Synthesis of Phase Pure Hexagonal YFeO3 Perovskite as Efficient Visible Light Active Photocatalyst
Abstract
:1. Introduction
2. Results and Discussion
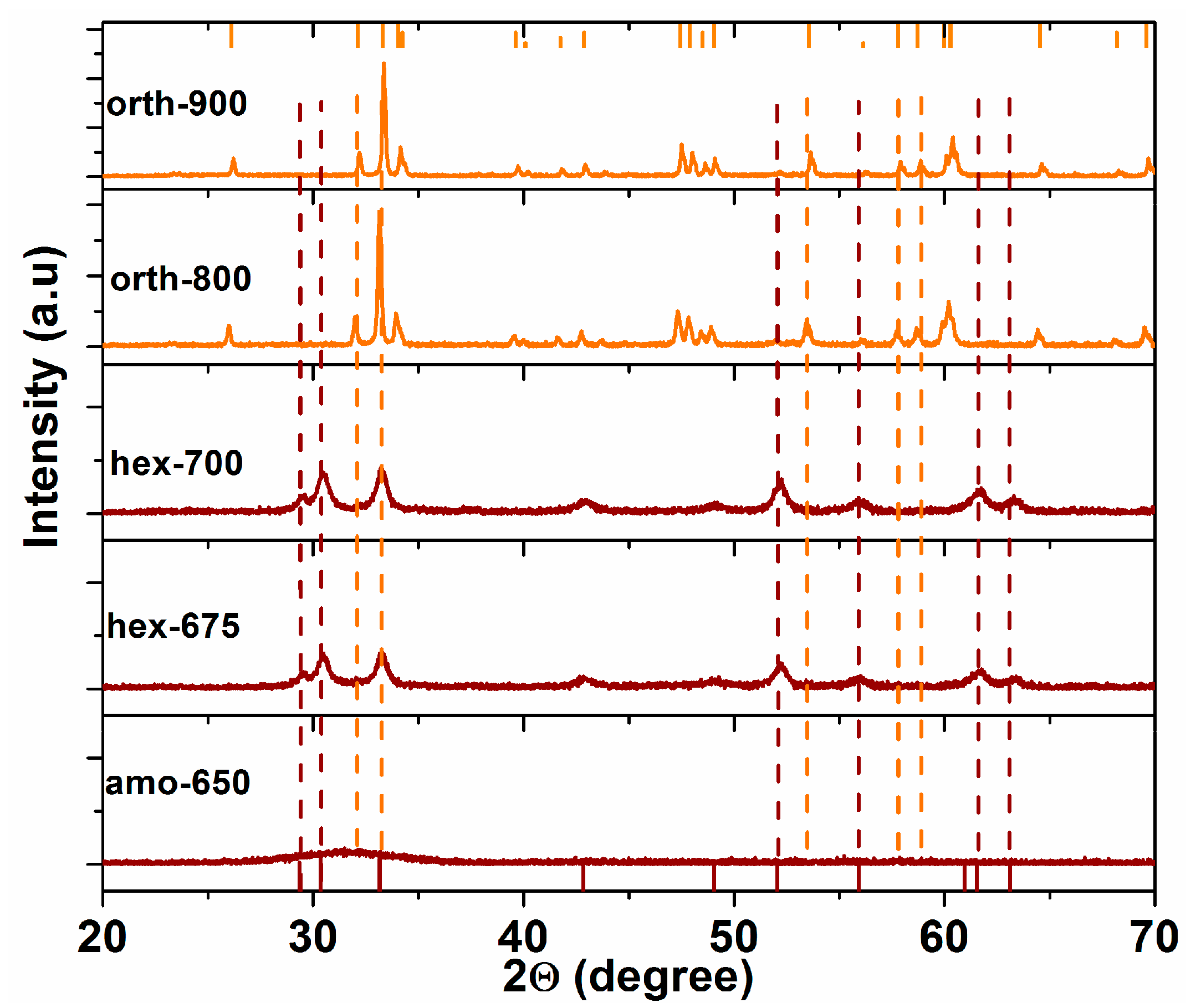
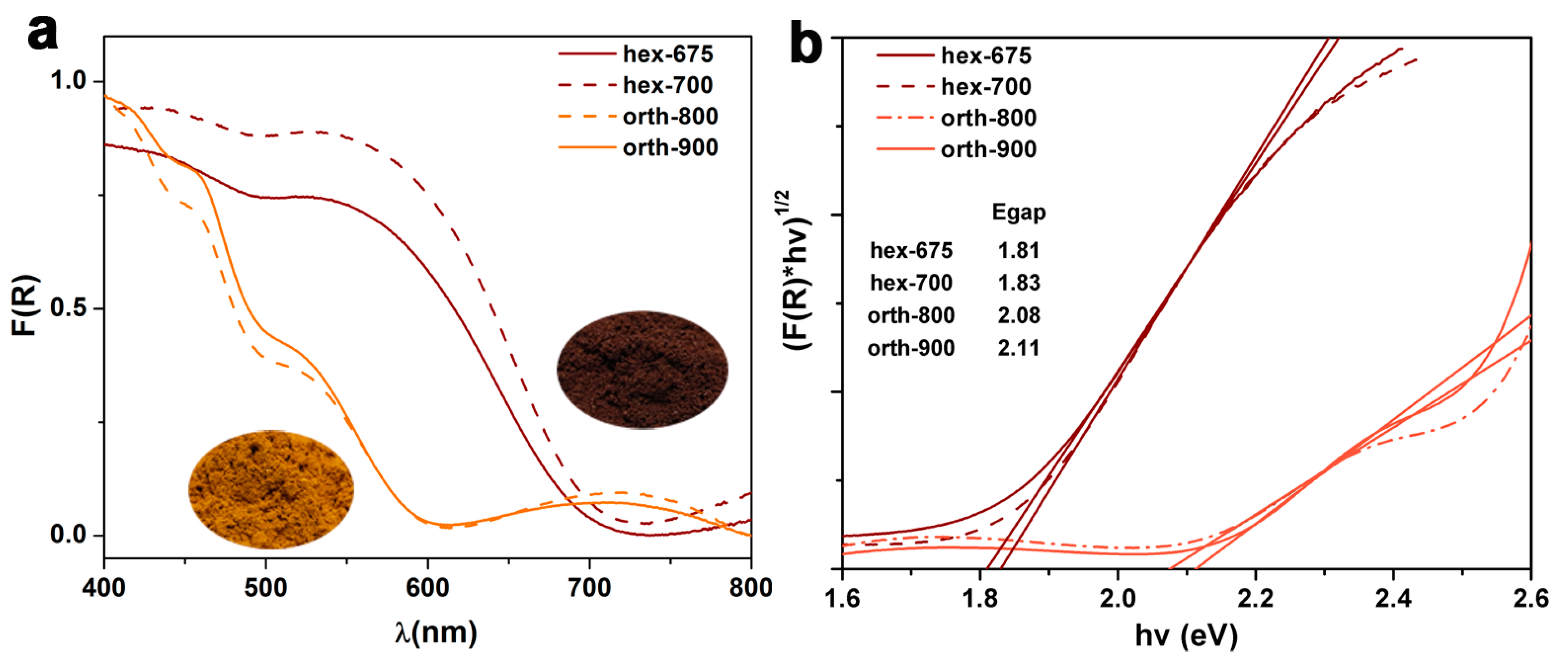
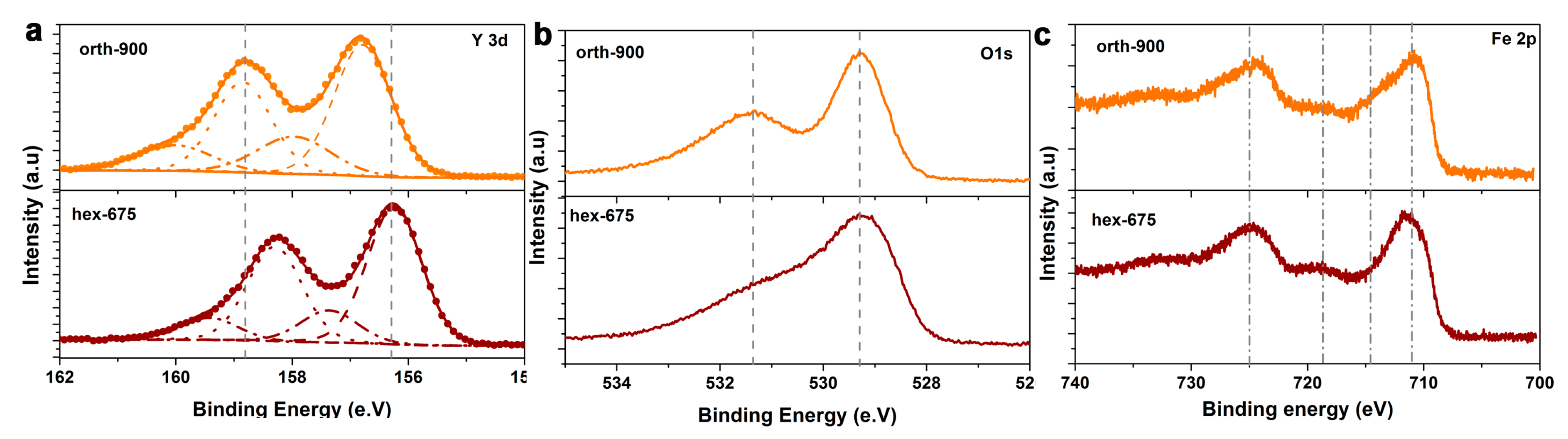
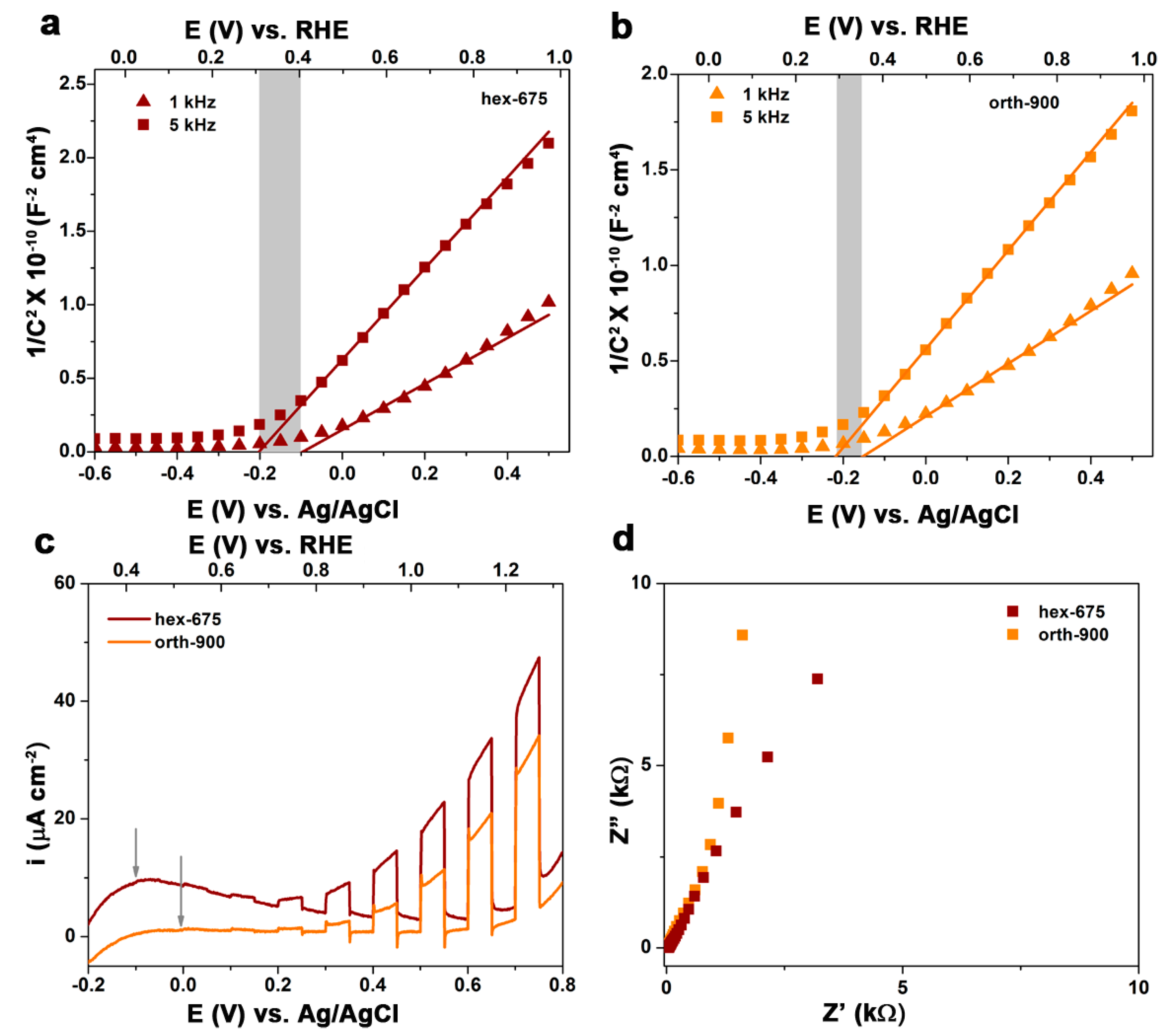
2.1. Structural, Optical, and Electrochemical Characterization of Hexagonal YFeO3
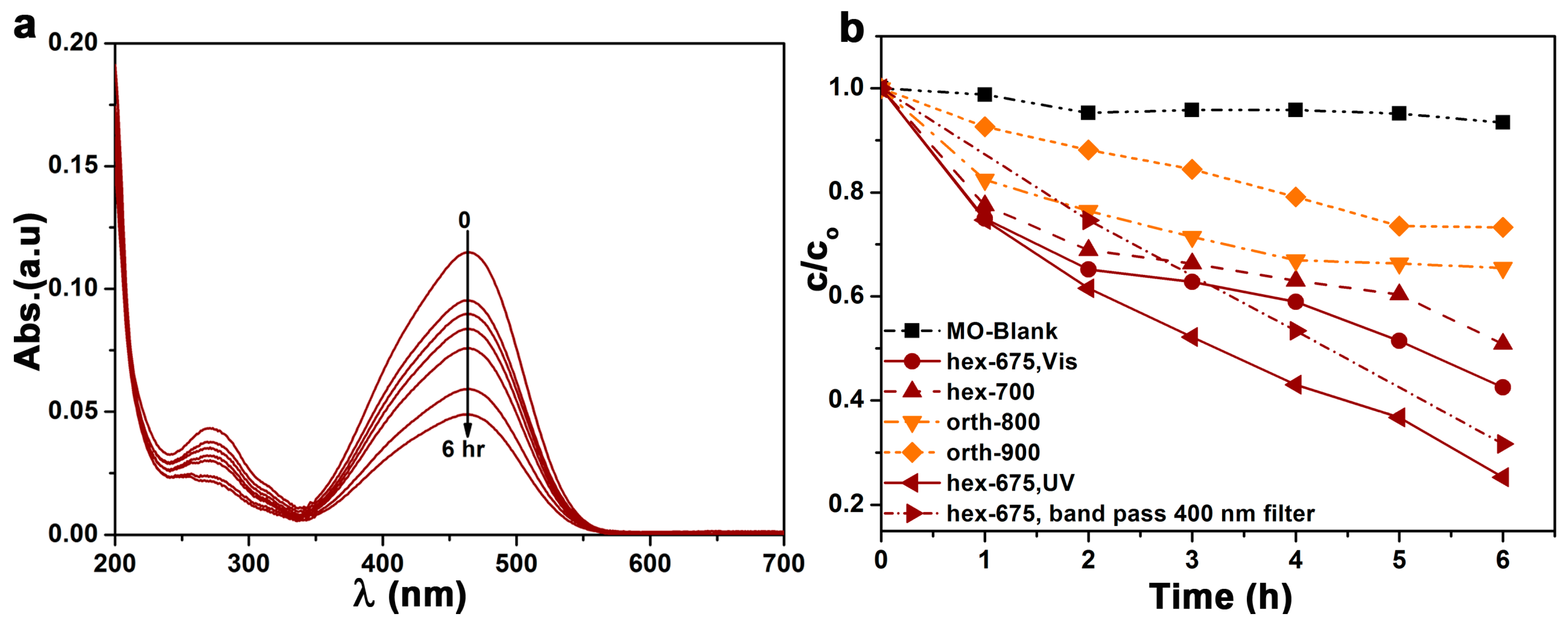
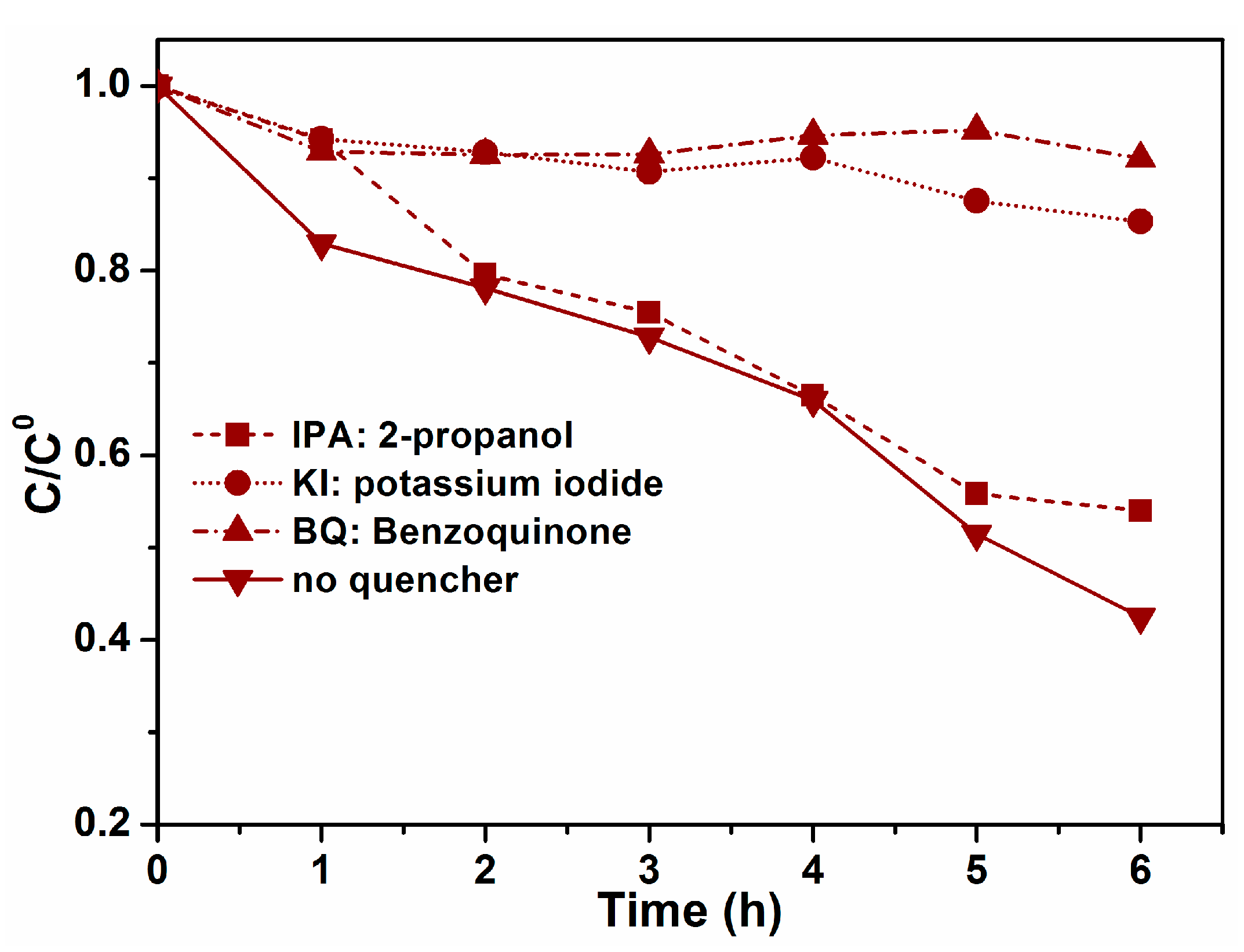
2.2. Photocatalytic Activity of Hexagonal YFeO3
3. Materials and Methods
3.1. Materials
3.2. Synthesis of YFeO3
3.3. Characterization
3.4. Photocatalytic Degradation Activity Measurement
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tang, P.; Chen, H.; Cao, F.; Pan, G. Magnetically recoverable and visible-light-driven nanocrystalline YFeO3 photocatalysts. J. Catal. Sci. Technol. 2011, 1, 1145–1148. [Google Scholar] [CrossRef]
- Li, X.; Kikugawa, N.; Ye, J. Nitrogen-doped lamellar niobic acid with visible light-responsive photocatalytic activity. J. Adv. Mater. 2008, 20, 3816–3819. [Google Scholar] [CrossRef]
- Varghese, O.K.; Paulose, M.; La Tempa, T.J.; Grimes, C.A. High-rate solar photocatalytic conversion of CO2 and water vapor to hydrocarbon fuels. Nano Lett. 2009, 9, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhang, Y.; Kudo, A. Synthesis and photocatalytic performances of BiVO4 by ammonia co-precipitation process. J. Solid State Chem. 2009, 182, 223–228. [Google Scholar] [CrossRef]
- Konta, R.; Ishii, T.; Kato, H.; Kudo, A. Photocatalytic Activities of Noble Metal Ion Doped SrTiO3 under Visible Light Irradiation. J. Phys. Chem. B 2004, 108, 8992–8995. [Google Scholar] [CrossRef]
