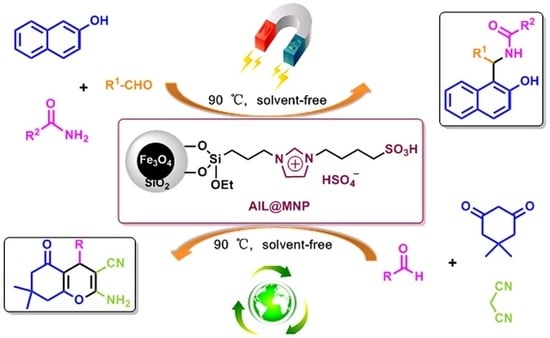
Facile One-Pot Synthesis of Amidoalkyl Naphthols and Benzopyrans Using Magnetic Nanoparticle-Supported Acidic Ionic Liquid as a Highly Efficient and Reusable Catalyst
Abstract
:1. Introduction
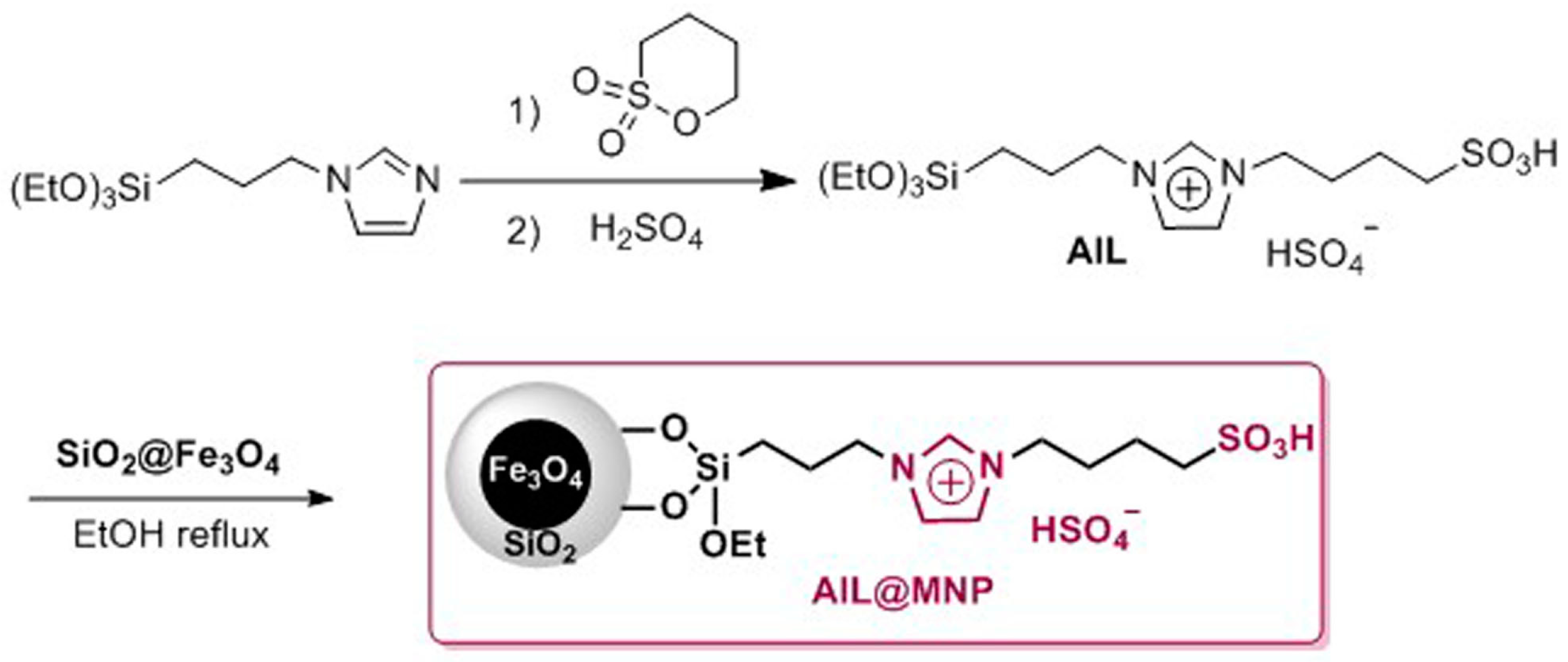
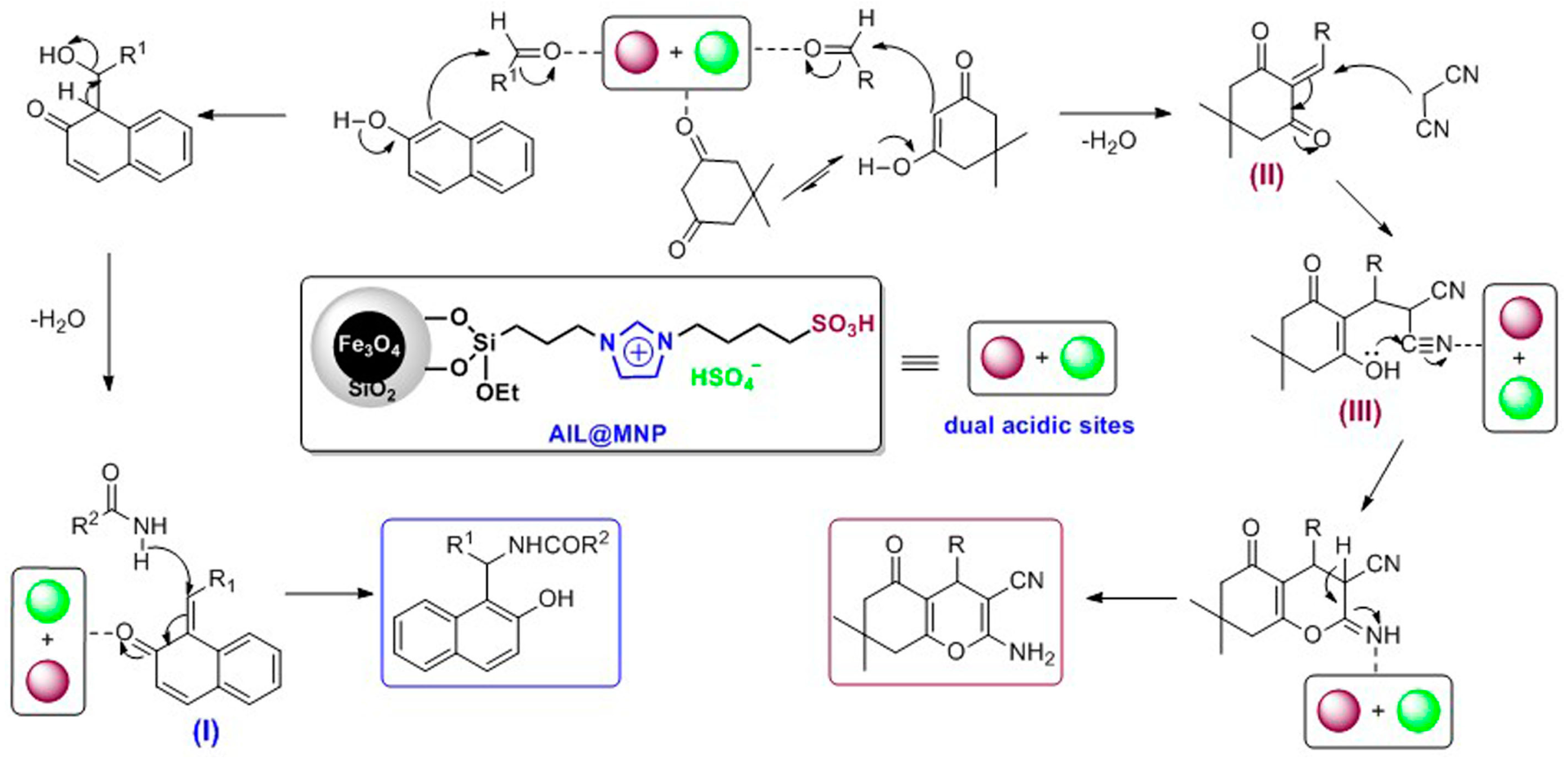
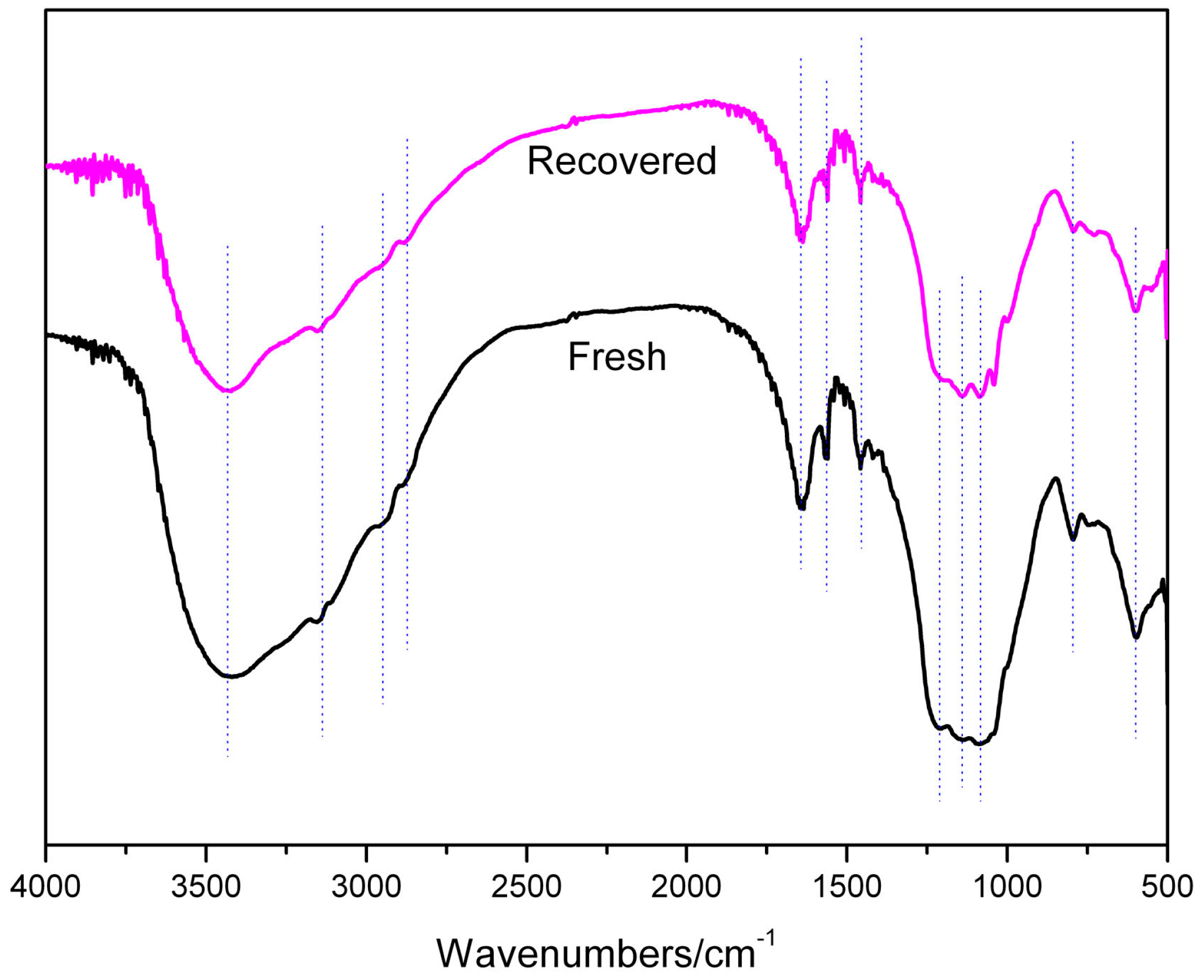
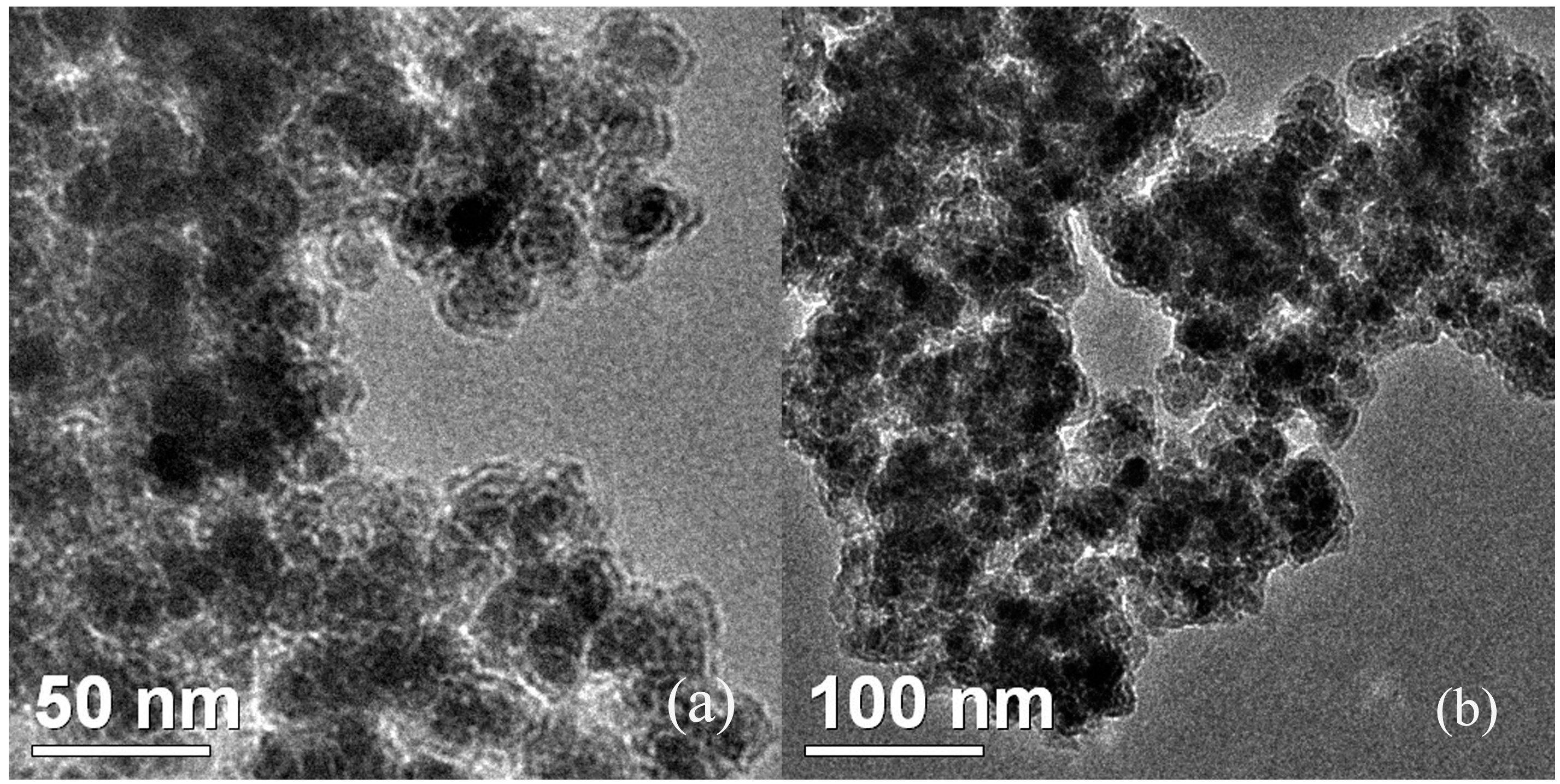
2. Results and Discussion
3. Experimental
3.1. General Procedure for the Synthesis of Amidoalkyl Naphthols
3.2. General Procedure for the Synthesis of Benzopyrans
4. Conclusions
Supplementary Materials
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Poliakoff, M.; Fitzpatrick, J.M.; Farren, T.R.; Anastas, P.T. Green chemistry: Science and politics of change. Science 2002, 297, 807–810. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A.; Arends, I.; Hanefeld, U. Green Chemistry and Catalysis; Wiely-VCH: Weinheim, Germany, 2007. [Google Scholar]
- Christensen, C.H.; Nørskov, J.K. Green gold catalysis. Science 2010, 327, 278–279. [Google Scholar] [CrossRef] [PubMed]
- Dömling, A.; Wang, W.; Wang, K. Chemistry and biology of multicomponent reactions. Chem. Rev. 2012, 112, 3083–3135. [Google Scholar] [CrossRef] [PubMed]
- Levi, L.; Müller, T.J.J. Multicomponent syntheses of functional chromophores. Chem. Soc. Rev. 2016, 45, 2825–2846. [Google Scholar] [CrossRef] [PubMed]
- Cioc, R.C.; Ruijter, E.; Orru, R.V.A. Multicomponent reactions: Advanced tools for sustainable organic synthesis. Green Chem. 2014, 16, 2958–2975. [Google Scholar] [CrossRef]
- Shaterian, H.R.; Yarahmadi, H.; Ghashang, M. An efficient, simple and expedition synthesis of 1-amidoalkyl-2-naphthols as ‘drug like’ molecules for biological screening. Bioorg. Med. Chem. Lett. 2008, 18, 788–792. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Jiang, B.; Li, T.; Qin, B.; Feng, X.; Zhang, H.; Wang, C.; Tu, S. Design and an efficient synthesis of natural product-based cyclopenta[b]pyran derivatives with potential bioactivity. Bioorg. Med. Chem. Lett. 2011, 21, 599–601. [Google Scholar] [CrossRef] [PubMed]
- Erichsen, M.N.; Huynh, T.H.V.; Abrahamsen, B.; Bastlund, J.F.; Bundgaard, C.; Monrad, O.; Jensen, A.B.; Nielsen, C.W.; Frydenvang, K.; Jensen, A.A.; et al. Structure−Activity Relationship Study of First Selective Inhibitor of Excitatory Amino Acid Transporter Subtype 1:2-Amino-4-(4-methoxyphenyl)-7-(naphthalen-1-yl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile (UCPH-101). J. Med. Chem. 2010, 53, 7180–7191. [Google Scholar] [CrossRef] [PubMed]
