Indoor Light Enhanced Photocatalytic Ultra-Thin Films on Flexible Non-Heat Resistant Substrates Reducing Bacterial Infection Risks
Abstract
:1. Introduction
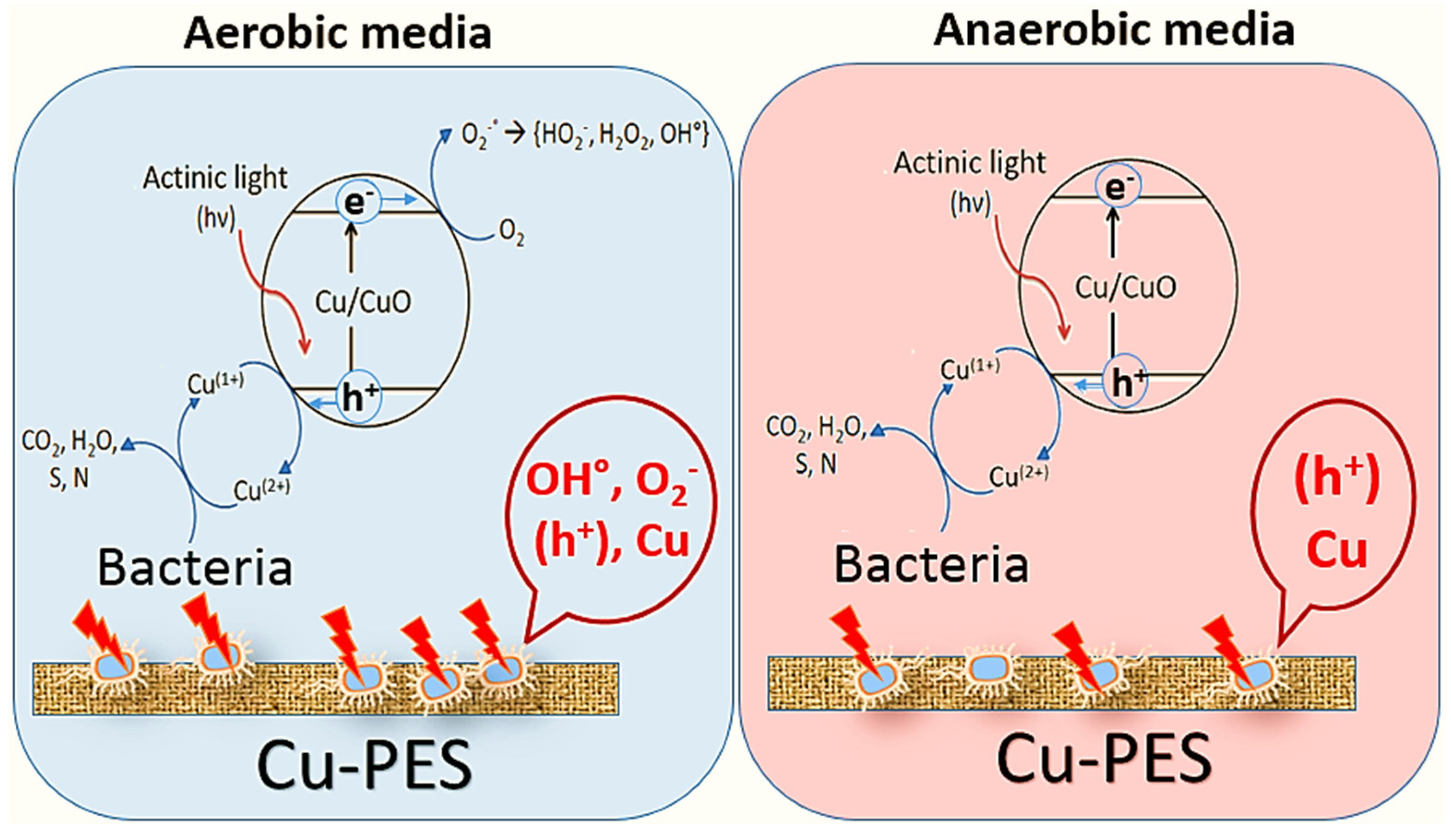
2. Cu-Coated Textiles: Surface Reactivity, Spectral Absorption and Mechanistic Consideration
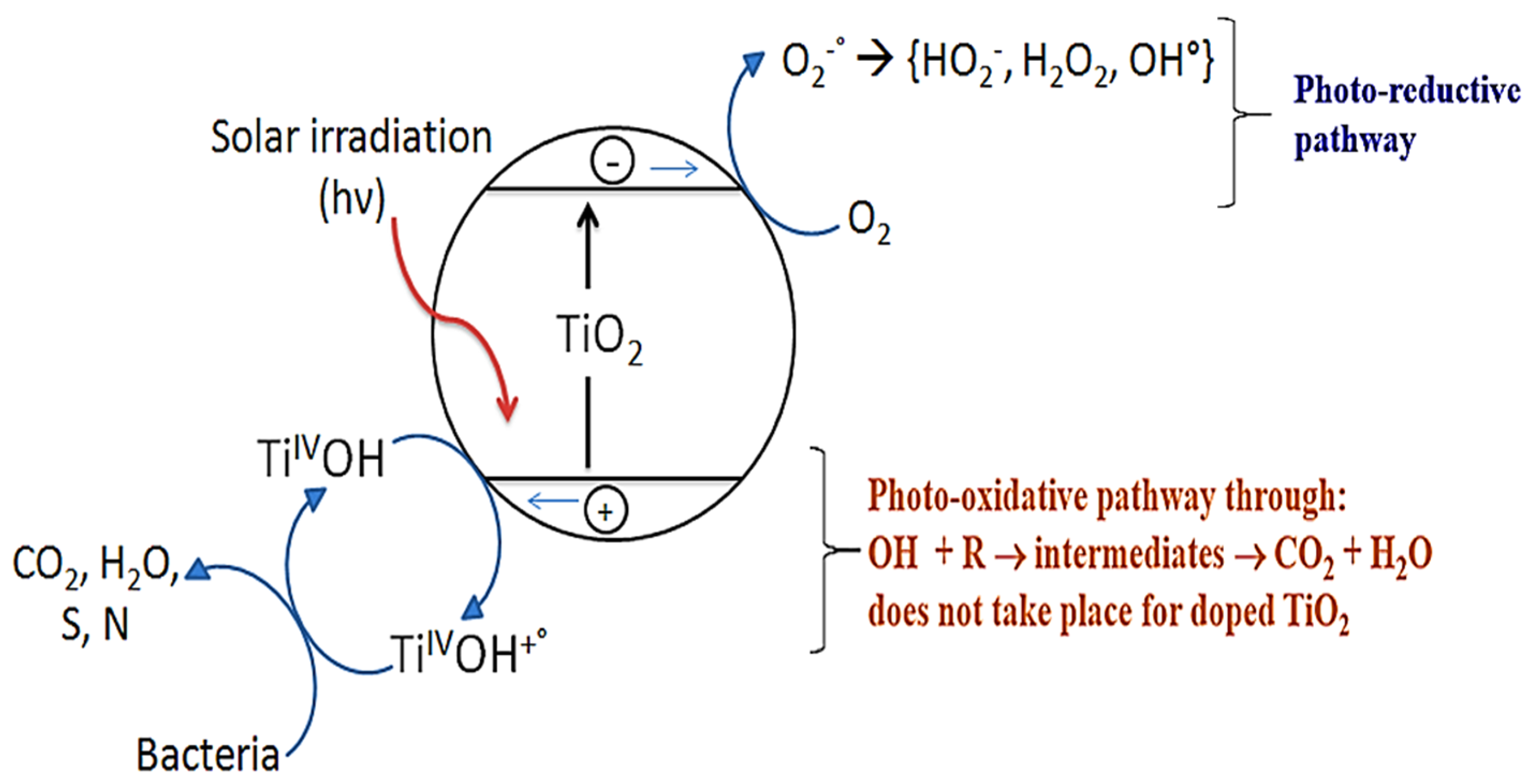
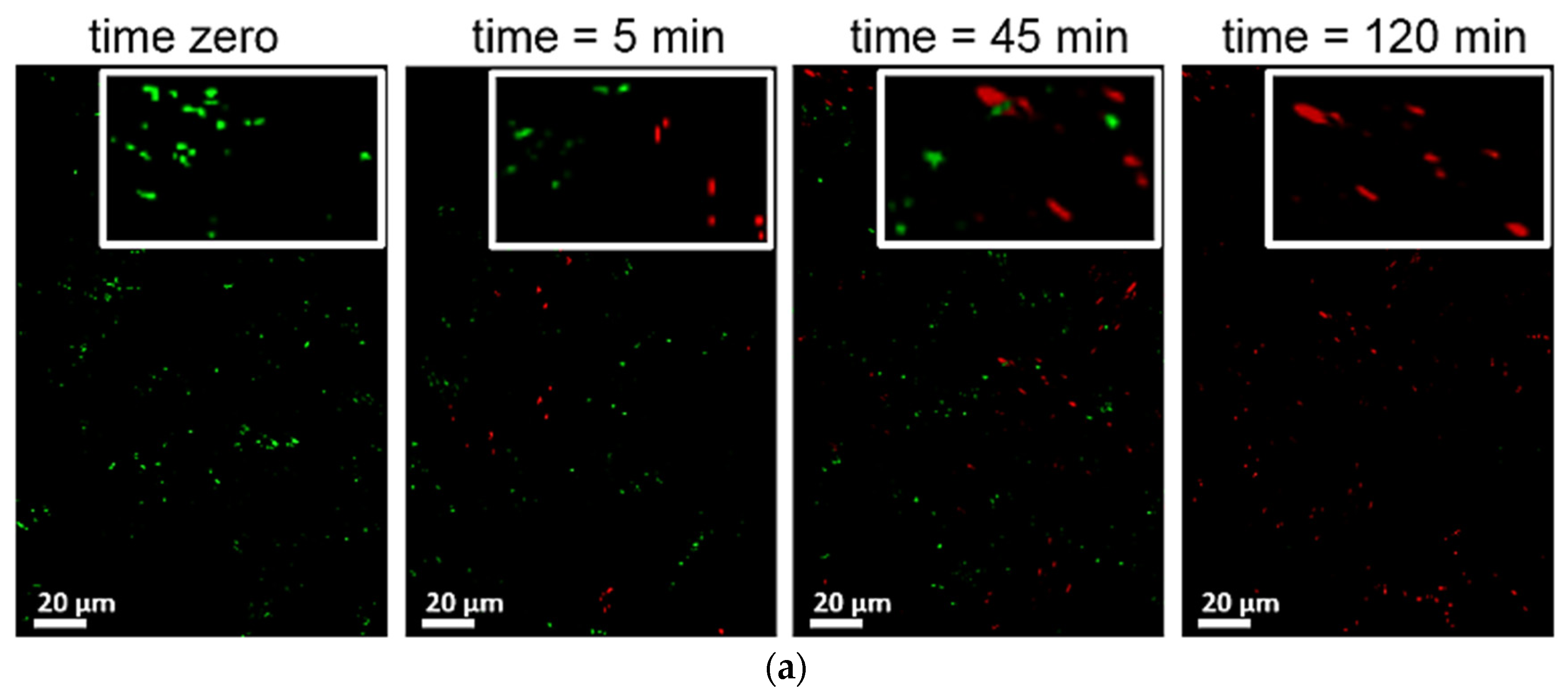
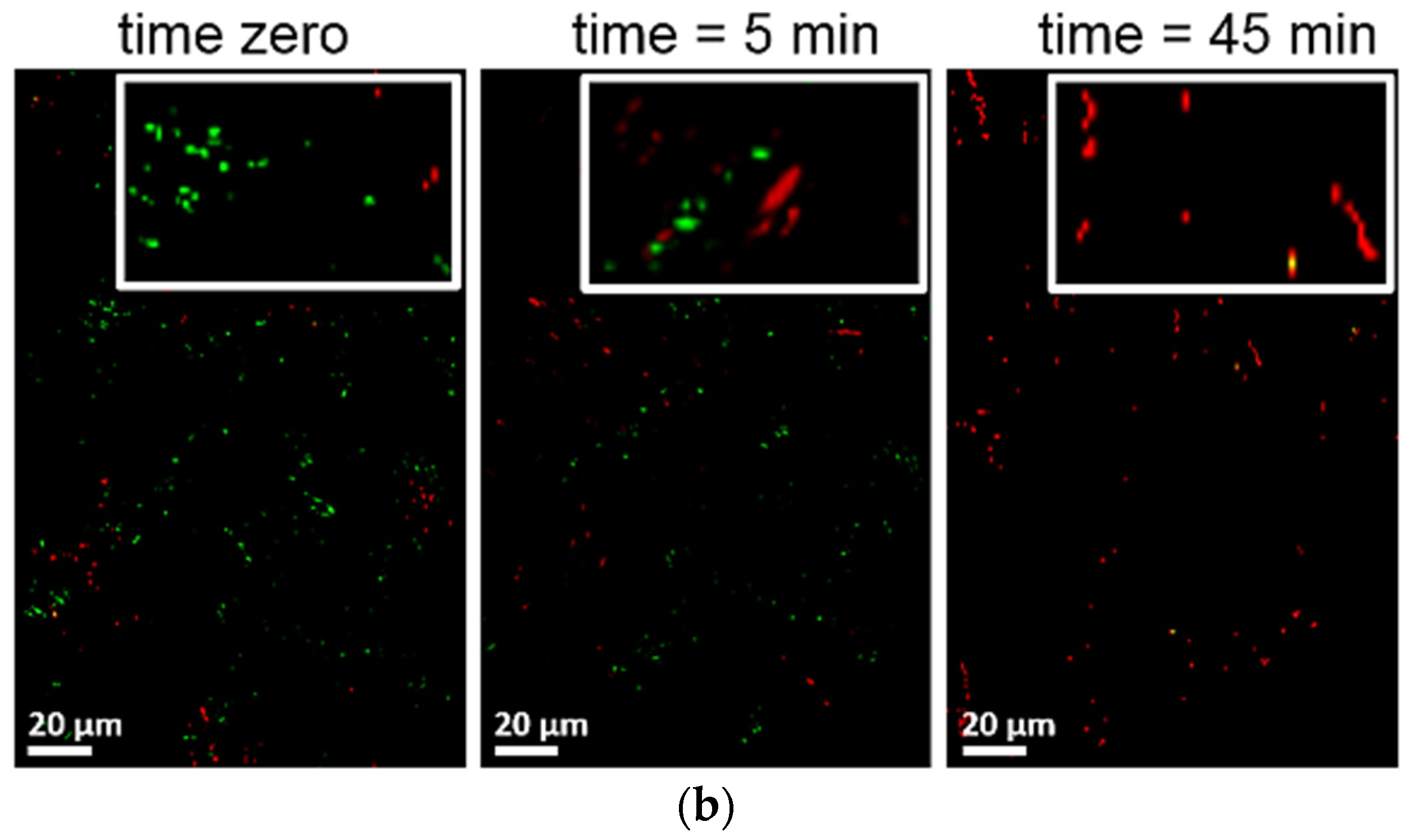
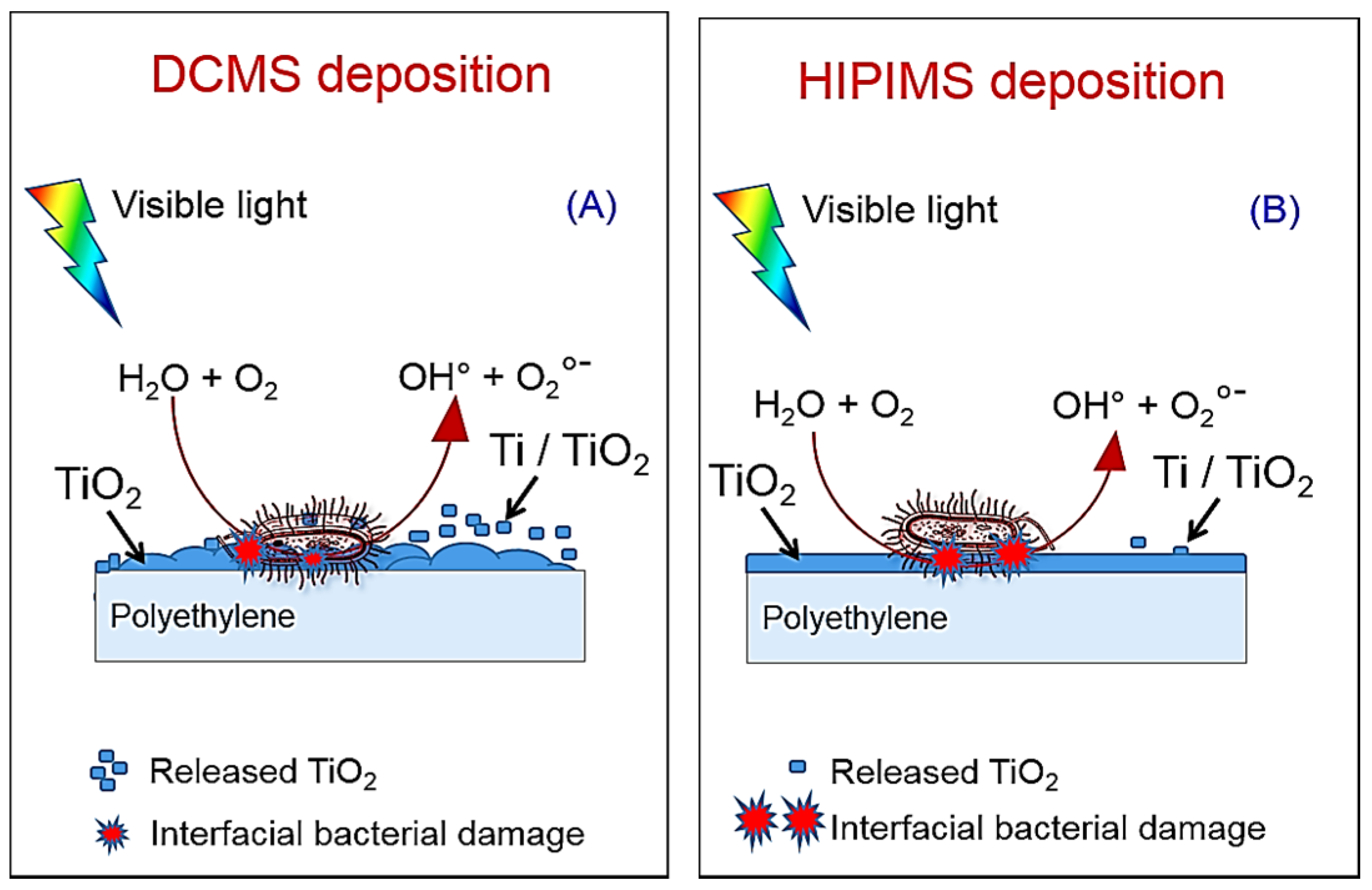
3. TiO2 Sputtered Thin Films Leading to Bacterial Inactivation: Effect of the Preparation Method
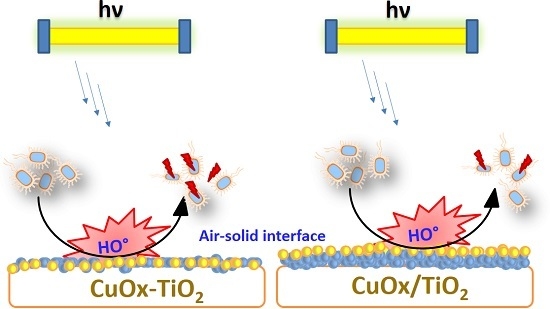
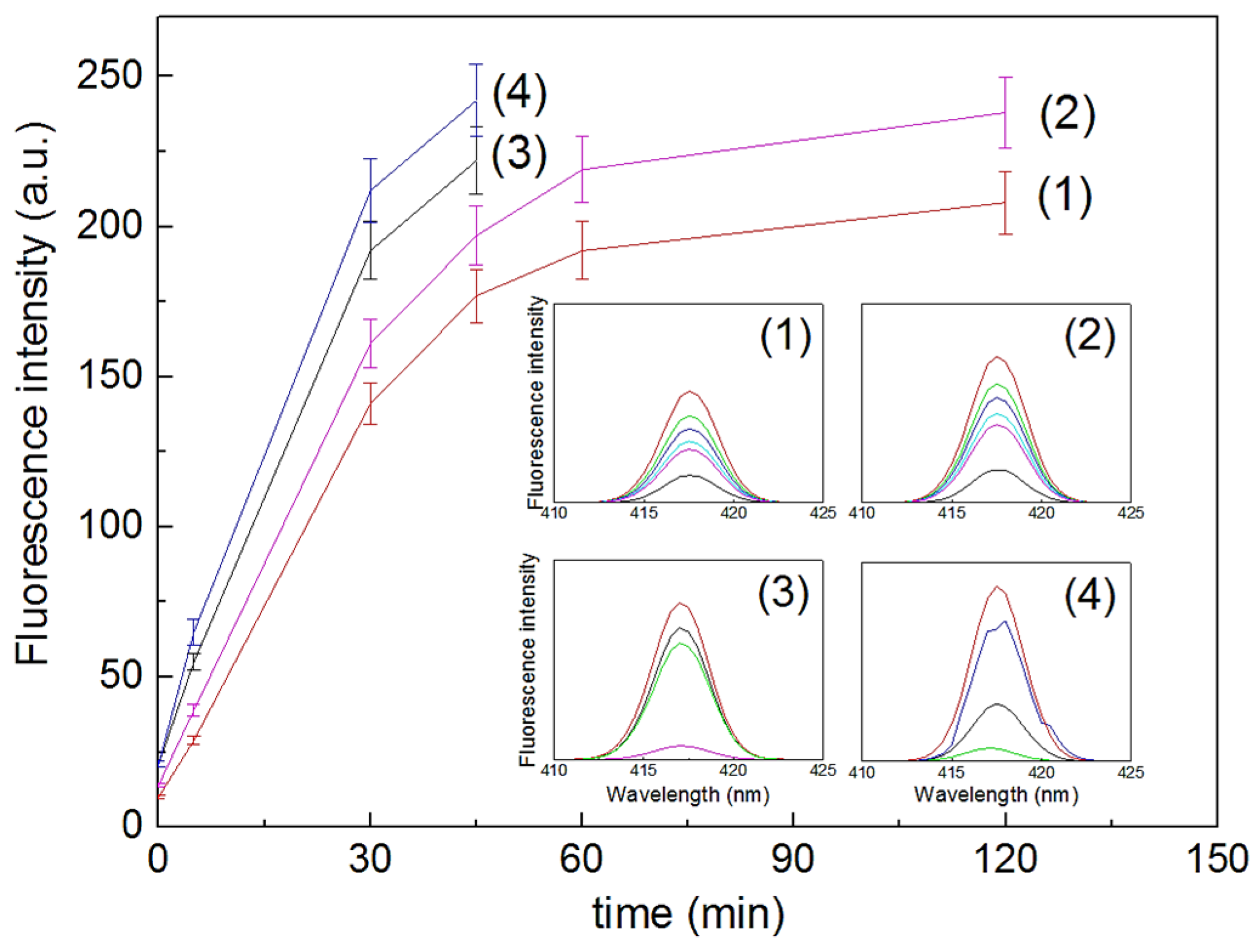
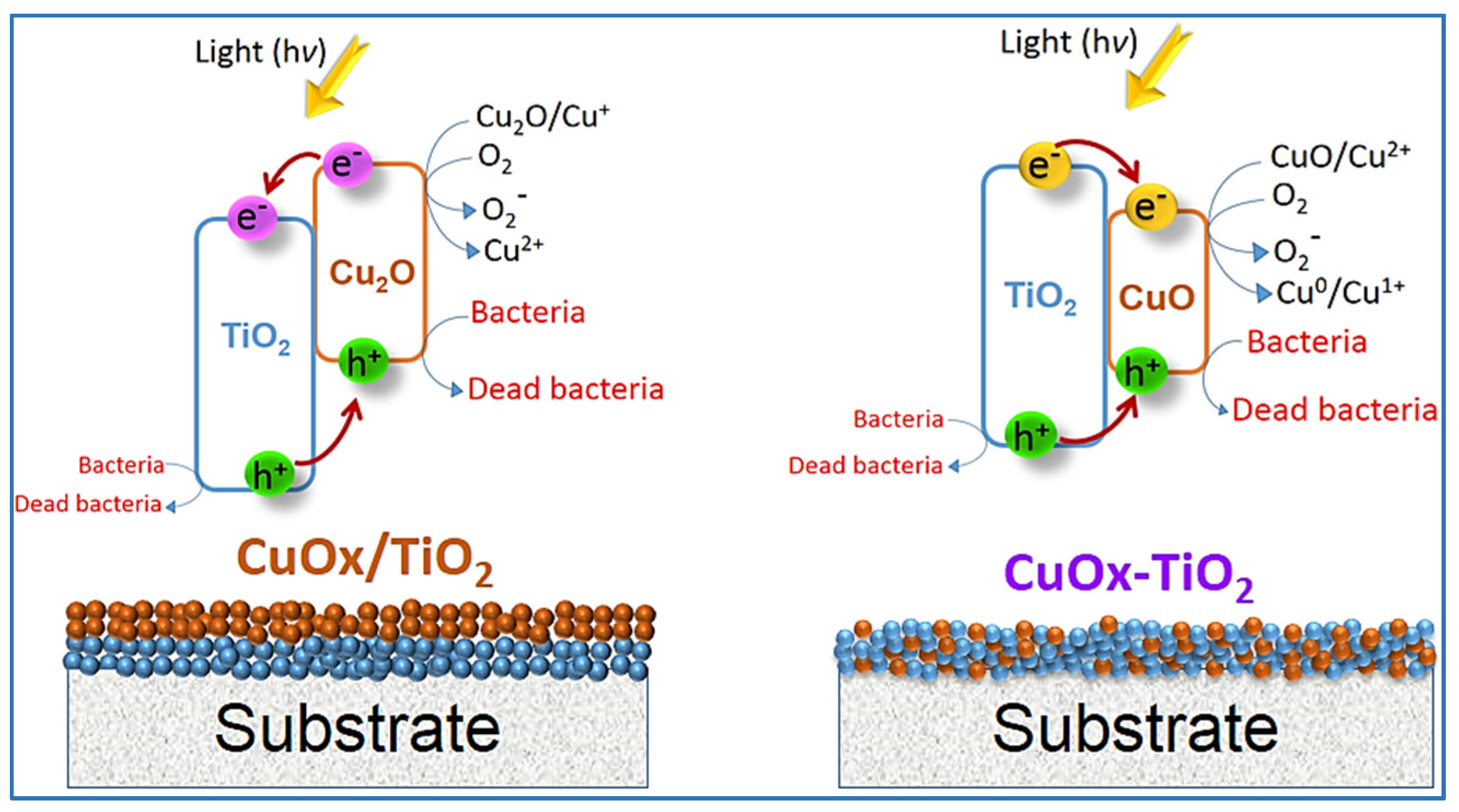
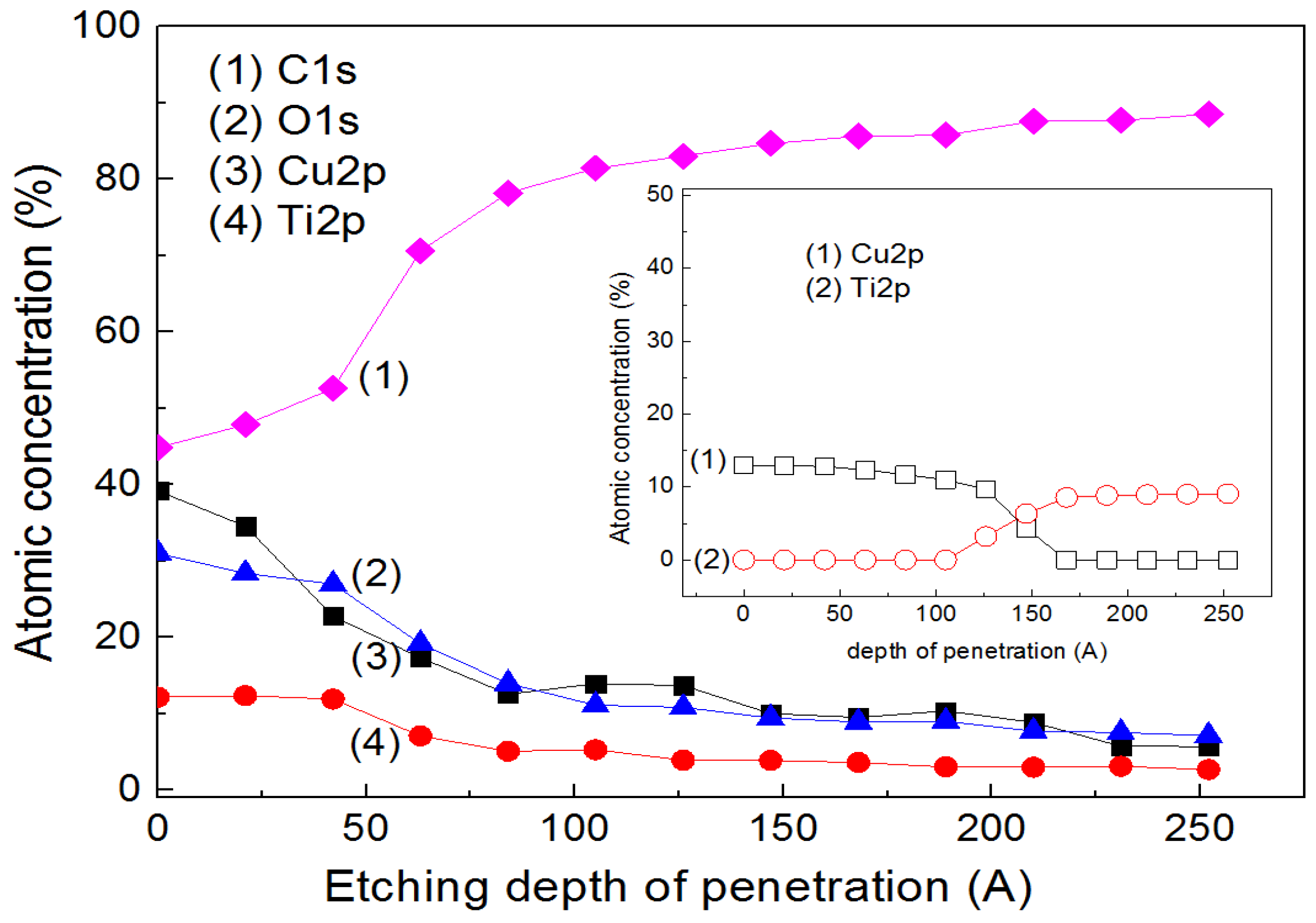
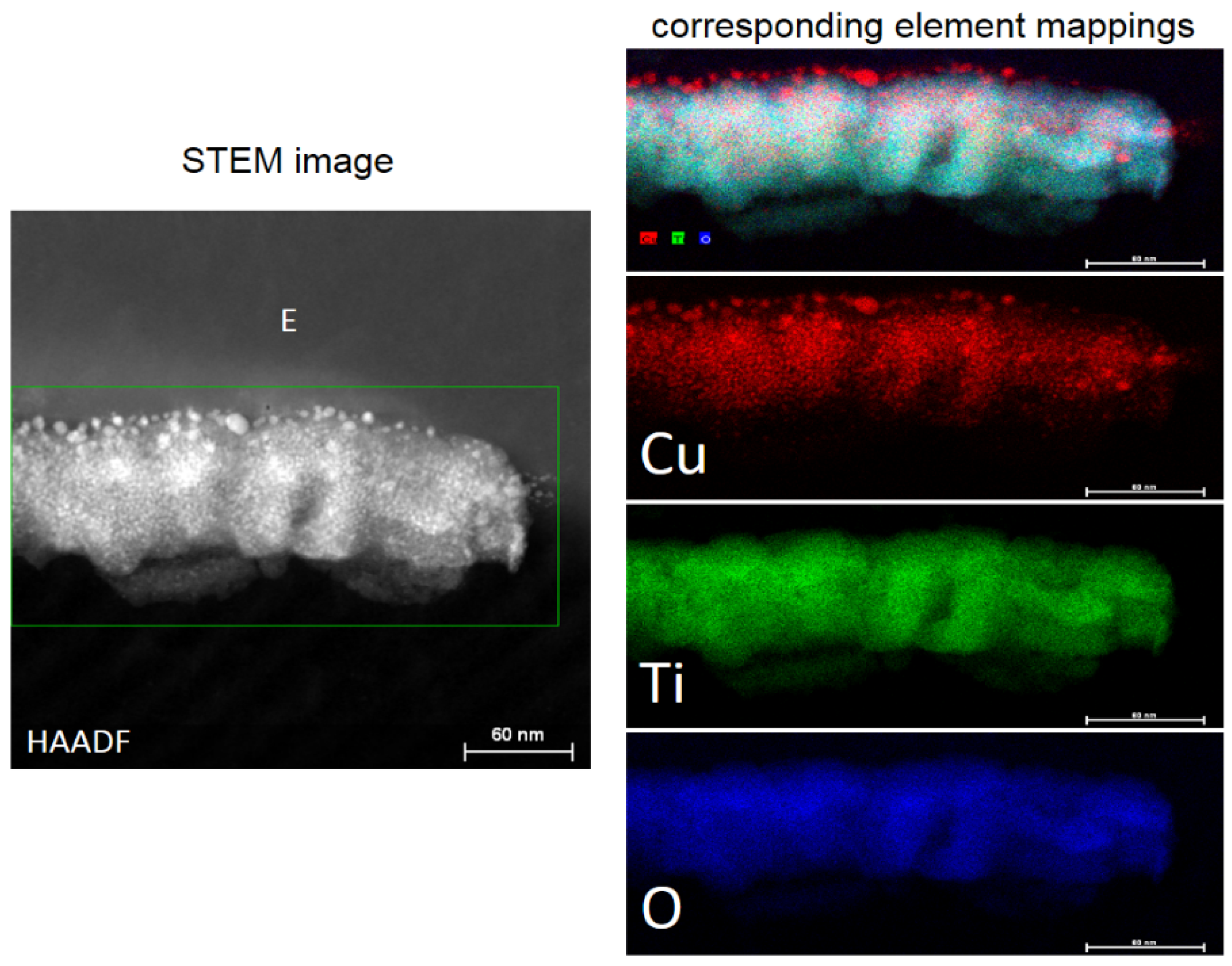
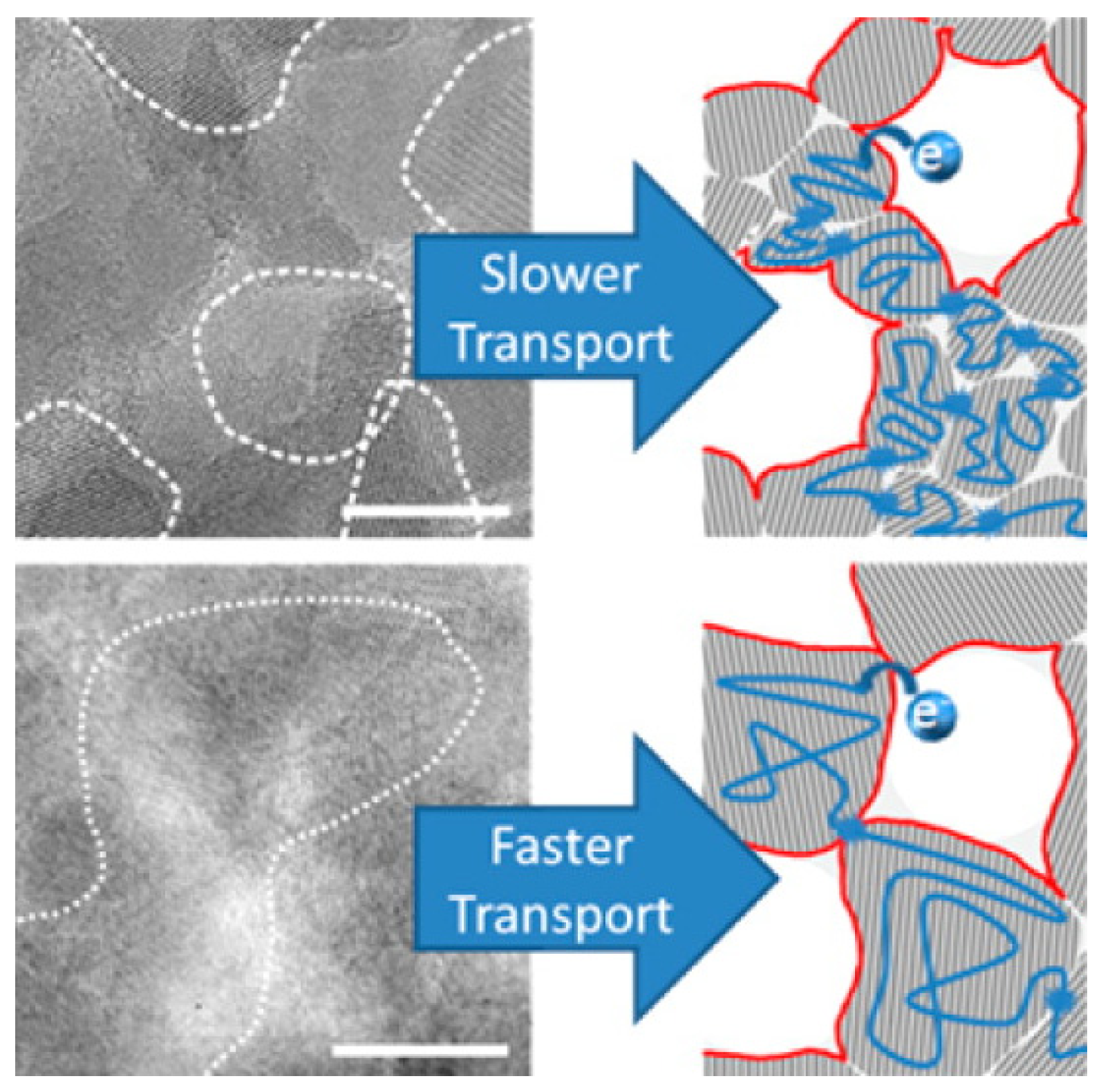
4. Cu Coupled with TiO2 Sputtered Surfaces Active in the Visible Region: Preparation, Mechanism and Microstructure
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Bernstein, R.; Freger, V.; Lee, J.; Kim, Y.; Lee, J.; Herzberg, M. ‘Should I stay or should I go?’ Bacterial attachment vs biofilm formation on surface-modified membranes. Biofouling 2014, 30, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Taylor, K.; Roberts, R.; Roberts, J. The Challenge of Hospital Acquired Infections (HAI); National Audit Office: London, UK, 2002. [Google Scholar]
- Plowman, R.; Graves, R.; Griffin, N.; Taylor, L. The rate ad cost of hospital acquired infections. J. Hosp. Infect. 2001, 47, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Dance, S. The role of environmental cleaning in the control of hospital acquired infections. J. Hosp. Infect. 2007, 73, 378–389. [Google Scholar] [CrossRef] [PubMed]
- Kramer, A.; Schwebke, I.; Kampf, G. How long do nosocomial pathogens persist on inanimate surfaces? Diseases 2006, 6, 137–146. [Google Scholar]
- Borkow, G.; Gabbay, J. Puttting copper into action. Copper impregnated products with potental biocidal activities. J. FASEB 2008, 18, 1728–1730. [Google Scholar]
- Borkow, G.; Gabbay, J. Biocidal Textiles can help fight nosocomial infections. Med. Hypothesis 2008, 70, 990–994. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.; Ruales, C.; Pulgarin, C.; Aimable, A.; Bowen, P.; Kiwi, J. Innovative High-Surface-Area CuO pretreated Cotton Effective in Bacterial Inactivation under Visible Light. Appl. Mater. Interfaces 2010, 2, 2547–2552. [Google Scholar] [CrossRef] [PubMed]