- Dho, J.; Qi, X.; Kim, H.; MacManus-Driscoll, J.L.; Blamire, M.G. Large electric polarization and exchange bias in multiferroic, BiFeO3. Adv. Mater. 2006, 18, 1445–1448. [Google Scholar] [CrossRef]
- Pimenov, A.; Mukhin, A.A.; Ivanov, V.Y.; Travkin, V.D.; Balbashov, A.M.; Loidl, A. Possible evidence for electromagnons in multiferroic manganites. Nat. Phys. 2006, 2, 97–100. [Google Scholar] [CrossRef]
- Peña, M.A.; Fierro, J.L.G. Chemical Structure and Performance of Pervoskite Oxides. Chem. Rev. 2001, 101, 1981–2018. [Google Scholar] [CrossRef] [PubMed]
- Goldschmidt, M.V. Die Gesetze der Kristallochemie. Die Naturwissenschaften 1926, 14, 477–485. [Google Scholar] [CrossRef]
- Dillert, R.; Taffa, D.H.; Wark, M.; Bredow, T.; Bahnemann, D.W. Research Update: Photoelectrochemical water splitting and photocatalytic hydrogen production using ferrites (MFe2O4) under visible light irradiation. APL Mater. 2015, 3. [Google Scholar] [CrossRef] [Green Version]
- Sharma, K.V.; He, C.; Doong, R.; Dionysiou, D.D. Green Materials for Energy, Products and Depollution. In Environmental Chemistry for a Sustainable World; Lichtfouse, E., Schwarzbauer, J., Robert, D., Eds.; Springer: Berlin, Germany, 2013; Chapter 4; Volume 3, pp. 135–150. [Google Scholar]
- Taffa, D.H.; Dillert, R.; Ulbe, A.C.; Bauerfeind, K.C.L.; Bredow, T.; Bahnemann, D.W.; Wark, M. Photoelectrochemical and theoretical investigations of spinel type ferrites (MxFe3−xO4) for water splitting: A mini-review. J. Photon. Energy 2016, 7. [Google Scholar] [CrossRef]
