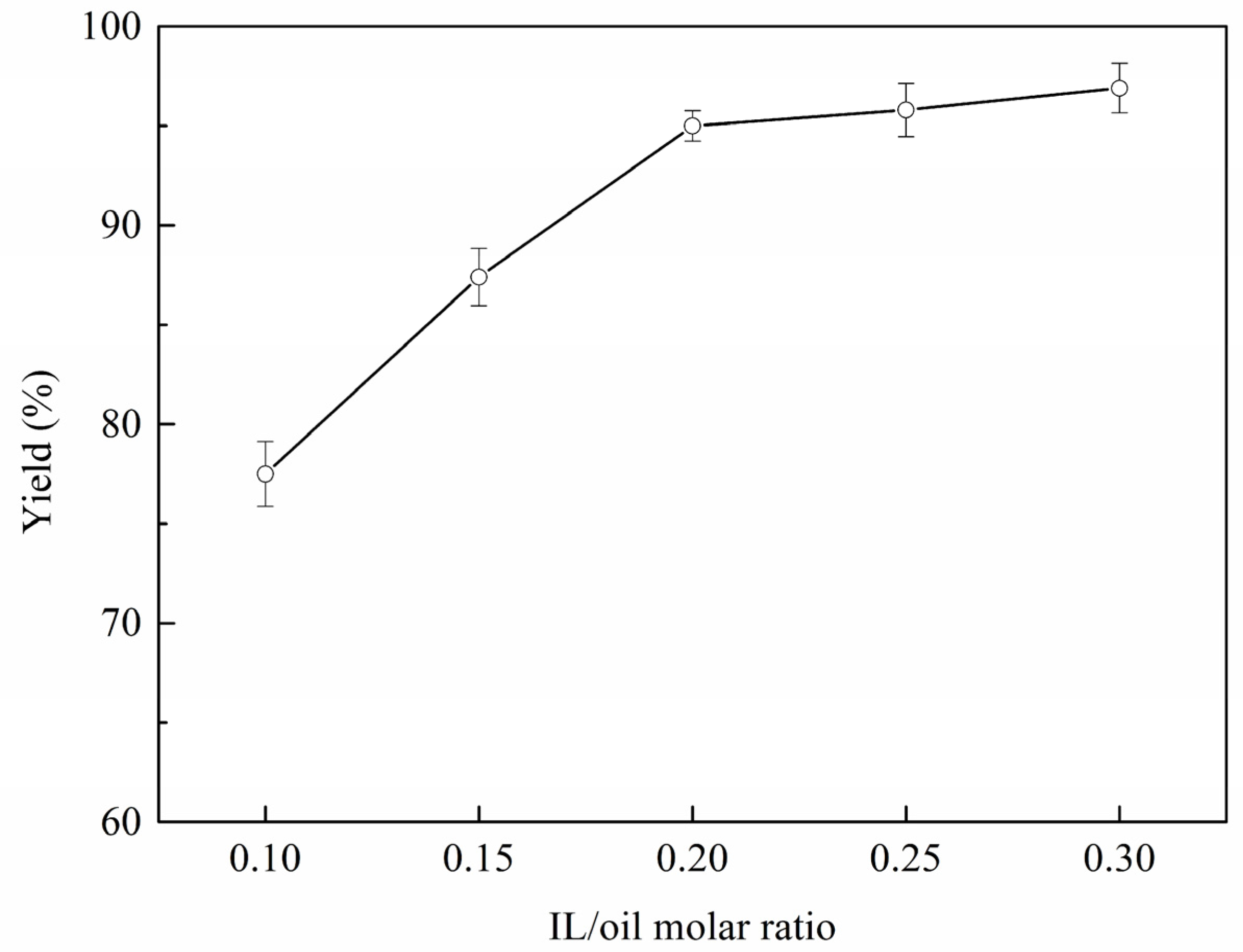
2.3. Reaction Parameter Analysis
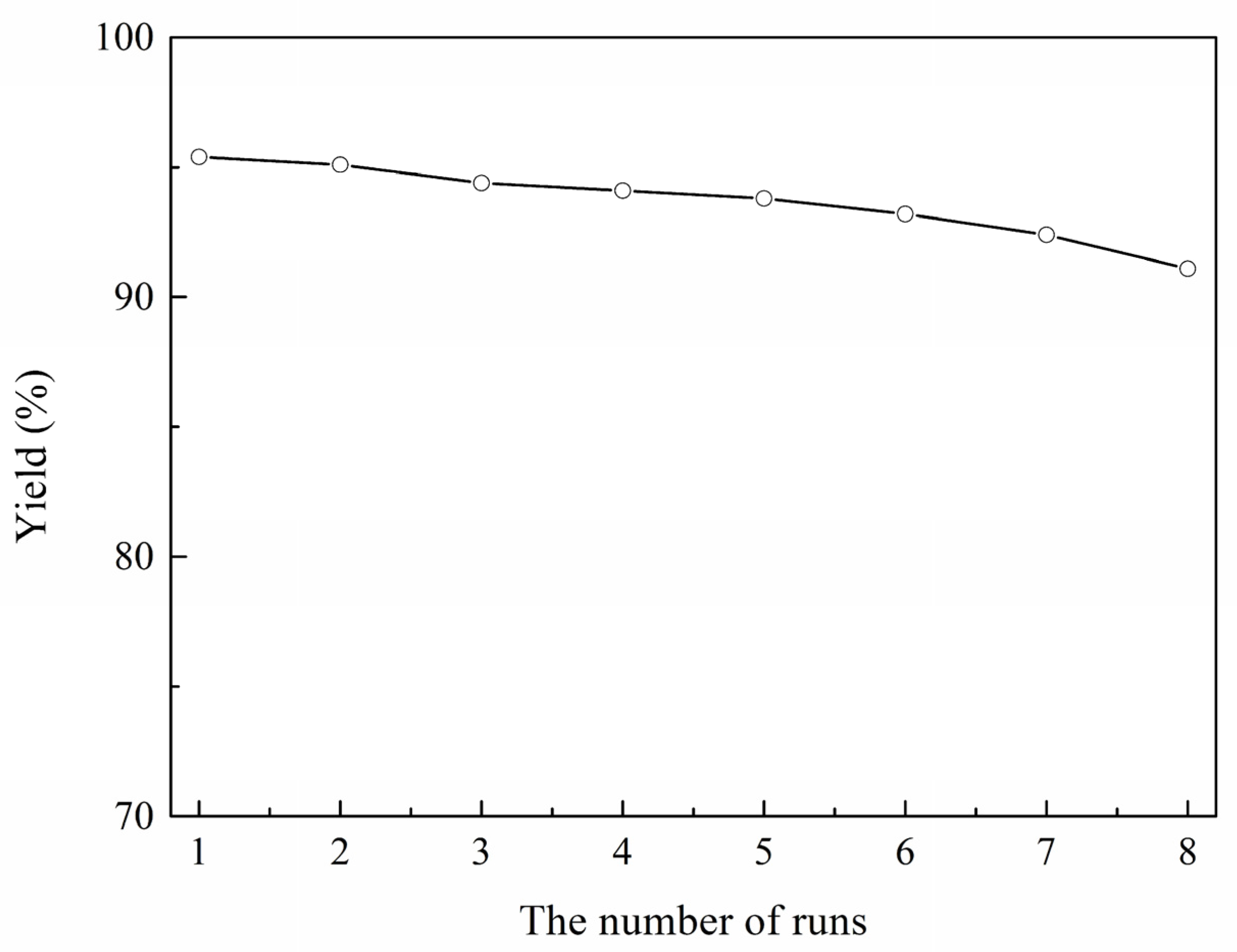
2.4. Reusability of IL [HSO3-bCPL][HSO4]
3. Materials and Methods
3.1. Materials
3.2. Preparation of Caprolactam-Based ILs
3.3. Determination of the Hammett Acidity function
3.4. Biodiesel Production
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ma, F.; Hanna, M.A. Biodiesel production: A review. Bioresour. Technol. 1999, 70, 1–15. [Google Scholar] [CrossRef]
- Sazzad, B.S.; Fazal, M.A.; Haseeb, A.S.M.A.; Masjukia, H.H. Retardation of oxidation and material degradation in biodiesel: A review. RSC Adv. 2016, 6, 60244–60263. [Google Scholar] [CrossRef]
- Vicente, G.; Martinez, M.; Aracil, J. Integrated biodiesel production: A comparison of different homogeneous catalysts systems. Bioresour. Technol. 2004, 92, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Qiu, F.; Li, Y.; Yang, D.; Li, X.; Sun, P. Biodiesel production from mixed soybean oil and rapeseed oil. Appl. Energy 2011, 88, 2050–2055. [Google Scholar] [CrossRef]
- Thanh, L.T.; Okitsu, K.; Boi, L.V.; Maeda, Y. Catalytic technologies for biodiesel fuel production and utilization of glycerol: A review. Catalysts 2012, 2, 191–222. [Google Scholar] [CrossRef]
- Lee, A.F.; Bennett, J.A.; Manayil, J.C.; Wilson, K. Heterogeneous catalysis for sustainable biodiesel production via esterification and transesterification. Chem. Soc. Rev. 2014, 43, 7887–7916. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Chen, H.; Han, M.; Wang, D.; Wang, J. Transesterification of Cottonseed Oil Catalyzed by Brønsted Acidic Ionic Liquids. Ind. Eng. Chem. Res. 2007, 46, 7955–7960. [Google Scholar] [CrossRef]
