On the Origin of Enhanced Photocatalytic Activity of Copper-Modified Titania in the Oxidative Reaction Systems
Abstract
:1. Introduction
2. Photocatalytic Systems Based on Cu/TiO2
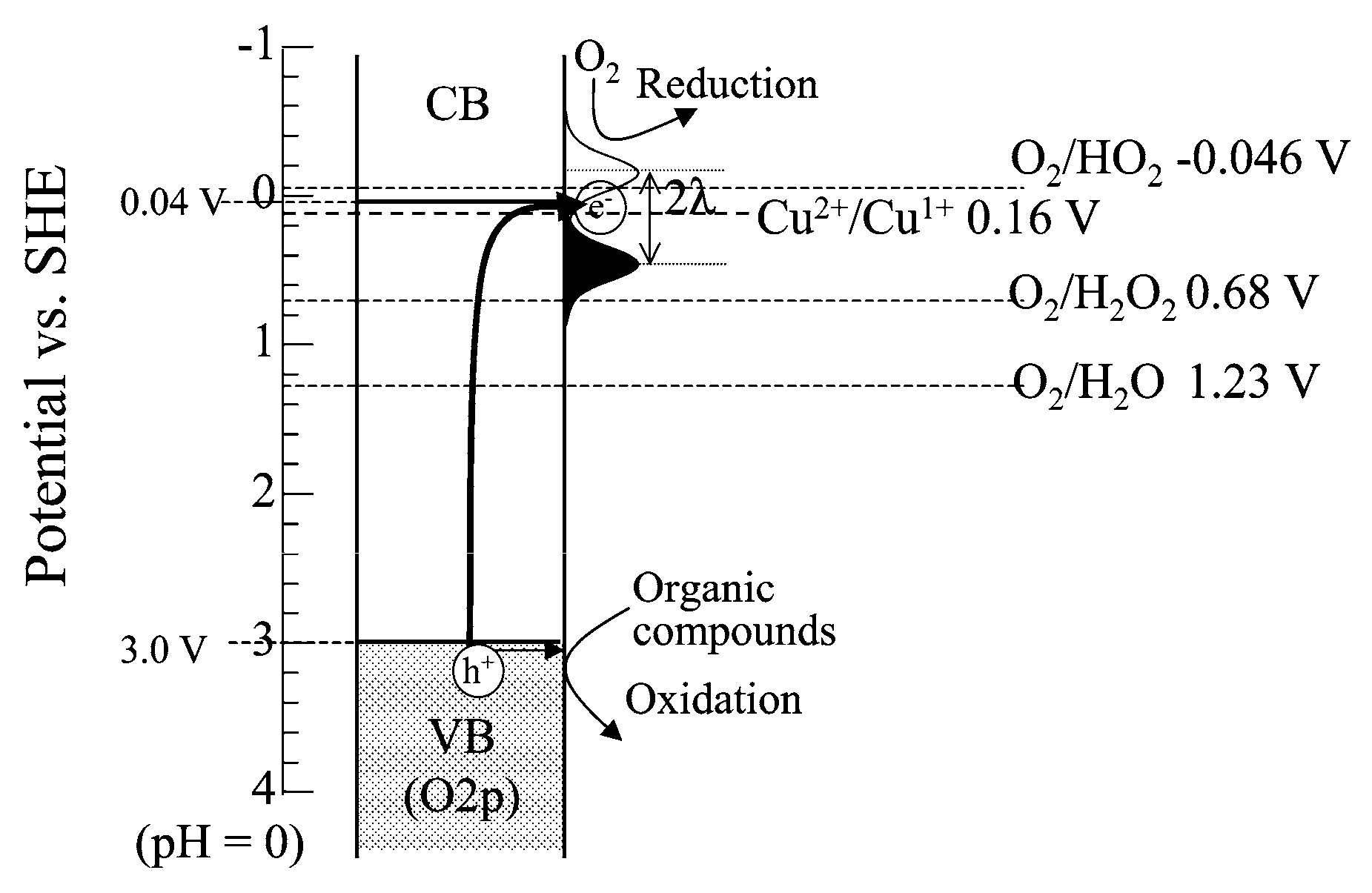
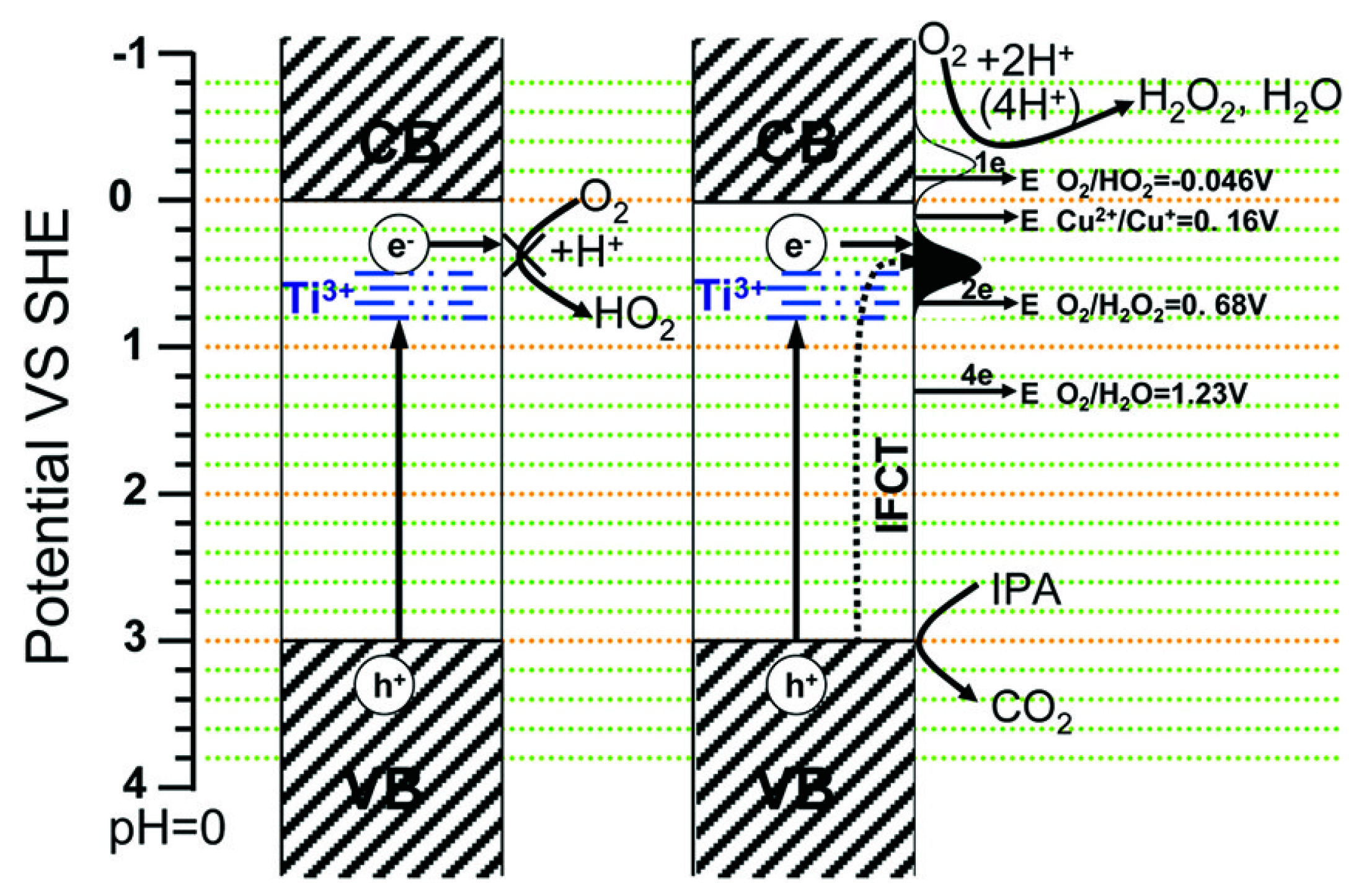