- Mathur, S.; Veith, M.; Rapalaviciute, R.; Shen, H.; Goya, G.F.; Fliho, W.M.; Berquo, T.S. Molecule derived synthesis of nanocrystalline YFeO3 and investigations on its weak ferromagnetic behavior. Chem. Mater. 2004, 16, 1906–1913. [Google Scholar] [CrossRef]
- Maiti, R.; Basu, S.; Chakravorty, D. Synthesis of nanocrystalline YFeO3 and its magnetic properties. J. Magn. Mater. 2009, 321, 3274–3277. [Google Scholar] [CrossRef]
- Shen, H.; Xu, J.; Wu, A.; Zhao, J.; Shi, M. Magnetic and thermal properties of perovskite YFeO3 single crystals. Mater. Sci. Eng. B 2009, 157, 77–80. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, J.; Wang, X.; Feng, F.; Zhang, Y.; Tang, Y. Synthesis YFeO3 by salt-assisted solution combustion method and its photocatalytic activity. J. Ceram. Soc. Jpn. 2014, 122, 146–150. [Google Scholar] [CrossRef]
- Wang, W.; Li, S.; Wen, Y.; Gong, M.; Zhang, L.; Yao, Y. Synthesis and characterization of TiO2/YFeO3 and its photocatalytic oxidation of gaseous benzene. Acta Phys. Chim. Sin. 2008, 24, 1761–1766. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, J.; Xu, J.; Gao, Q. Controllable synthesis of hexagonal and orthorhombic YFeO3 and their visible-light photocatalytic activity. Mater. Lett. 2012, 81, 1–4. [Google Scholar] [CrossRef]
- Lu, X.; Xie, J.; Shu, H.; Liu, J.; Yin, G.; Lin, J. Microwave-assisted synthesis of nanocrystalline YFeO3 and study of its photoactivity. Mater. Sci. Eng. B 2007, 138, 289–292. [Google Scholar] [CrossRef]