- Taghrir, H.; Ghashang, M.; Biregan, M.N. Preparation of 1-amidoalkyl-2-naphthol derivatives using barium phosphate nano-powders. Chin. Chem. Lett. 2016, 27, 119–126. [Google Scholar] [CrossRef]
- Kiasat, A.R.; Hemat-Alian, L.; Saghanezhad, S.J. Nano Al2O3: An efficient and recyclable nanocatalyst for the one-pot preparation of 1-amidoalkyl-2-naphthols under solvent-free conditions. Res. Chem. Intermed. 2016, 42, 915–922. [Google Scholar] [CrossRef]
- Tayebee, R.; Amini, M.M.; Akbari, M.; Aliakbari, A. A novel inorganic–organic nanohybrid material H4SiW12O40/pyridino-MCM-41 as efficient catalyst for the preparation of 1-amidoalkyl-2-naphthols undersolvent-free conditions. Dalton Trans. 2015, 44, 9596–9609. [Google Scholar] [CrossRef] [PubMed]
- Esmaeilpour, M.; Javidi, J.; Zandi, M. Preparation and characterization of Fe3O4@SiO2@PMA:AS an efficient and recyclable nanocatalyst for the synthesis of 1-amidoalkyl-2-naphthols. Mater. Res. Bull. 2014, 55, 78–87. [Google Scholar] [CrossRef]
- Nasresfahani, Z.; Kassaee, M.Z.; Eidi, E. Homopiperazine sulfamic acid functionalized mesoporous silica nanoparticles (MSNs-HPZ-SO3H) as an efficient catalyst for one-pot synthesis of 1-amidoalkyl-2-naphthols. New J. Chem. 2016, 40, 4720–4726. [Google Scholar] [CrossRef]
- Cai, Z.; Shu, C.; Peng, Y. Magnetically recoverable nano-sized mesoporous solid acid: Effective catalysts for the synthesis of 1-amidoalkyl-2-naphthols. Monatshefte Chem. 2014, 145, 1681–1687. [Google Scholar] [CrossRef]
- Zolfagharinia, S.; Kolvari, E.; Salehi, M. Highly efficient and recyclable phosphoric acid functionalized zirconia encapsulated-Fe3O4 nanoparticles: Clean synthesis of 1,4-dihydropyridine and 1-amidoalkyl-2-naphthol derivatives. React. Kinet. Mech. Catal. 2017, 121, 701–718. [Google Scholar] [CrossRef]
- Gong, K.; Wang, H.; Ren, X.; Wang, Y.; Chen, J. β-Cyclodextrin-butane sulfonic acid: An efficient and reusable catalyst for the multicomponent synthesis of 1-amidoalkyl-2-naphthols under solvent-free conditions. Green Chem. 2015, 17, 3141–3147. [Google Scholar] [CrossRef]