- Kiwi, J.; Pulgarin, C. Innovative self-cleaning and bactericide textiles. Catal. Today 2010, 151, 2–7. [Google Scholar] [CrossRef]
- Pigeot-Rémy, S.; Simonet, F.; Errazuriz-Cerda, E.; Lazzaroni, E.; Atlan, D.; Guillard, C. Photocatalysis and disinfectionof water: Identification of potential bacterial targets. Appl. Catal. B 2011, 201, 390–398. [Google Scholar] [CrossRef]
- Markowska-Szczupak, A.; Ulfig, K.; Morawski, A. The application of TiO2 for deactivation of bioparticules: An overview. Catal. Today 2011, 169, 249–257. [Google Scholar] [CrossRef]
- Foster, H.; Ditta, I.; Varghese, S.; Steele, A. Photocatalytic disinfection using titanium dioxide: Spectrum and mechanism of antimicrobial activity. Appl. Microbiol. Biotech. 2010, 90, 1847–1868. [Google Scholar] [CrossRef] [PubMed]
- Applerot, G.; Abu-Mukh, R.; Irzh, R.; Charmet, J.; Keppner, J.; Laux, L.; Guilbert, G.; Gedanken, A. Decorative parylene coated glass with ZnO nanoparticles for antibacterial applications. ACS Appl. Mater. Interfaces 2010, 2, 1052–1059. [Google Scholar] [CrossRef] [PubMed]
- Perelshtein, I.; Applerot, N.; Perkas, N.; Wehrshuetz-Sigl, E.; Hasman, E.G.; Guebitz, G.; Gedanken, A. CuO-cotton nanocomposite: Formation, Morphology and bacterial activity. Surf. Coat. Technol. 2009, 204, 54–57. [Google Scholar] [CrossRef]
- Yuranova, T.; Rincon, G.; Bozzi, A.; Parra, S.; Pulgarin, C.; Albers, P.; Kiwi, J. Antibacterial textiles prepared by RF-plasma and vacuum-UV mediated deposition of silver. J. Photochem. Photobiol. A Chem. 2003, 161, 27–34. [Google Scholar] [CrossRef]
- Yuranova, T.; Rincon, G.; Pulgarin, C.; Laub, D.; Xanthopoulos, N.; Mathieu, H.-J.; Kiwi, J. Bactericide cotton textiles active in E coli abatement prepared under mild preparation conditions. J. Photochem. Photobiol. A 2006, 181, 363–369. [Google Scholar] [CrossRef]
- Zhang, L.; Dillert, R.; Bahnemann, D. Photoinduced hydrophylicity and self-cleaning: Models and reality. Energy Environ. Sci. 2012, 5, 7491–7507. [Google Scholar] [CrossRef]