- Yan, C.-H.; Xu, Z.-G.; Cheng, F.-X.; Wang, Z.-M.; Sun, L.-D.; Liao, C.-S.; Jia, J.-T. Nanophased CoFe2O4 prepared by combustion method. Solid State Commun. 1999, 111, 287–291. [Google Scholar] [CrossRef]
- Zhang, R.; Chen, C.; Jin, K.; Niu, L.; Xing, H.; Luo, B. Dielectric behavior of hexagonal and orthorhombic YFeO3 prepared by modified sol-gel method. J. Electroceram. 2014, 32, 187–191. [Google Scholar] [CrossRef]
- Wu, L.; Yu, J.; Zhang, L.; Wang, X.; Li, S. Selective self-propagating combustion synthesis of hexagonal and orthorhombic nanocrystalline yttrium iron oxide. J. Solid State Chem. 2004, 177, 3666–3674. [Google Scholar] [CrossRef]
- Downie, L.J.; Goff, R.J.; Kockelmann, W.; Forder, S.D.; Parker, J.E.; Morrison, F.D.; Lightfoot, P. Structural, magnetic and electrical properties of the hexagonal ferrites MFeO3 (M = Y, Yb, In). J. Solid State Chem. 2012, 190, 52–60. [Google Scholar] [CrossRef]
- Gil, D.; Navarro, C.; Cristina, A.; Lagarrigue, C.; Guimpel, J.; Carbonio, R.; Gomez, I. Synthesis and structural characterization of perovskite YFeO3 by thermal decomposition of a cyano complex precursor, Y[Fe(CN)6]·4H2O. J. Therm. Anal. Calorim. 2011, 103, 889–896. [Google Scholar] [CrossRef]
- Popkov, V.I.; Almjasheva, O.V.; Gusarov, V.V. The Investigation of the Structure Control Possibility of Nanocrystalline Yttrium Orthoferrite in Its Synthesis from Amorphous Powders. Russ. J. Appl. Chem. 2014, 2, 1417–1421. [Google Scholar] [CrossRef]