- Chinnappan, A.; Jadhav, A.H.; Chung, W.J.; Kim, H. Synthesis of 1-amidoalkyl 2-naphthols using ionic liquid with metal complex as an efficient and reusable catalyst under solvent free conditions. J. Mol. Liq. 2015, 212, 413–417. [Google Scholar] [CrossRef]
- Rakhtshah, J.; Salehzadeh, S. Multi-wall carbon nanotube supported Co(II) Schiff base complex: An efficient and highly reusable catalyst for synthesis of 1-amidoalkyl-2-naphthol and tetrahydrobenzo[b]pyran derivatives. Appl. Organomet. Chem. 2017, 31, e3560. [Google Scholar] [CrossRef]
- Pourmousavi, S.A.; Moghimi, P.; Ghorbani, F.; Zamani, M. Sulfonated polynaphthalene as an effective and reusable catalyst for the one-pot preparation of amidoalkyl naphthols: DFT and spectroscopic studies. J. Mol. Struct. 2017, 1144, 87–102. [Google Scholar] [CrossRef]
- Gupta, A.; Kour, D.; Gupta, V.K.; Kapoor, K.K. Graphene oxide mediated solvent-free three component reaction for the synthesis of 1-amidoalkyl-2-naphthols and 1,2-dihydro-1- arylnaphth[1,2-e][1,3]oxazin-3-ones. Tetrahedron Lett. 2016, 57, 4869–4872. [Google Scholar] [CrossRef]
- Abdollahi-Alibeik, M.; Nezampour, F. Synthesis of 4H-benzo[b]pyrans in the presence of sulfated MCM-41 nanoparticles as efficient and reusable solid acid catalyst. React. Kinet. Mech. Catal. 2013, 108, 213–229. [Google Scholar] [CrossRef]
- Maleki, B.; Ashrafi, S.S. Nano α-Al2O3 supported ammonium dihydrogen phosphate (NH4H2PO4/Al2O3): Preparation, characterization and its application as a novel and heterogeneous catalyst for the one-pot synthesis of tetrahydrobenzo[b]pyran and pyrano[2,3-c]pyrazole derivatives. RSC Adv. 2014, 4, 42873–42891. [Google Scholar] [CrossRef]
- Pandit, K.S.; Chavan, P.V.; Desai, U.V.; Kulkarni, M.A.; Wadgaonkar, P.P. Tris-hydroxymethylaminomethane (THAM): A novel organocatalyst for an environmentally benign synthesis of medicinally important tetrahydrobenzo[b]pyrans and pyran-annulated heterocycles. New J. Chem. 2015, 39, 4452–4463. [Google Scholar] [CrossRef]
- Brahmachari, G.; Banerjee, B. Facile and one-pot access to diverse and densely functionalized 2-amino-3-cyano-4H-pyrans and pyran-annulated heterocyclic scaffolds via an eco-friendly multicomponent reaction at room temperature using urea as a novel organo-catalyst. ACS Sustain. Chem. Eng. 2014, 2, 411–422. [Google Scholar] [CrossRef]