- Mills, A.; Hill, P.; Robertson, P. Overview of the current ISO tests for photocatalytic materials. J. Photochem. Photobiol. A 2012, 237, 7–23. [Google Scholar] [CrossRef]
- Page, K.; Wilson, W.; Parkin, I.P. Antimicrobial surfaces and their potential in reducing the role of the inanimate environment in the incidence of hospital-acquired infections. J. Mater. Chem. 2009, 19, 3819–3831. [Google Scholar] [CrossRef]
- Foster, H.A.; Sheel, P.; Sheel, D.W.; Evans, P.; Varghese, S.; Rutschke, N.; Yates, H.M. Antimicrobial activity of titatnia/silver and titania/copper films prepared by CVD. J. Photochem. Photobiol. A 2010, 216, 283–289. [Google Scholar] [CrossRef]
- Dunlop, P.S.M.; Sheeran, C.P.; Byrne, J.A.; McMahon, M.A.S.; Boyle, M.A.; McGuigan, K.G. Inactivation of clinically relevant pathogens by photocatalytic coatings. J. Photochem. Photobiol. A 2010, 216, 303–310. [Google Scholar] [CrossRef]
- Yates, M.H.; Brook, L.A.; Ditta, I.B.; Evans, P.; Foster, A.H.; Sheel, W.D.; Steele, A. Photo-induced self–cleaning and biocidal behviour of titania and copper oxide multilayers. J. Photochem. Photobiol. A 2008, 197, 197–208. [Google Scholar] [CrossRef]
- Sarakinos, K.; Alami, J.; Konstantinidis, D. High power pulsed magnetron sputtering: A review on scientific and engineering state of the art. Surf. Coat. Technol. 2010, 204, 1661–1684. [Google Scholar] [CrossRef]
- Lin, J.; Moore, J.; Sproul, W.; Mishra, B.; Wu, Z.; Wang, L. The structure and properties of chromium nitride coatings deposited using dc, pulsed dc and modulated pulse power magnetron sputtering. Surf. Coat. Technol. 2010, 204, 2230–2239. [Google Scholar] [CrossRef]
- Alami, J.; Persson, P.; Gudmunsson, P.; Bohlmark, J.; Helmersson, J. Ion-assisted physical vapor deposition for enhanced film properties on nonflat surfaces. J. Vac. Technol. A 2005, 23, 278–280. [Google Scholar] [CrossRef]
- Kelly, J.P.; Arnell, D.R. Magnetron sputtering: A review of recent developments and applications. Vacuum 2000, 56, 159–172. [Google Scholar] [CrossRef]