- Nishimura, T.; Hosokawa, S.; Masuda, Y.; Wada, K.I. Masashi, Synthesis of metastable rare-earth-iron mixed oxide with the hexagonal crystal structure. J. Solid State Chem. 2013, 197, 402–407. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.A.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution. Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Marschall, R.; Soldat, J.; Wark, M. Enhanced photocatalytic hydrogen generation from barium tantalate composites. Photochem. Photobiol. Sci. 2013, 12, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.; Sun, H.; Chen, H.; Cao, F. Hydrothermal processing-assisted synthesis of nanocrystalline YFeO3 and its visible-light photocatalytic activity. Curr. Nanosci. 2012, 8, 64–67. [Google Scholar] [CrossRef]
- Majumdar, D.; Chatterjee, D. X-ray photoelectron spectroscopic studies on yttria, zirconia, and yttria-stabilized zirconia. J. Appl. Phys. 1991, 70, 988–992. [Google Scholar] [CrossRef]
- Vasquez, R.P.; Foote, M.C.; Hunt, B.D. Reaction of non-aqueous halogen solutions with YBa2Cu3O7−x. J. Appl. Phys. 1989, 66, 4867–4877. [Google Scholar] [CrossRef]
- Li, J.; Singh, U.G.; Schladt, T.U.; Stalick, J.K.; Scott, S.L.; Seshadri, R. Hexagonal YFe1−x PdxO3−δ: Nonperovskite Host Compounds for Pd2+ and Their Catalytic Activity for CO Oxidation. Chem. Mater. 2008, 20, 6567–6576. [Google Scholar] [CrossRef]
- Lu, X.; Liu, J.; Zhu, J.; Jiang, D.; Xie, J. Preparation and characterization of heterojunction semiconductor YFeO3/TiO2 with an enhanced photocatalytic activity. J. Mater. Res. 2010, 25, 104–109. [Google Scholar] [CrossRef]
- Wang, C.W.; Badylevich, M.; Afanasev, V.V.; Stesmans, A.; Adelmann, C.; Van Elshocht, S.; Kittl, T.A.; Lukosius, M.; Walczyk, C.; Wenger, C. Band alignment and electron traps in Y2O3 layers on (100) Si. Appl. Phys. Lett. 2009, 95. [Google Scholar] [CrossRef]