- Keshavarz, M.; Iravani, N.; Azqhandi, M.H.A.; Nazari, S. Ion-pair immobilization of l-prolinate anion onto cationic polymer support and a study of its catalytic activity as an efficient heterogeneous catalyst for the synthesis of 2-amino-4H-chromene derivatives. Res. Chem. Intermed. 2016, 42, 4591–4604. [Google Scholar] [CrossRef]
- Shamsi, T.; Amoozadeh, A.; Sajjadi, S.M.; Tabrizian, E. Novel type of SO3H-functionalized nano-titanium dioxide as a highly efficient and recyclable heterogeneous nanocatalyst for the synthesis of tetrahydrobenzo[b]pyrans. Appl. Organomet. Chem. 2017, 31, e3636. [Google Scholar] [CrossRef]
- Shahbazi, F.; Amani, K. Synthesis, characterization and heterogeneous catalytic activity of diamine-modified silica-coated magnetite-polyoxometalate nanoparticles as a novel magnetically-recoverable nanocatalyst. Catal. Commun. 2014, 55, 57–64. [Google Scholar] [CrossRef]
- Esmaeilpour, M.; Javidi, J.; Dehghani, F.; Dodeji, F.N. A green one-pot three-component synthesis of tetrahydrobenzo[b]pyran and 3,4-dihydropyrano[c]-chromene derivatives using a Fe3O4@SiO2-imid-PMAn magnetic nanocatalyst under ultrasonic irradiation or reflux conditions. RSC Adv. 2015, 5, 26625–26633. [Google Scholar] [CrossRef]
- Shirini, F.; Langarudi, M.S.N.; Daneshvar, N. Preparation of a new DABCO-based ionic liquid [H2-DABCO][H2PO4]2 and its application in the synthesis of tetrahydrobenzo[b]pyran and pyrano[2,3-d]pyrimidinonederivatives. J. Mol. Liq. 2017, 234, 268–278. [Google Scholar] [CrossRef]
- Dai, C.; Zhang, J.; Huang, C.; Lei, Z. Ionic liquids in selective oxidation: Catalysts and solvents. Chem. Rev. 2017, 117, 6929–6983. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.M.; Xu, M.D.; Wang, X. PEG1000-based dicationic acidic ionic liquid/solvent-free conditions: An efficient catalytic system for the synthesis of bis(indolyl)methanes. Catalysts 2017, 7, 300. [Google Scholar] [CrossRef]
- Zhang, S.G.; Zhang, J.H.; Zhang, Y.; Deng, Y.Q. Nanoconfined ionic liquids. Chem. Rev. 2017, 117, 6755–6833. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.K.; Dutta, S.; Sharma, S.; Zboril, R.; Varma, R.S.; Gawande, M.B. Fe3O4 (iron oxide)-supported nanocatalysts: Synthesis, characterization and applications in coupling reactions. Green Chem. 2016, 18, 3184–3209. [Google Scholar] [CrossRef]
- Gawande, M.B.; Monga, Y.; Zboril, R.; Sharma, R.K. Silica-decorated magnetic nanocomposites for catalytic applications. Coord. Chem. Rev. 2015, 288, 118–143. [Google Scholar] [CrossRef]