- Han, M.; Yi, W.; Wu, Q.; Liu, Y.; Hong, Y.; Wang, D. Preparation of biodiesel from waste oils catalyzed by a Brønsted acidic ionic liquid. Bioresour. Technol. 2009, 100, 2308–2310. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Chen, L.; Yan, Z.; Wang, H. Application of Pyridinium Ionic Liquid as a Recyclable Catalyst for Acid-Catalyzed Transesterification of Jatropha Oil. Catal. Lett. 2010, 139, 151–156. [Google Scholar] [CrossRef]
- Ghiaci, M.; Aghabarari, B.; Habibollahi, S.; Gil, A. Highly efficient Brønsted acidic ionic liquid-based catalysts for biodiesel synthesis from vegetable oils. Bioresour. Technol. 2011, 102, 1200–1204. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Peng, X.; Luo, M.; Zhao, C.; Gu, C.; Zu, Y.; Fu, Y. Biodiesel production from Camptotheca acuminata seed oil catalyzed by novel Brönsted–Lewis acidic ionic liquid. Appl. Energy 2014, 115, 438–444. [Google Scholar] [CrossRef]
- Olah, G.A.; Mathew, T.; Goeppert, A.; Török, B.; Bucsi, I.; Li, X.; Wang, Q.; Marinez, E.R.; Batamack, P.; Aniszfeld, R.; et al. Ionic Liquid and Solid HF Equivalent Amine-Poly(Hydrogen Fluoride) Complexes Effecting Efficient Environmentally Friendly Isobutane−Isobutylene Alkylation. J. Am. Chem. Soc. 2005, 127, 5964–5969. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhao, W.; Li, H.; Guo, L. Solvent-free synthesis of unsaturated ketones by the Saucy–Marbet reaction using simple ammonium ionic liquid as a catalyst. Green Chem. 2009, 11, 843–847. [Google Scholar] [CrossRef]