- Zhao, Y.; Kita, K.; Kyuno, K.; Toriumi, A. Design of higher-k and more stable rare earth oxides as gate dielectrics for advanced CMOS devices. Appl. Phys. Lett. 2009, 94, 1413–1438. [Google Scholar] [CrossRef]
- Hisatomi, T.; Formal, F.L.; Cornuz, M.; Brillet, J.; Tetreault, N.; Sivula, K.; Graetzel, M. Cathodic shift in onset potential of solar oxygen evolution on hematite by 13-group oxide overlayers. Energy Environ. Sci. 2011, 4, 2512–2515. [Google Scholar] [CrossRef]
- Kay, A.; Graetzel, M. Dye-sensitized core-shell nanocrystals: Improved efficiency of mesoporous tin oxide electrodes coated with a thin layer of an insulating oxide. Chem. Mater. 2002, 14, 2930–2935. [Google Scholar] [CrossRef]
- Wu, W.; Liang, S.; Shen, L.; Ding, Z.; Zheng, H.; Su, W.; Wu, L. Preparation, characterization and enhanced visible light photocatalytic activities of polyaniline/Bi3NbO7 nanocomposites. J. Alloys Compod. 2012, 520, 213–219. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, X.; Li, G.; Cao, Y.; Shao, Y.; Li, D. Highly efficient Bi2O2CO3/BiOCl photocatalyst based on heterojunction with enhanced dye-sensitization under visible light. Appl. Catal. B Environ. 2016, 187, 301–309. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, B.; Yang, S.; Wu, H.; Wu, L.; Pan, J.; Xiong, X. In Situ construction of an SnO2/g-C3N4 heterojunction for enhanced visible-light photocatalytic activity. RSC Adv. 2015, 5, 68953–68963. [Google Scholar] [CrossRef]