2.1. Presence of Copper Ions in the TiO2 Suspension
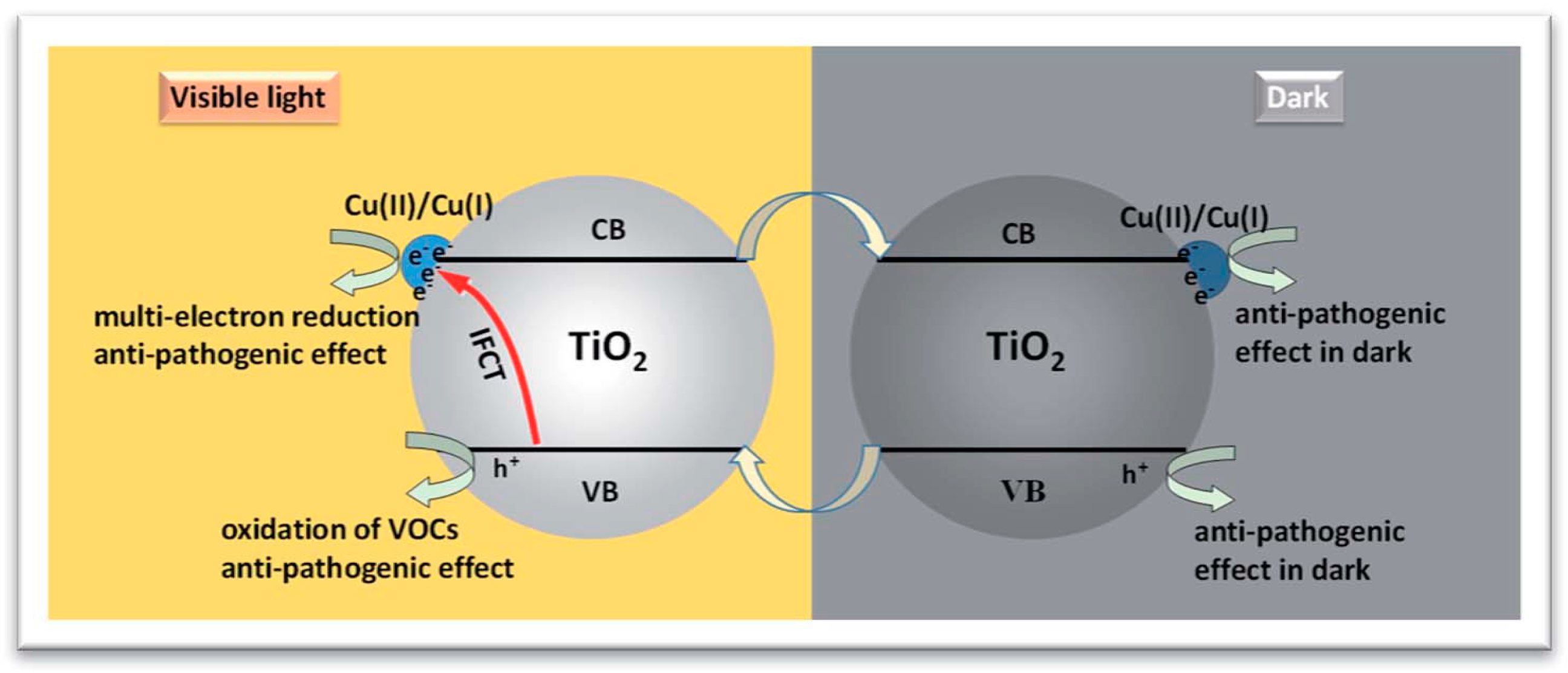
2.2. Copper Species