- Sydnes, M.O. The use of palladium on magnetic supports as catalyst for Suzuki-Miyaura cross-coupling reactions. Catalysts 2017, 7, 35. [Google Scholar] [CrossRef]
- Baig, R.B.N.; Nadagouda, M.N.; Varma, R.S. Magnetically retrievable catalysts for asymmetric synthesis. Coord. Chem. Rev. 2015, 287, 137–156. [Google Scholar] [CrossRef]
- Zhang, Q.; Su, H.; Luo, J.; Wei, Y.Y. A magnetic nanoparticle supported dual acidic ionic liquid: A “quasi-homogeneous” catalyst for the one-pot synthesis of benzoxanthenes. Green Chem. 2012, 14, 201–208. [Google Scholar] [CrossRef]
- Dadhania, H.N.; Raval, D.K.; Dadhania, A.N. Magnetically retrievable magnetite (Fe3O4) immobilized ionic liquid: An efficient catalyst for the preparation of 1-carbamatoalkyl-2-naphthols. Catal. Sci. Technol. 2015, 5, 4806–4812. [Google Scholar] [CrossRef]
- Yarie, M.; Zolfigol, M.A.; Bayat, Y.; Asgari, A.; Alonso, D.A.; Khoshnood, A. Novel magnetic nanoparticles with ionic liquid tags as a reusable catalyst in the synthesis of polyhydroquinolines. RSC Adv. 2016, 6, 82842–82853. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, X.; Wei, H.X.; Li, J.H.; Luo, J. Silica-coated nano-Fe3O4-supported iminopyridine palladium complex as an active, phosphine-free and magnetically separable catalyst for Heck reactions. Appl. Organomet. Chem. 2017, 31, e3608. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, J.H.; Zhao, X. Magnetic nanoparticle supported imino-pyridine palladium catalyzed Suzuki reactions. Chin. J. Org. Chem. 2016, 36, 130–136. [Google Scholar] [CrossRef]
- Zhang, Q.; Su, H.; Luo, J.; Wei, Y.Y. Recyclable palladium(II) imino-pyridine complex immobilized on mesoporous silica as a highly active and recoverable catalyst for Suzuki-Miyaura coupling reactions in aqueous medium. Tetrahedron 2013, 69, 447–454. [Google Scholar] [CrossRef]
- Zhang, Q.; Su, H.; Luo, J.; Wei, Y.Y. “Click” magnetic nanoparticle-supported palladium catalyst: A phosphine-free, highly efficient and magnetically recoverable catalyst for Suzuki–Miyaura coupling reactions. Catal. Sci. Technol. 2013, 3, 235–243. [Google Scholar] [CrossRef]
- Zhang, Q.; Wei, H.X.; Li, J.H.; Zhao, X.; Luo, J. One-pot synthesis of benzopyrans catalyzed by silica supported dual acidic ionic liquid under solvent-free conditions. Heterocycl. Commun. 2017. [Google Scholar] [CrossRef]