- Chhotaray, P.K.; Jella, S.; Gardas, R.L. Physicochemical properties of low viscous lactam based ionic liquids. J. Chem. Thermodyn. 2014, 74, 255–262. [Google Scholar] [CrossRef]
- Du, Z.; Li, Z.; Guo, S.; Zhang, J.; Zhu, L.; Deng, Y. Investigation of Physicochemical Properties of Lactam-Based Brønsted Acidic Ionic Liquids. J. Phys. Chem. B 2005, 109, 19542–19546. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Cheng, G.; Lu, C.; Qian, D. Nitration of Simple Aromatics with NO2 under Air Atmosphere in the Presence of Novel Brønsted Acidic Ionic Liquids. Synth. Commun. 2008, 38, 537–545. [Google Scholar] [CrossRef]
- Zhang, C.; Yu, M.J.; Pan, X.Y.; Wu, G.; Jin, L.; Gao, W.; Du, M.; Zhang, J. Regioselective mononitration of chlorobenzene using caprolactam-based Brønsted acidic ionic liquids. J. Mol. Catal. A Chem. 2014, 383, 101–105. [Google Scholar] [CrossRef]
- Huang, P.; Gu, A.; Wang, J. Efficient production of 5-hydroxymethylfurfural through the dehydration of sugars with caprolactam hydrogen sulfate ([CPL] HSO4) ionic liquid catalyst in a water/proprylene glycol monomethyl ether mixed solvent. Res. Chem. Intermed. 2015, 41, 5311–5321. [Google Scholar] [CrossRef]
- Luo, H.; Xue, K.; Fan, W.; Li, C.; Nan, G.; Li, Z. Hydrolysis of Vegetable Oils to Fatty Acids Using Brønsted Acidic Ionic Liquids as Catalysts. Ind. Eng. Chem. Res. 2014, 53, 11653–11658. [Google Scholar] [CrossRef]