- Xu, N.; Shi, Z.; Fan, Y.; Dong, J.; Shi, J.; Hu, M. Effects of Particle Size of TiO2 on Photocatalytic Degradation of Methylene Blue in Aqueous Suspensions. Ind. Eng. Chem. Res. 1999, 38, 373–379. [Google Scholar] [CrossRef]
- Ghobadi, A.; Gamze Ulusoy, T.; Garifullin, R.; Mustafa, O.G.; Okyay, A.K. Heterojunction Design of Single Layer Hole Tunneling ZnO Passivation Wrapping around TiO2 Nanowires for Superior Photocatalytic Performance. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Krumova, K.; Cosa, G. Overview of reactive oxygen species. In Singlet Oxygen, Applications in Biosciences and Nanosciences; Nonell, S., Flors, C., Eds.; Royal Society of Chemistry: London, UK, 2016; Chapter 1; Volume 1, pp. 1–21. [Google Scholar]
- Liu, W.; Wang, M.; Xu, C.; Chen, S. Facile synthesis of g-C3N4/ZnO composite with enhanced visible light photooxidation and photoreduction properties. Chem. Eng. J. 2012, 209, 386–393. [Google Scholar] [CrossRef]
- Ju, L.; Chen, Z.; Fang, L.; Dong, W.; Zheng, F.; Shen, M. Sol-gel synthesis and Photo-Fenton-like catalytic activity of EuFeO3 nanoparticles. J. Am. Ceram. Soc. 2011, 94, 3418–3424. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, L.; Lin, H.; Nong, Q.; Wu, Y.; Wu, T.; He, Y. Synthesis and characterization of a ZrO2/g-C3N4 composite with enhanced visible-light photoactivity for rhodamine degradation. RSC Adv. 2014, 4, 40029–40035. [Google Scholar] [CrossRef]
- Yang, X.; Cui, H.; Li, Y.; Qin, J.; Zhang, R.; Tang, H. Fabrication of Ag3PO4-graphene composites with highly efficient and stable visible light photocatalytic performance. ACS Catal. 2013, 3, 363–369. [Google Scholar] [CrossRef]