Deposited on TiO2 Surface
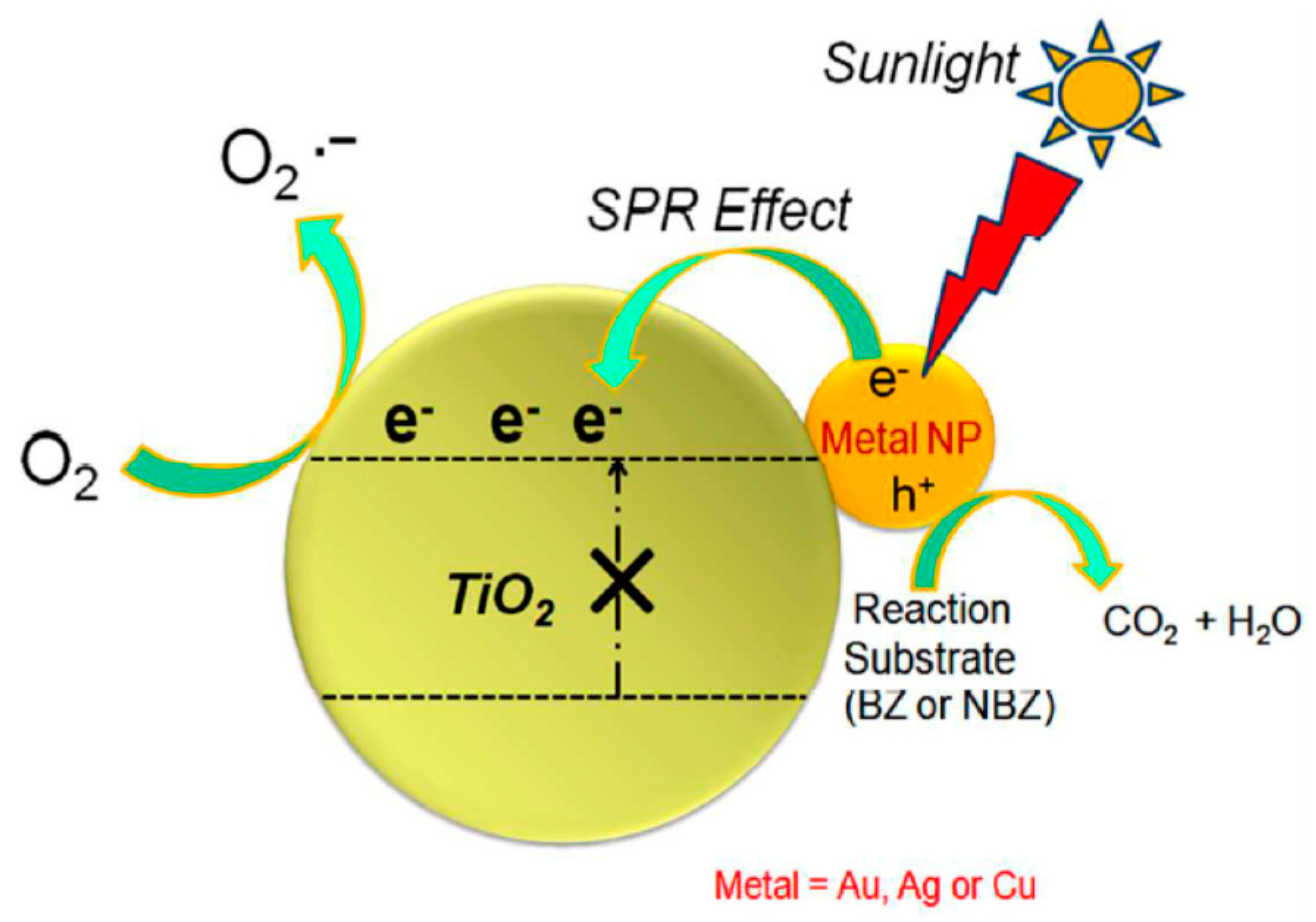
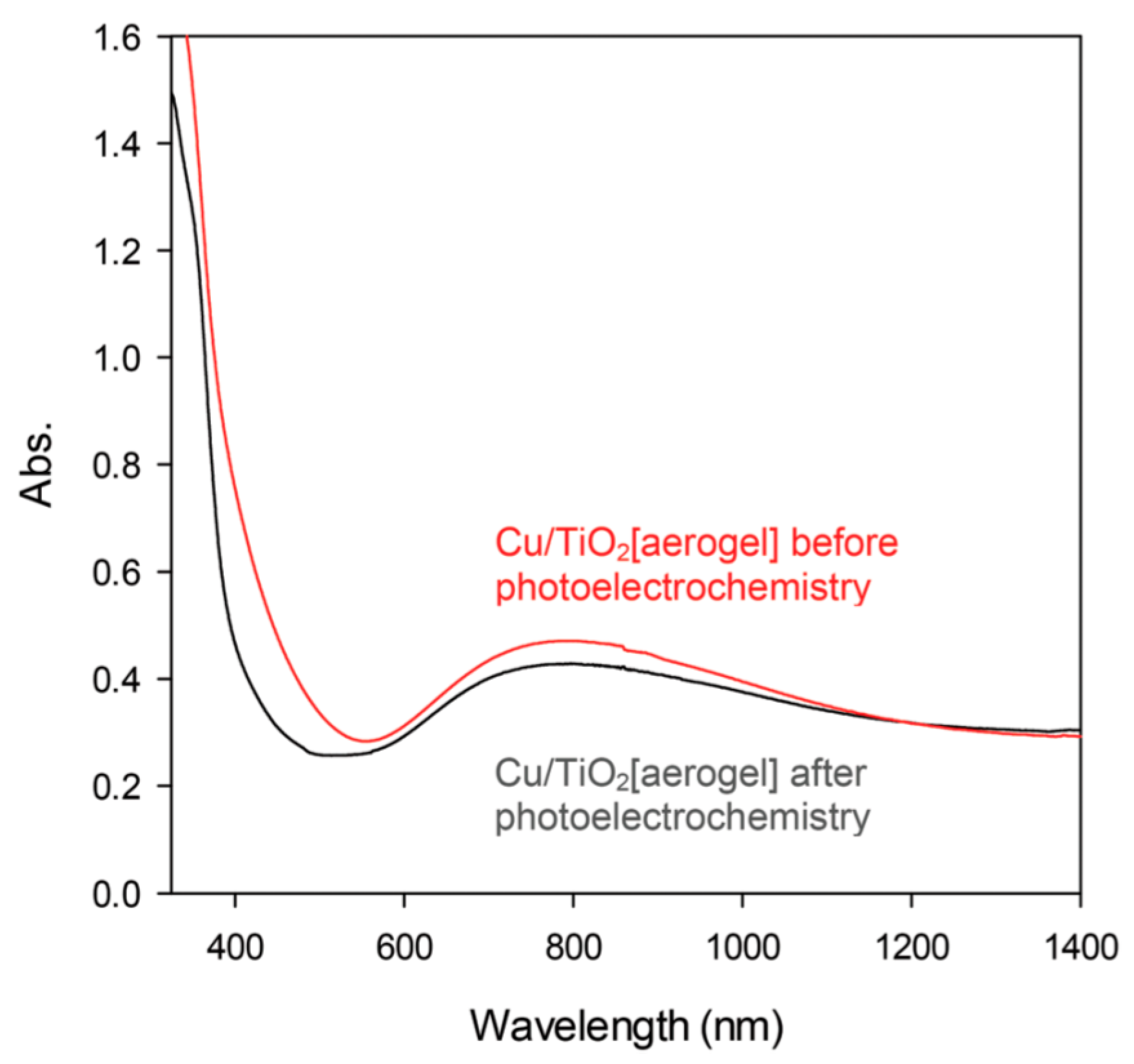