- Rtimi, S.; Sanjines, R.; Pulgarin, C.; Kiwi, J. Quasi-Instantaneous Bacterial Inactivation on Cu-Ag Nano-particulate 3D-Catheters in the Dark and Under Light: Mechanism and Dynamics. ACS Appl. Mater. Interfaces 2016, 8, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Castro, C.; Pulgarin, C.; Osorio, P.; Giraldo, S.; Kiwi, J. Structure-reactivity relations of the Cu-cotton sputtered layers during E. coli inactivation in the dark and under light. J. Photochem. Photobiol. A 2010, 216, 295–302. [Google Scholar] [CrossRef]
- Rtimi, S.; Sanjines, R.; Pulgarin, C.; Kiwi, J. Effect of Light and Oxygen on repetitive bacterial inactivation on uniform, adhesive, robust and stable Cu-polyester surfaces. J. Adv. Oxid. Technol. 2017. [Google Scholar] [CrossRef]
- Rtimi, S.; Sanjines, R.; Bensimon, M.; Pulgarin, C.; Kiwi, J. Accelerated Escherichia coli inactivation in the dark on uniform copper flexible surfaces. Biointerphases 2014, 9, 029012. [Google Scholar] [CrossRef] [PubMed]
- Osorio, P.; Sanjines, R.; Ruales, C.; Castro, C.; Pulgarin, C.; Rengifo, J.-A.; Lavanchy, J.-C.; Kiwi, J. Antimicrobial Cu-functionalized surfaces prepared by bipolar asymmetric DC-pulsed magnetron sputtering (PMS). J. Photochem. Photobiol. A 2011, 220, 70–76. [Google Scholar] [CrossRef]
- Kusiak-Nejman, E.; Morawski, A.; Ehiasarian, A.; Baghriche, O.; Pulgarin, C.; Mielczarski, E.; Mielczarski, J.; Kulik, A.; Kiwi, J. E. coli Inactivation by High Power Impulse Magnetron Sputtered (HIPIMS) Cu-Surfaces. J. Phys. Chem. C 2011, 115, 21113–21119. [Google Scholar] [CrossRef]
- Rio, L.; Kusiak, E.; Kiwi, J.; Pulgarin, C.; Trampuz, A.; Bizzini, A. Comparative methods to evaluate the bactericidal activity of copper-sputtered surfaces against methicillin-resistant staphylococcus aureus. J. Appl. Microbiol. 2012, 78, 8176–8182. [Google Scholar] [CrossRef] [PubMed]
- Hardee, K.; Bard, J. Semiconductor electrodes: X. Photochemical behavior of polycrystalline metal-oxides electrodesin aqueous solutions. J. Electrochem. Soc. 1977, 124, 215–224. [Google Scholar] [CrossRef]
- Kiwi, J.; Rtimi, S.; Pulgarin, C. Cu, Cu/TiO2 thin films sputtered by up to date methods on non-thermal thin resistant substrates leading to bacterial inactivation. In Microbial Pathogens and Strategies for Combating Them: Science, Technology and Education; Méndez-Vilas, A., Ed.; Formatex Research Center: Extremadura, Spain, 2013; Volume 1, pp. 74–82. [Google Scholar]