- Thomazeau, C.; Olivier-Bourbigou, H.; Magna, L.; Luts, S.; Gilbert, B. Determination of an Acidic Scale in Room Temperature Ionic Liquids. J. Am. Chem. Soc. 2003, 125, 5264–5265. [Google Scholar] [CrossRef] [PubMed]
- Duan, Z.; Gu, Y.; Zhang, J.; Zhu, L.; Deng, Y. Protic pyridinium ionic liquids: Synthesis, acidity determination and their performances for acid catalysis. J. Mol. Catal. A Chem. 2006, 250, 163–168. [Google Scholar] [CrossRef]
- Zhao, Y.; Long, J.; Deng, F.; Liu, X.; Li, Z.; Xia, C.; Peng, J. Catalytic amounts of Brønsted acidic ionic liquids promoted esterification: Study of acidity–activity relationship. Catal. Commun. 2009, 10, 732–736. [Google Scholar] [CrossRef]
- D’Anna, F.; La Marca, S.; Noto, R. p-Nitrophenolate: A Probe for Determining Acid Strength in Ionic Liquids. J. Org. Chem. 2009, 74, 1952–1956. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.K.; Kumar, A. Do protic ionic liquids and water display analogous behavior in terms of Hammett acidity function. Chem. Phys. Lett. 2013, 566, 12–16. [Google Scholar] [CrossRef]
- Maçaira, J.; Santana, A.; Recasens, F.; Larrayoz, M.A. Biodiesel production using supercritical methanol/carbon dioxide mixtures in a continuous reactor. Fuel 2011, 90, 2280–2288. [Google Scholar] [CrossRef]
- Zaramello, L.; Kuhnen, C.A.; Dall’Oglio, E.L.; de Sousa, P.T. DFT study of gas phase acid-catalyzed ethanolysis of butyric acid triglyceride. Fuel 2012, 94, 473–479. [Google Scholar] [CrossRef]