- Zhang, Q.; Luo, J.; Wei, Y.Y. A silica gel supported dual acidic ionic liquid: An efficient and recyclable heterogeneous catalyst for the one-pot synthesis of amidoalkyl naphthols. Green Chem. 2010, 12, 2246–2254. [Google Scholar] [CrossRef]
- Zhi, H.Z.; Lü, C.X.; Zhang, Q.; Luo, J. A new PEG-1000-based dicationic ionic liquid exhibiting temperature-dependent phase behavior with toluene and its application in one-pot synthesis of benzopyrans. Chem. Commun. 2009, 2878–2880. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Zhang, Q. A one-pot multicomponent reaction for synthesis of 1-amidoalkyl-2-naphthols catalyzed by PEG-based dicationic acidic ionic liquids under solvent-free conditions. Monatshefte Chem. 2011, 142, 923–930. [Google Scholar] [CrossRef]
- Shaterian, H.R.; Yarahmadi, H.; Ghashang, M. Silica supported perchloric acid (HClO4–SiO2): An efficient and recyclable heterogeneous catalyst for the one-pot synthesis of amidoalkyl naphthols. Tetrahedron 2008, 64, 1263–1269. [Google Scholar] [CrossRef]
- Nandi, G.C.; Samai, S.; Kumar, R.; Singh, M.S. Atom-efficient and environment-friendly multicomponent synthesis of amidoalkyl naphthols catalyzed by P2O5. Tetrahedron Lett. 2009, 50, 7220–7222. [Google Scholar] [CrossRef]
- Hajipour, A.R.; Ghayeb, Y.; Sheikhan, N.; Ruoho, A.E. Brønsted acidic ionic liquid as an efficient and reusable catalyst for one-pot synthesis of 1-amidoalkyl 2-naphthols under solvent-free conditions. Tetrahedron Lett. 2009, 50, 5649–5651. [Google Scholar] [CrossRef]
- Baghbanian, S.M.; Rezaei, N.; Tashakkorian, H. Nanozeolite clinoptilolite as a highly efficient heterogeneous catalyst for the synthesis of various 2-amino-4H-chromene derivatives in aqueous media. Green Chem. 2013, 15, 3446–3458. [Google Scholar] [CrossRef]
- Wang, Y.L.; Li, Z.; Luo, J.; Liu, Z.L. One-pot synthesis of tetrahydrobenzo[b]pyrans catalyzed by PEG-1000 bridged primary amine functionalized dicationic ionic liquid in Water. J. Chin. Chem. Soc. 2013, 60, 1431–1436. [Google Scholar] [CrossRef]
- Hekmatshoar, R.; Majedi, S.; Bakhtiari, K. Sodium selenate catalyzed simple and efficient synthesis of tetrahydrobenzo[b]pyran derivatives. Catal. Commun. 2008, 9, 307–310. [Google Scholar] [CrossRef]
- Bhosale, R.S.; Magar, C.V.; Solanke, K.S.; Mane, S.B.; Choudhary, S.S.; Pawar, R.P. Molecular iodine: An efficient catalyst for the synthesis of tetrahydrobenzo[b]pyrans. Synth. Commun. 2007, 37, 4353–4357. [Google Scholar] [CrossRef]