- Bandara, J.; Guasaquillo, I.; Bowen, P.; Soare, L.; Jardim, W.; Kiwi, J. Photocatalytic Storing of O2 as H2O2 Mediated by High Surface Area CuO. Evidence for the Reductive-Oxidative Interfacial Mechanism of Reaction. Langmuir 2005, 21, 8554–8559. [Google Scholar] [CrossRef] [PubMed]
- Sunada, K.; Watanabe, S.; Hashimoto, K. Bactericidal Activity of Copper Deposited TiO2 Film under UV Light Illumination. Environ. Sci. Technol. 2003, 40, 4785–4789. [Google Scholar] [CrossRef]
- Ishiguro, H.; Yao, Y.; Nakano, Y.; Hara, M.; Sunada, K.; Hashimoto, K.; Kajioka, J.; Fujishima, A.; Kubota, Y. Photocatalytic activity of Cu2+/TiO2-coated cordierite foam inactivates bacteriophages and Legionella pneumophila. Appl. Catal. B 2013, 129, 56–61. [Google Scholar] [CrossRef]
- Ditta, I.B.; Steele, A.; Liptrot, C.; Tobin, J.; Tyler, H.; Yates, H.M.; Sheel, D.W.; Foster, H.A. Photocatalytic antimicrobial activity of thin surface films of TiO2, CuO and TiO2/CuO dual layers on Escherichia coli and bacteriophage T4. Appl. Microbiol. Biotechnol. 2008, 79, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Irie, H.; Miura, S.; Kamiya, S.; Hashimoto, K. Efficient visible light-sensitive photocatalysis: Grafting Cu(II) ions onto TiO2 and WO3 photocatalysts. Chem. Phys. Letts. 2008, 457, 202–205. [Google Scholar] [CrossRef]
- Qiu, X.; Miyauchi, M.; Sunada, K.; Minoshima, M.; Liu, M.; Lu, Y.; Ding, L.; Shomodaira, Y.; Hosogi, Y.; Kuroda, Y.; et al. Hybrid CuxO/TiO2 Nanocomposites As Risk-Reduction Materials in Indoor Environments. ACS Nano 2012, 6, 1609–1618. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Dennehy, J. Differential Bacteriophage Mortality on Exposure to Copper. Appl. Environ. Microbiol. 2011, 77, 6878–6883. [Google Scholar] [CrossRef] [PubMed]
- Rtimi, S.; Pulgarin, C.; Sanjines, R.; Kiwi, J. Kinetics and mechanism for transparent polyethylene-TiO2 films mediated self-cleaning leading to MB dye discoloration under sunlight irradiation. Appl. Catal. B Environ. 2015, 162, 236–244. [Google Scholar] [CrossRef]