- Da Silva, A.C.H.; Dall’Oglio, E.L.; de Sousa, P.T., Jr.; da Silva, S.C.; Kuhnen, C.A. DFT study of the acid-catalyzed ethanolysis of butyric acid monoglyceride: Solvent effects. Fuel 2014, 119, 1–5. [Google Scholar] [CrossRef]
- Xing, H.B.; Wang, T.; Zhou, Z.H.; Dai, Y.Y. Novel Brønsted-Acidic Ionic Liquids for Esterifications. Ind. Eng. Chem. Res. 2005, 44, 4147–4150. [Google Scholar] [CrossRef]
- Zabeti, M.; Wan Daud, W.M.A.; Aroua, M.K. Activity of solid catalysts for biodiesel production: A review. Fuel Process. Technol. 2009, 90, 770–777. [Google Scholar] [CrossRef]
- Luo, H.; Fan, W.; Li, Y.; Nan, G. Biodiesel production using alkaline ionic liquid and adopted as lubricity additive for low-sulfur diesel fuel. Bioresour. Technol. 2013, 140, 337–341. [Google Scholar] [CrossRef] [PubMed]


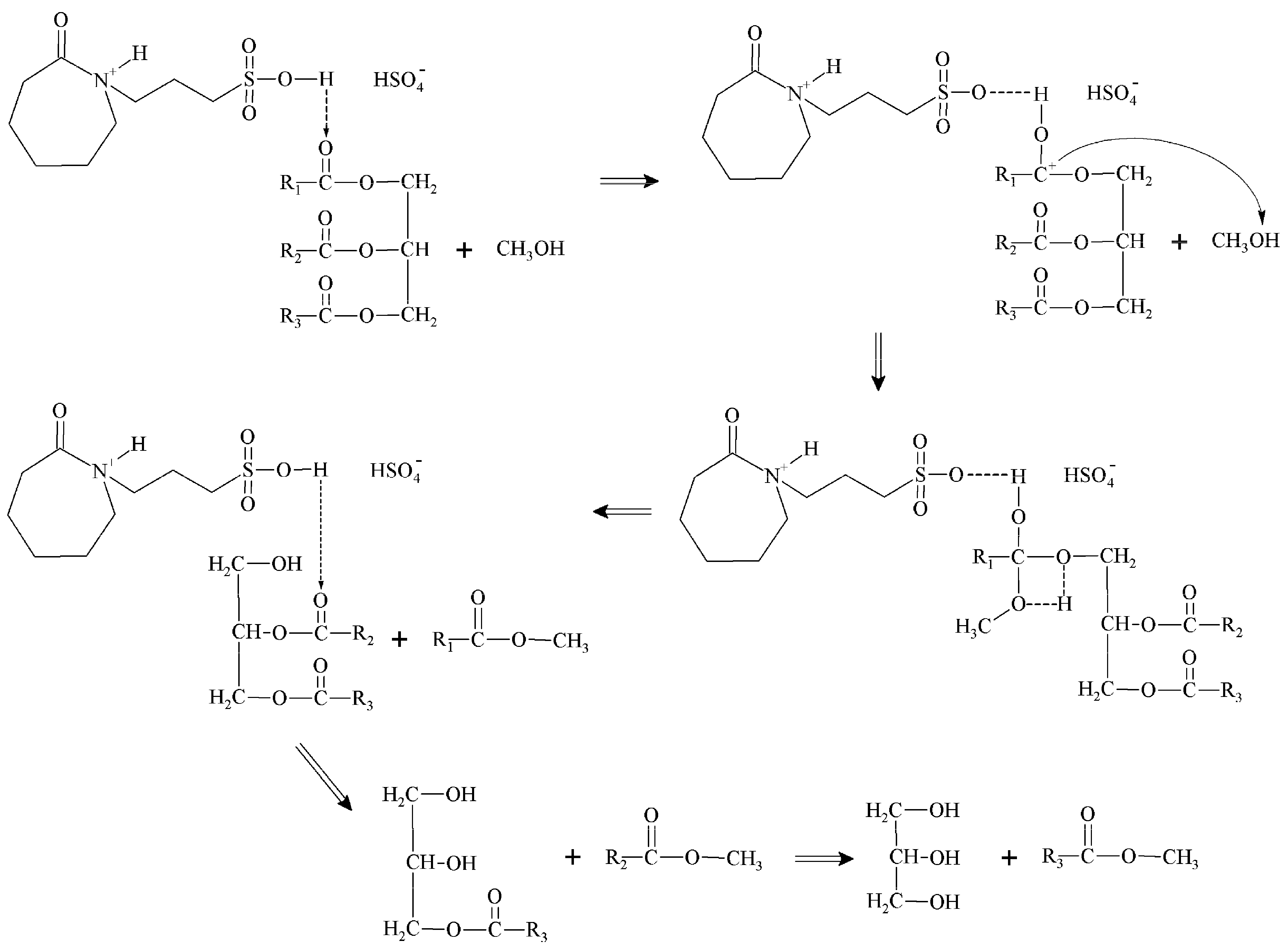


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, H.; Yin, H.; Wang, R.; Fan, W.; Nan, G. Caprolactam-Based Brønsted Acidic Ionic Liquids for Biodiesel Production from Jatropha Oil. Catalysts 2017, 7, 102. https://doi.org/10.3390/catal7040102
Luo H, Yin H, Wang R, Fan W, Nan G. Caprolactam-Based Brønsted Acidic Ionic Liquids for Biodiesel Production from Jatropha Oil. Catalysts. 2017; 7(4):102. https://doi.org/10.3390/catal7040102
Chicago/Turabian StyleLuo, Hui, Heng Yin, Rui Wang, Weiyu Fan, and Guozhi Nan. 2017. "Caprolactam-Based Brønsted Acidic Ionic Liquids for Biodiesel Production from Jatropha Oil" Catalysts 7, no. 4: 102. https://doi.org/10.3390/catal7040102