| Entry | Catalyst (mg) | Temperature (°C) | Time (min) | Isolated Yield (%) |
|---|---|---|---|---|
| 1 | None | 90 | 60 | Trace |
| 2 | AIL@MNP (20) | 90 | 30 | 52 |
| 3 | AIL@MNP (40) | 90 | 20 | 77 |
| 4 | AIL@MNP (60) | 90 | 10 | 91 |
| 5 | AIL@MNP (80) | 90 | 10 | 92 |
| 6 | AIL@MNP (100) | 90 | 10 | 90 |
| 7 | AIL@MNP (60) | r.t. | 120 | 18 |
| 8 | AIL@MNP (60) | 50 | 60 | 54 |
| 9 | AIL@MNP (60) | 75 | 30 | 83 |
| 10 | AIL@MNP (60) | 100 | 10 | 91 |
| 11 | SiO2@Fe3O4 (60) | 90 | 60 | 22 |
| 12 | AIL@SiO2 (60) | 90 | 10 | 90 |
| Entry | Aldehyde R1 | Amide R2 | Time (min) | Product | Yield (%) b | M.p. (°C) | |
|---|---|---|---|---|---|---|---|
| Found | Reported | ||||||
| 1 | Ph | CH3 | 10 |  | 91 | 230–232 | 227–229 [17] |
| 2 | 4-NO2-C6H4 | CH3 | 7 |  | 94 (93) c | 243–245 | 245–247 [19] |
| 3 | 2,4-Cl2-C6H3 | CH3 | 20 |  | 85 | 201–203 | 201–203 [7] |
| 4 | 3-Cl-C6H4 | CH3 | 20 |  | 83 | 235–237 | 237–238 [48] |
| 5 | 4-Br-C6H4 | CH3 | 20 |  | 90 | 227–229 | 229–231 [11] |
| 6 | 3-MeO-C6H4 | CH3 | 15 |  | 87 | 201–203 | 201–204 [7] |
| 7 | 4-Me-C6H4 | CH3 | 25 |  | 84 | 221–223 | 222–223 [49] |
| 8 | n-C3H7 | CH3 | 12 |  | 85 (82) c | 221–222 | 222–223 [46] |
| 9 | i-C4H9 | CH3 | 15 |  | 87 | 194–196 | 195–197 [46] |
| 10 | 3-NO2-C6H4 | Ph | 10 |  | 95 | 241–243 | 242–243 [50] |
| 11 | 4-Me-C6H4 | Ph | 20 |  | 86 (84) c | 213–215 | 212–215 [14] |
| 12 | n-C3H7 | Ph | 10 |  | 86 | 219–221 | 220–222 [46] |
| 13 | 4-Cl-C6H4 | CH2=CH | 15 |  | 89 | 211–213 | 212–213 [17] |
| 14 | 4-NO2-C6H4 | CH2=CH | 8 |  | 93 | 224–226 | 223–225 [48] |
| 15 | n-C3H7 | CH2=CH | 10 |  | 83 | 188–190 | 190–191 [48] |
| Entry | Catalyst | Conditions | Time (min) | Yield (%) | Ref. |
|---|---|---|---|---|---|
| 1 | Nano Al2O3 | Solvent-free/110 °C | 30 | 80 | [11] |
| 2 | [TEBSA][HSO4] | Solvent-free/120 °C | 10 | 88 | [51] |
| 3 | β-CD-BSA | Solvent-free/100 °C | 7 | 95 | [17] |
| 4 | Ba3(PO4)2 | Solvent-free/100 °C | 35 | 88 | [10] |
| 5 | Fe3O4@SiO2-Imid-PMA | Solvent-free/100 °C | 20 | 96 | [13] |
| 6 | MWCNT@Co-complex | Solvent-free/75 °C | 20 | 95 | [19] |
| 7 | HClO4-SiO2 | Solvent-free/110 °C | 30 | 95 | [49] |
| 8 | Fe(HSO4)3 | Solvent-free/85 °C | 25 | 92 | [7] |
| 9 | [C6(MPy)2][CoCl4]2− | Solvent-free/120 °C | 15 | 93 | [18] |
| 10 | AIL@MNP | Solvent-free/90 °C | 7 | 94 | This work |