- Nesic, J.; Rtimi, S.; Laub, D.; Roglic, G.M.; Pulgarin, C.; Kiwi, J. New evidence for TiO2 uniform surfaces leading to complete bacterial reduction in the dark: Critical issues. Colloids Surf. B Biointerfaces 2014, 123, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Kiwi, J.; Rtimi, S.; Nesic, J.; Pulgarin, C.; Sanjines, R.; Bensimon, M.; Kiwi, J. Effect of surface pretreatment of TiO2 films on interfacial processes leading to bacterial inactivation in the dark and under light irradiation. Interface Focus 2014, 5, 20140046. [Google Scholar]
- Rtimi, S.; Sanjines, R.; Andrzejczuk, M.; Pulgarin, C.; Kulik, A.; Kiwi, J. Innovative transparent non-scattering TiO2 bactericide thin films inducing increased E. coli cell wall fluidity. Surf. Coat. Technol. 2014, 254, 333–343. [Google Scholar] [CrossRef]
- Petronella, F.; Rtimi, S.; Comparelli, R.; Sanjines, R.; Pulgarin, C.; Curri, M.L.; Kiwi, J. Uniform TiO2/In2O3 surface films effective in bacterial inactivation under visible light. J. Photochem. Photobiol. A Chem. 2014, 279, 1–7. [Google Scholar] [CrossRef]
- Kiwi, J.; Rtimi, S. Environmentally mild self-cleaning processes on textile surfaces under daylight irradiation: Critical issues. In Active Coatings for Smart Textiles; Hu, J., Ed.; Elsevier Limited: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Baghriche, O.; Rtimi, S.; Pulgarin, C.; Roussel, C.; Kiwi, J. RF-plasma pretreatment of surfaces leading to TiO2 coatings with improved optical absorption and OH-radical production. Appl. Catal. B Environ. 2013, 130–131, 65–72. [Google Scholar] [CrossRef]
- Rtimi, S.; Pulgarin, C.; Sanjines, R.; Kiwi, J. Innovative semi-transparent nanocomposite films presenting photo-switchable behavior and leading to a reduction of the risk of infection under sunlight. RSC Adv. 2013, 3, 16345–16348. [Google Scholar] [CrossRef]
- Rtimi, S.; Giannakis, S.; Bensimon, M.; Pulgarin, C.; Sanjines, R.; Kiwi, J. Supported TiO2 films deposited at different energies: Implications of the surface compactness on the catalytic kinetics. Appl. Catal. B Environ. 2016, 191, 42–52. [Google Scholar] [CrossRef]
- Chan, C.; Ko, T.; Hiroaka, N. Polymer surface modification by plasma and photons. Surf. Sci. Rep. 1996, 24, 1–54. [Google Scholar] [CrossRef]
- Winkler, J. Titaniun Oxide; Vincentz: Hannover, Germany, 2003. [Google Scholar]
- Page, K.; Palgrave, R.G.; Parkin, I.P.; Wilson, M.; Savin, P.S.L.; Chadwick, V.A. Titania and silver titania composite films on glass antimicrobial coating. J. Mater. Chem. 2007, 17, 95–104. [Google Scholar] [CrossRef]