2.2.1. Metallic Copper—Plasmonic Photocatalysis
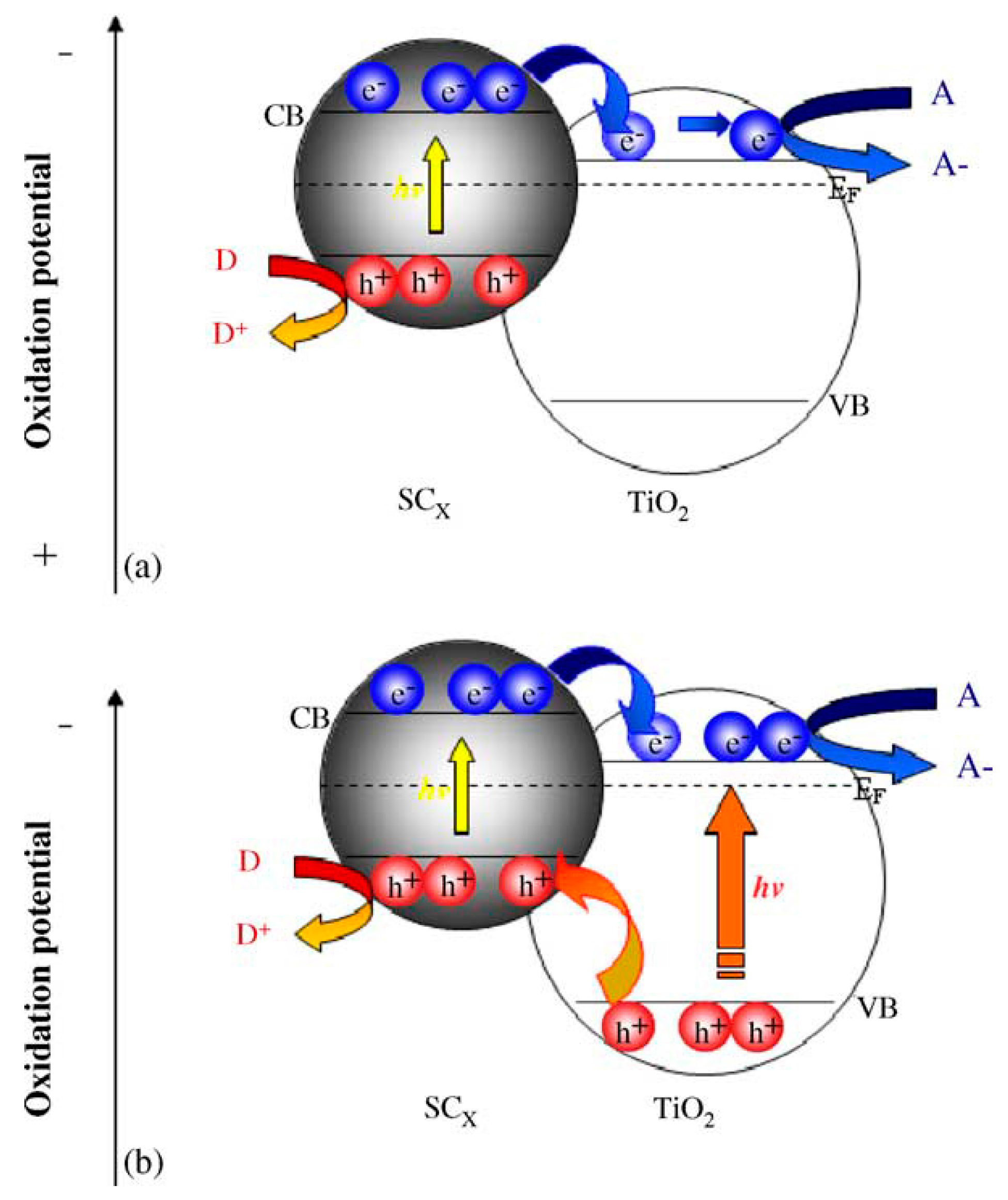
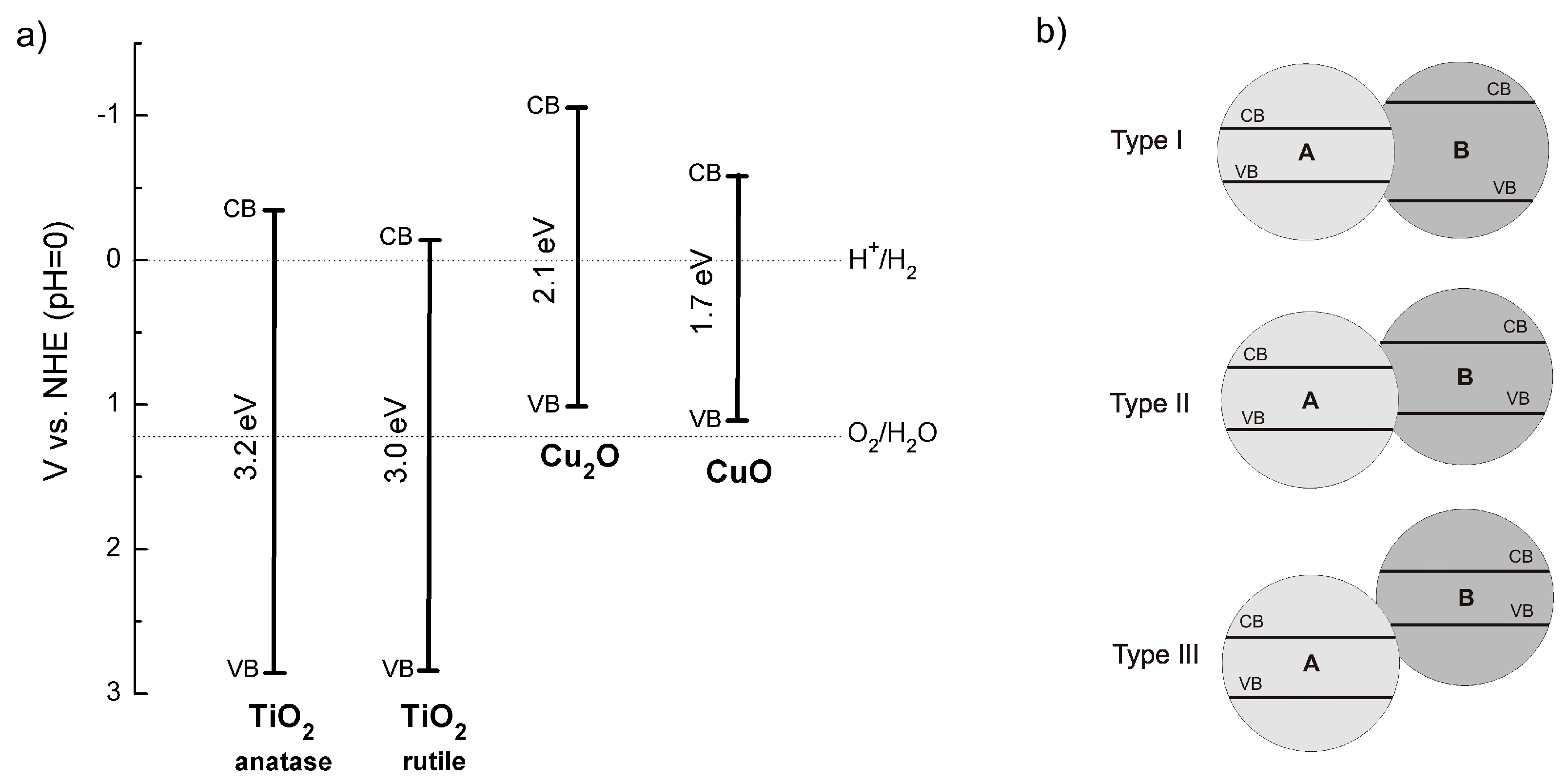