| Entry | R | Time (min) | Product | Yield (%) b | M.p. (°C) | |
|---|---|---|---|---|---|---|
| Found | Reported | |||||
| 1 | Ph | 25 |  | 89 | 230–232 | 227–229 [27] |
| 2 | 4-F-C6H4 | 22 |  | 90 | 190–192 | 188–189 [29] |
| 3 | 3-Br-C6H4 | 20 |  | 92 (90) c | 229–230 | 227–228 [25] |
| 4 | 3,4-Cl2-C6H3 | 20 |  | 93 | 224–226 | 225–227 [47] |
| 5 | 4-Cl-C6H4 | 22 |  | 91 | 212–214 | 213–214 [52] |
| 6 | 3-NO2-C6H4 | 20 |  | 94 (92) c | 213–215 | 214–216 [24] |
| 7 | 2-NO2-C6H4 | 20 |  | 92 | 223–225 | 223–224 [27] |
| 8 | 4-OH-C6H4 | 30 |  | 88 | 224–226 | 225–227 [29] |
| 9 | 4-Me-C6H4 | 35 |  | 86 (83) c | 216–218 | 217–219 [24] |
| 10 | 3-OH-4-OMe-C6H3 | 40 |  | 86 | 237–238 | 238–240 [53] |
| 11 | 4-Me2N-C6H4 | 40 |  | 85 | 212–214 | 210–213 [52] |
| 12 | 2-Furyl | 30 |  | 90 | 221–223 | 219–221 [29] |
| 13 | C3H7 | 60 |  | 76 | 171–172 | 170–172 [24] |
| Entry | Catalyst | Conditions | Time (min) | Yield (%) | Reference |
|---|---|---|---|---|---|
| 1 | SO42−/MCM-41 | EtOH/Reflux | 60 | 80 | [22] |
| 2 | Na2SeO4 | EtOH-H2O/Reflux | 180 | 90 | [54] |
| 3 | Iodine | DMSO/120 °C | 210 | 88 | [55] |
| 4 | Fe3O4@SiO2@NH-NH2-PW | H2O/Reflux | 25 | 92 | [28] |
| 5 | Fe3O4@SiO2-Imid-PMA | H2O/Reflux | 10 | 95 | [29] |
| 6 | NH4H2PO4/Al2O3 | Solvent-free/80 °C | 30 | 88 | [23] |
| 7 | H3PMo12O40 | H2O/Reflux | 25 | 76 | [16] |
| 8 | Nanozeolite CP | H2O/Reflux | 15 | 98 | [52] |
| 9 | AIL@MNP | Solvent-free/90 °C | 22 | 91 | This work |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Gao, Y.-H.; Qin, S.-L.; Wei, H.-X. Facile One-Pot Synthesis of Amidoalkyl Naphthols and Benzopyrans Using Magnetic Nanoparticle-Supported Acidic Ionic Liquid as a Highly Efficient and Reusable Catalyst. Catalysts 2017, 7, 351. https://doi.org/10.3390/catal7110351
Zhang Q, Gao Y-H, Qin S-L, Wei H-X. Facile One-Pot Synthesis of Amidoalkyl Naphthols and Benzopyrans Using Magnetic Nanoparticle-Supported Acidic Ionic Liquid as a Highly Efficient and Reusable Catalyst. Catalysts. 2017; 7(11):351. https://doi.org/10.3390/catal7110351
Chicago/Turabian StyleZhang, Qiang, Yin-Hong Gao, Shan-Lin Qin, and Huai-Xin Wei. 2017. "Facile One-Pot Synthesis of Amidoalkyl Naphthols and Benzopyrans Using Magnetic Nanoparticle-Supported Acidic Ionic Liquid as a Highly Efficient and Reusable Catalyst" Catalysts 7, no. 11: 351. https://doi.org/10.3390/catal7110351