- Rtimi, S.; Robyr, M.; Pulgarin, C.; Lavanchy, J.-C.; Kiwi, J. A New Perspective in the Use of FeOx-TiO2 Photocatalytic Films: Indole Degradation in the Absence of Fe-Leaching. J. Catal. 2016, 342, 184–192. [Google Scholar] [CrossRef]
- Baghriche, O.; Rtimi, S.; Sanjines, R.; Pulgarin, C.; Kiwi, J. Effect of the spectral properties of TiO2, Cu, TiO2/Cu sputtered films on the bacterial inactivation under low intensity actinic light. J. Photochem. Photobiol. A 2013, 251, 50–56. [Google Scholar] [CrossRef]
- Baghriche, O.; Rtimi, S.; Pulgarin, C.; Sanjines, R.; Kiwi, J. Innovative TiO2/Cu Nanosurfaces Inactivating Bacteria in the Minute Range under Low-Intensity Actinic Light. ACS Appl. Mater. Interfaces 2012, 4, 5234–5240. [Google Scholar] [CrossRef] [PubMed]
- Negishi, N.; Iyoda, T.; Hashimoto, K.; Fujishima, A. Preparation of Transparent TiO2 Thin Film Photocatalyst and Its Photocatalytic Activity. Chem. Lett. 1995, 24, 841–842. [Google Scholar] [CrossRef]
- Nikaido, H.J. Prevention of Drug Access to Bacterial Targets. Permeability Barriers and Active Flux. Biol. Chem. 1994, 269, 3905–3909. [Google Scholar]
- Pelaez, M.; Nolan, N.; Pillai, S.; Seery, M.; Falaras, P.; Kontos, A.; Dunlop, P.S.M.; Hamilton, J.; Byrne, J.-A.; O’Shea, K.; et al. A Review on the Visible Light Active Titanium Dioxide Photocatalysts for Environmental Applications. Appl. Catal. B 2012, 125, 331–349. [Google Scholar] [CrossRef]
- Kühn, K.; Chaberny, I.; Massholder, K.; Sticker, M.; Benz, V.; Sonntag, V.H.-G.; Erdinger, I. Disinfection of surfaces by photocatalytic oxidation with titanium dioxide and UVA light. Chemosphere 2003, 53, 71–77. [Google Scholar] [CrossRef]
- Espirito Santo, C.; Lam, E.; Elowsky, C.; Quarnta, D.; Domaille, D.; Chang, C.; Grass, G. Bacterial Killing by Dry Metallic Copper Surfaces. Appl. Environ. Microbiol. 2011, 77, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Rentz, E. Viral Pathogens and Severe Acute Respiratory Syndrome: Oligodynamic Ag+ for Direct Immune Intervention. J. Nutr. Environ. Med. 2003, 13, 109–118. [Google Scholar] [CrossRef]