2.2.2. Copper Oxides—Heterojunction Systems with TiO2
The Concept of Heterojunction between Copper Oxides and Titania
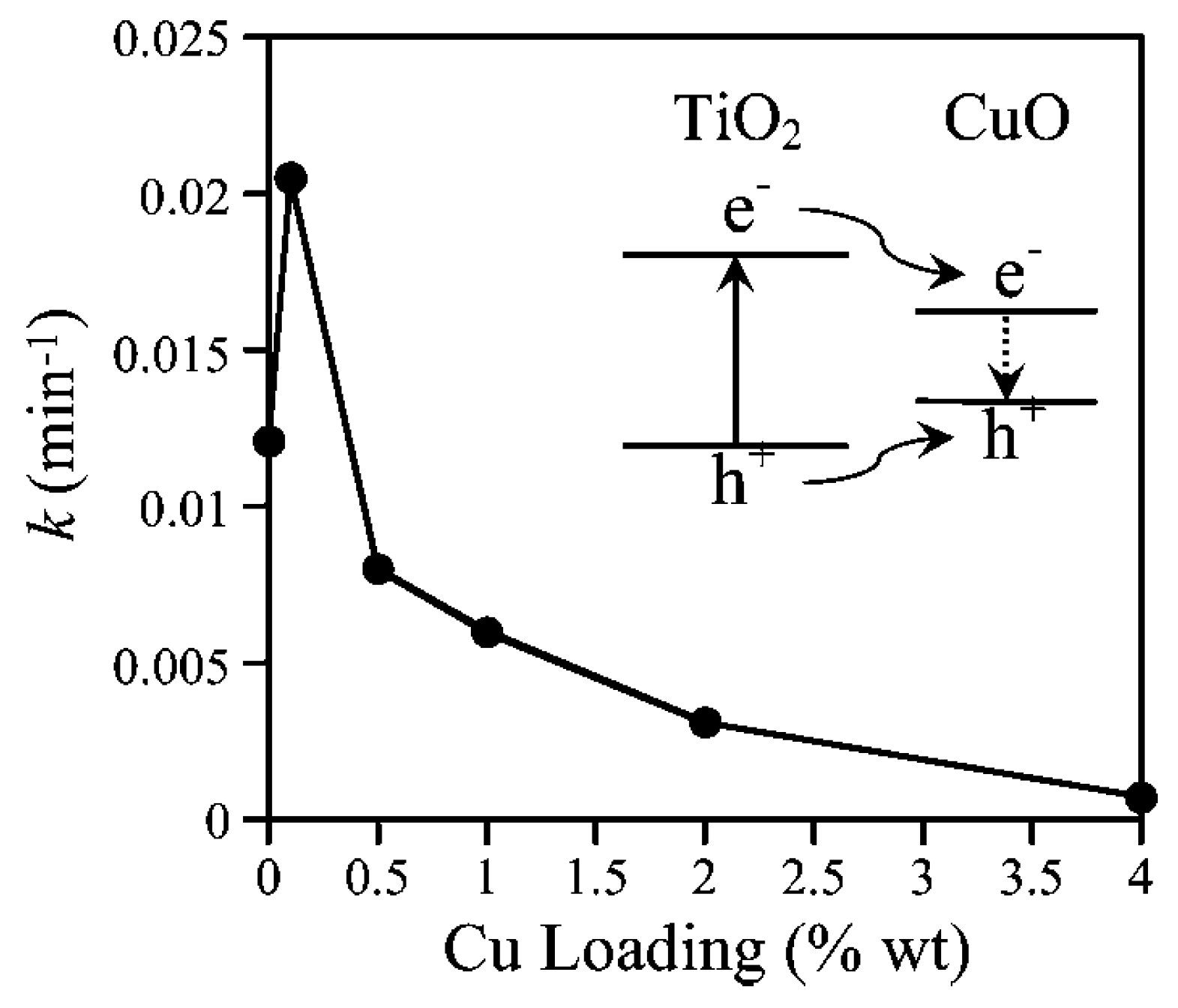
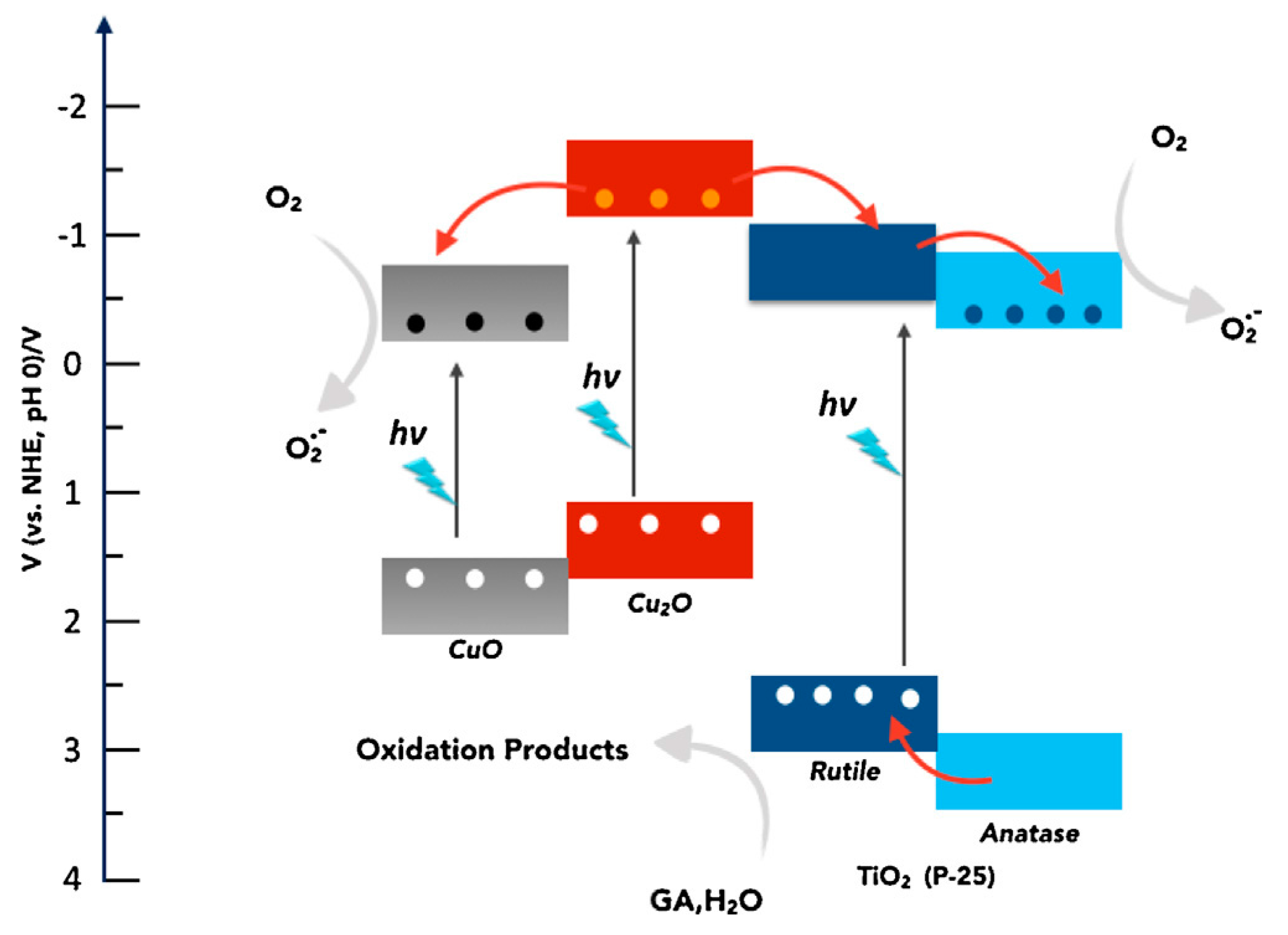
CuO-TiO2 Heterojunction
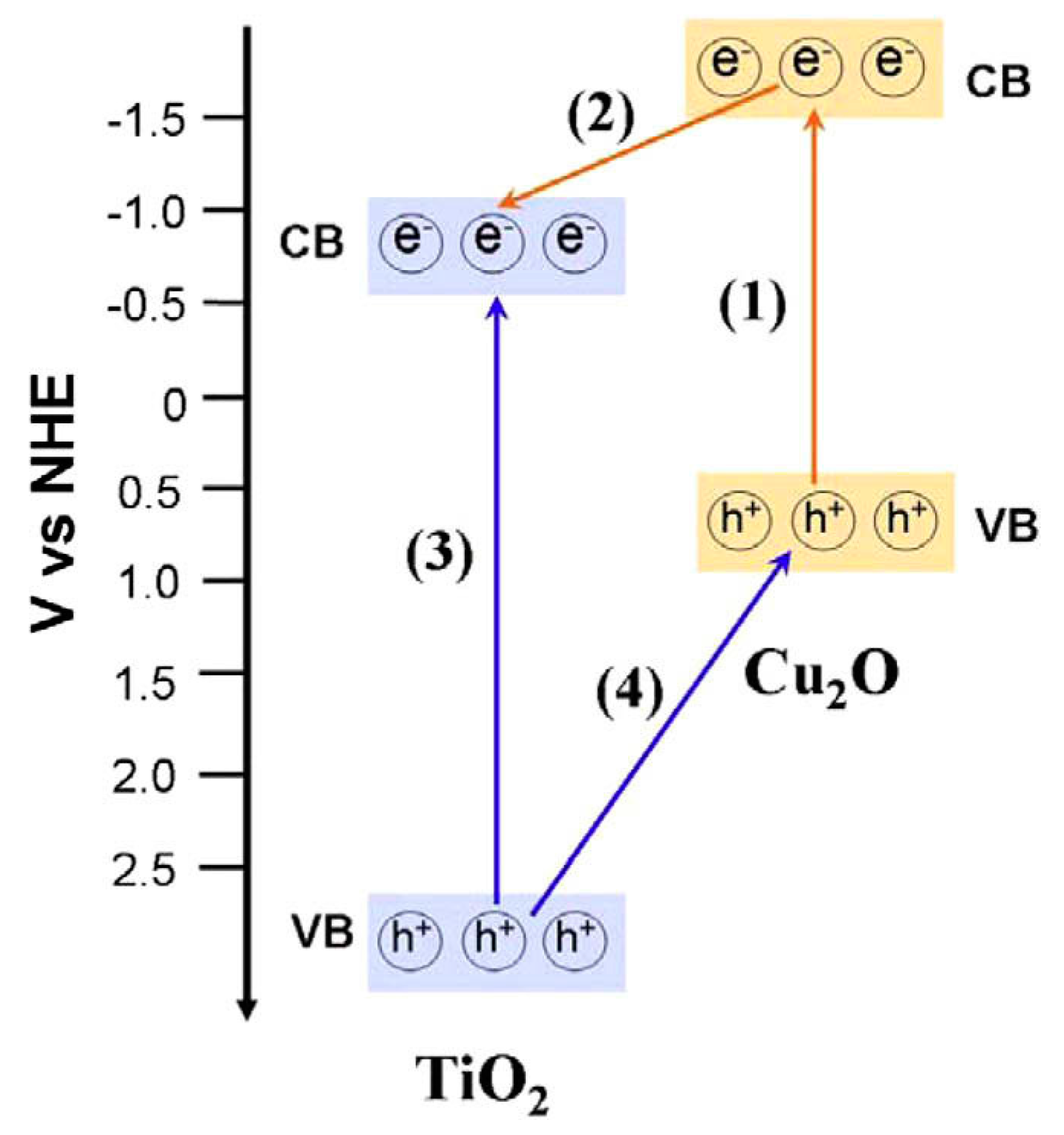