- Wang, D.; Kako, T.; Ye, J. Efficient photocatalytic decomposition of acetaldehyde over a solid-solution perovskite (Ag0.75Sr0.25)(Nb0.75Ti0.25)O3 under visible-light irradiation. J. Am. Chem. Soc. 2008, 130, 2724–2725. [Google Scholar] [CrossRef] [PubMed]
- Neubert, S.; Ramakrishnan, A.; Strunk, J.; Mei, B.; Wang, L.; Bledowski, M.; Guschin, D.A.; Wang, Y.; Muhler, M.; Beranek, R. Surface-modified TiO2 photocatalysts prepared by a photosynthetic route: Mechanism, enhancement and limits. ChemPlusChem 2013, 79, 163–170. [Google Scholar] [CrossRef]
- Cui, M.; Nong, Q.; Yu, J.; Lin, H.; Wu, Y.; Jiang, X.; Liu, X.; He, Y. Preparation, characterization, and photocatalytic activity of CdV2O6 nanorods decorated g-C3N4 composite. J. Mol. Catal. A Chem. 2016, 423, 240–247. [Google Scholar] [CrossRef]

| Samples | % Fe | % Y | % O |
| hex-675 | 20.2 | 26.6 | 53.2 |
| orth-900 | 20.1 | 27.6 | 52.2 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ismael, M.; Elhaddad, E.; Taffa, D.H.; Wark, M. Synthesis of Phase Pure Hexagonal YFeO3 Perovskite as Efficient Visible Light Active Photocatalyst. Catalysts 2017, 7, 326. https://doi.org/10.3390/catal7110326
Ismael M, Elhaddad E, Taffa DH, Wark M. Synthesis of Phase Pure Hexagonal YFeO3 Perovskite as Efficient Visible Light Active Photocatalyst. Catalysts. 2017; 7(11):326. https://doi.org/10.3390/catal7110326
Chicago/Turabian StyleIsmael, Mohammed, Engy Elhaddad, Dereje H. Taffa, and Michael Wark. 2017. "Synthesis of Phase Pure Hexagonal YFeO3 Perovskite as Efficient Visible Light Active Photocatalyst" Catalysts 7, no. 11: 326. https://doi.org/10.3390/catal7110326