- Thill, A.; Zeyons, O.; Spalla, O.; Chauvat, F.; Rose, J.; Auffan, M.; Flank, A.-M. Cytotoxicity of CeO2 Nanoparticles for Escherichia coli. Physico-Chemical Insight of the Cytotoxicity Mechanism. Environ. Sci. Technol. 2006, 40, 6151–6156. [Google Scholar] [CrossRef] [PubMed]
- Nadtochenko, V.; Rincon, G.; Stanka, S.; Kiwi, J. Dynamics of E. coli membrane cell peroxidation during TiO2 photocatalysis studied by ATR-FTIR spectroscopy and AFM microscopy. J. Photochem. Photobiol. A 2005, 169, 131–137. [Google Scholar] [CrossRef]
- Rtimi, S.; Baghriche, O.; Pulgarin, C.; Lavanchy, J.-C.; Kiwi, J. Growth of TiO2/Cu films by HiPIMS for accelerated bacterial loss of viability. Surf. Coat. Technol. 2013, 232, 804–813. [Google Scholar] [CrossRef]
- Rtimi, S.; Ballo, M.; Laub, D.; Pulgarin, C.; Entenza, J.; Bizzini, A.; Sanjines, R.; Kiwi, J. Duality in the Escherichia coli and methicillin resistant Staphylococcus aureus reduction mechanism under actinic light on innovative co-sputtered surfaces. Appl. Catal. A Gen. 2015, 498, 185–191. [Google Scholar] [CrossRef]
- Docampo, P.; Guldin, S.; Steiner, U.; Snaith, H.J. Charge Transport Limitations in Self-Assembled TiO2 Photo-anodes for Dye-Sensitized Solar Cells. J. Phys. Chem. Lett. 2013, 4, 698–703. [Google Scholar] [CrossRef] [PubMed]
- Alhussein, A.; Achache, S.; Deturche, R.; Sanchette, F.; Pulgarin, C.; Kiwi, J.; Rtimi, S. Beneficial effect of Cu on Ti-Nb-Ta-Zr sputtered uniform/adhesive gum films accelerating bacterial inactivation under indoor visible light. Collids Surf. B Biointerface 2017, 152, 152–158. [Google Scholar] [CrossRef] [PubMed]


© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rtimi, S. Indoor Light Enhanced Photocatalytic Ultra-Thin Films on Flexible Non-Heat Resistant Substrates Reducing Bacterial Infection Risks. Catalysts 2017, 7, 57. https://doi.org/10.3390/catal7020057
Rtimi S. Indoor Light Enhanced Photocatalytic Ultra-Thin Films on Flexible Non-Heat Resistant Substrates Reducing Bacterial Infection Risks. Catalysts. 2017; 7(2):57. https://doi.org/10.3390/catal7020057
Chicago/Turabian StyleRtimi, Sami. 2017. "Indoor Light Enhanced Photocatalytic Ultra-Thin Films on Flexible Non-Heat Resistant Substrates Reducing Bacterial Infection Risks" Catalysts 7, no. 2: 57. https://doi.org/10.3390/catal7020057