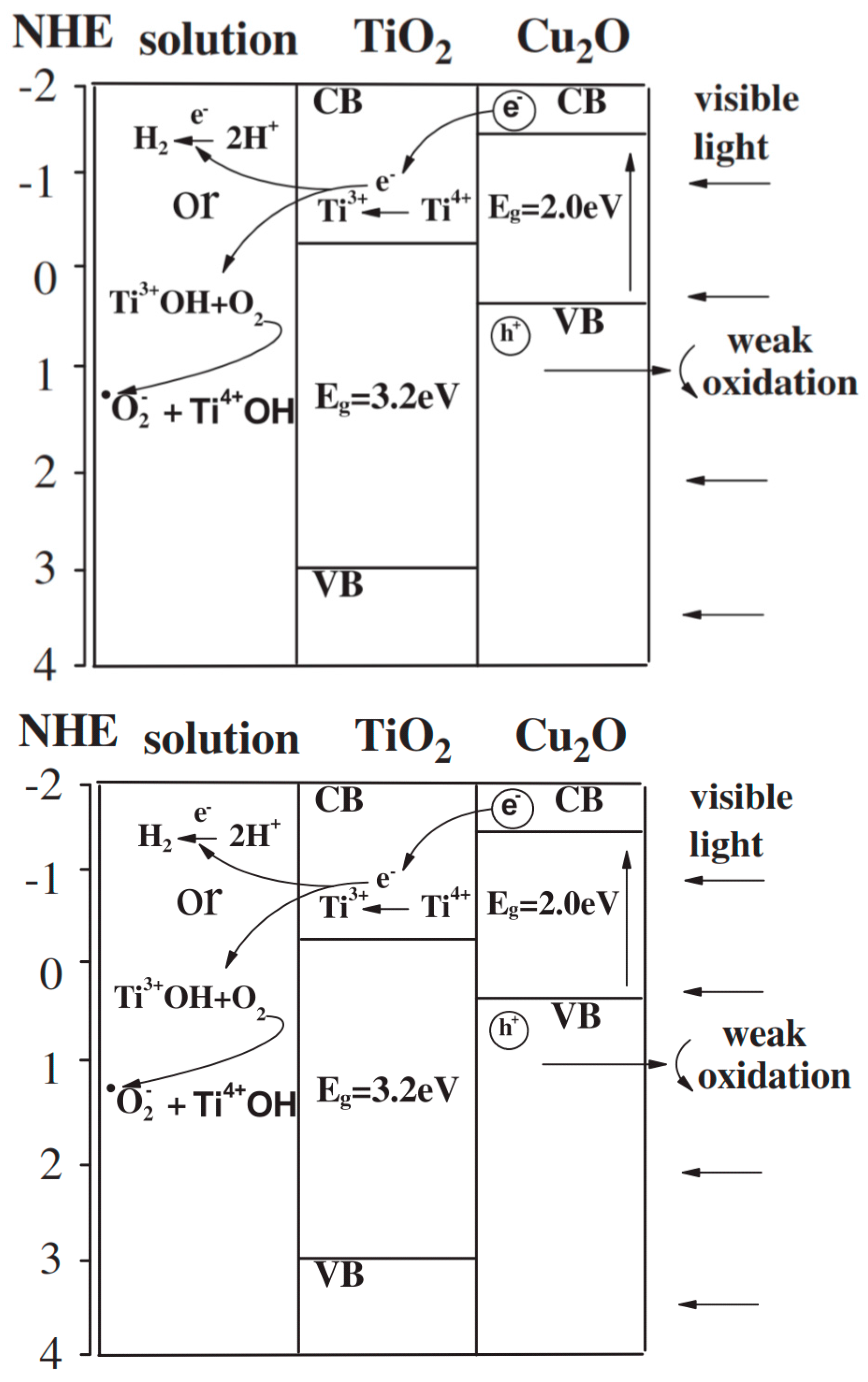
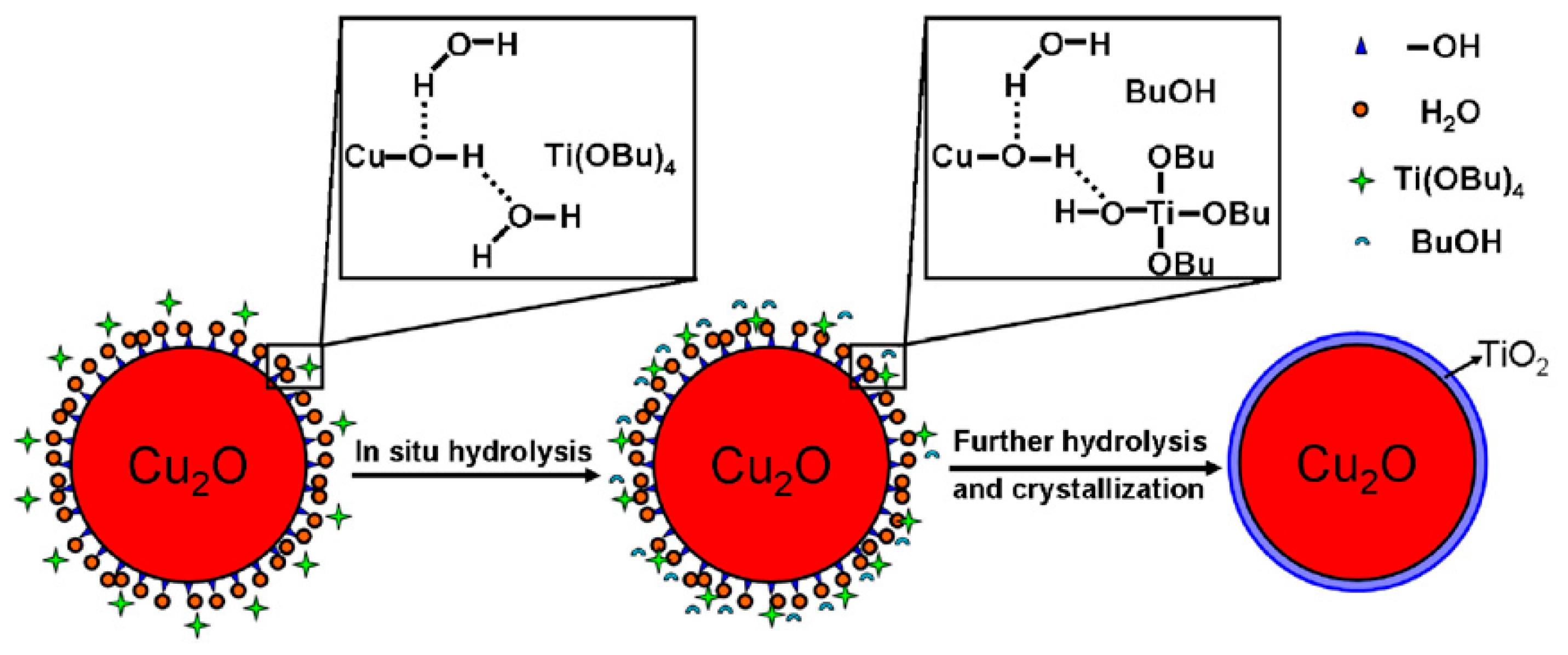
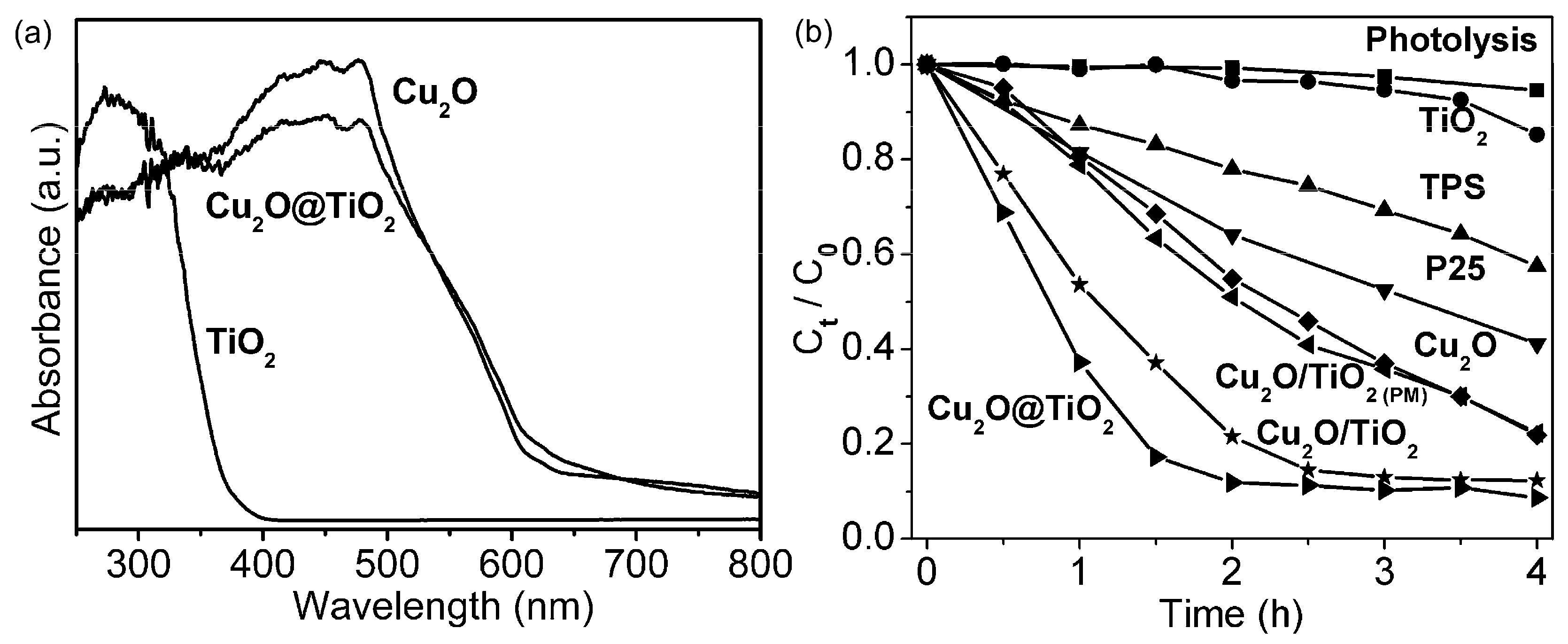
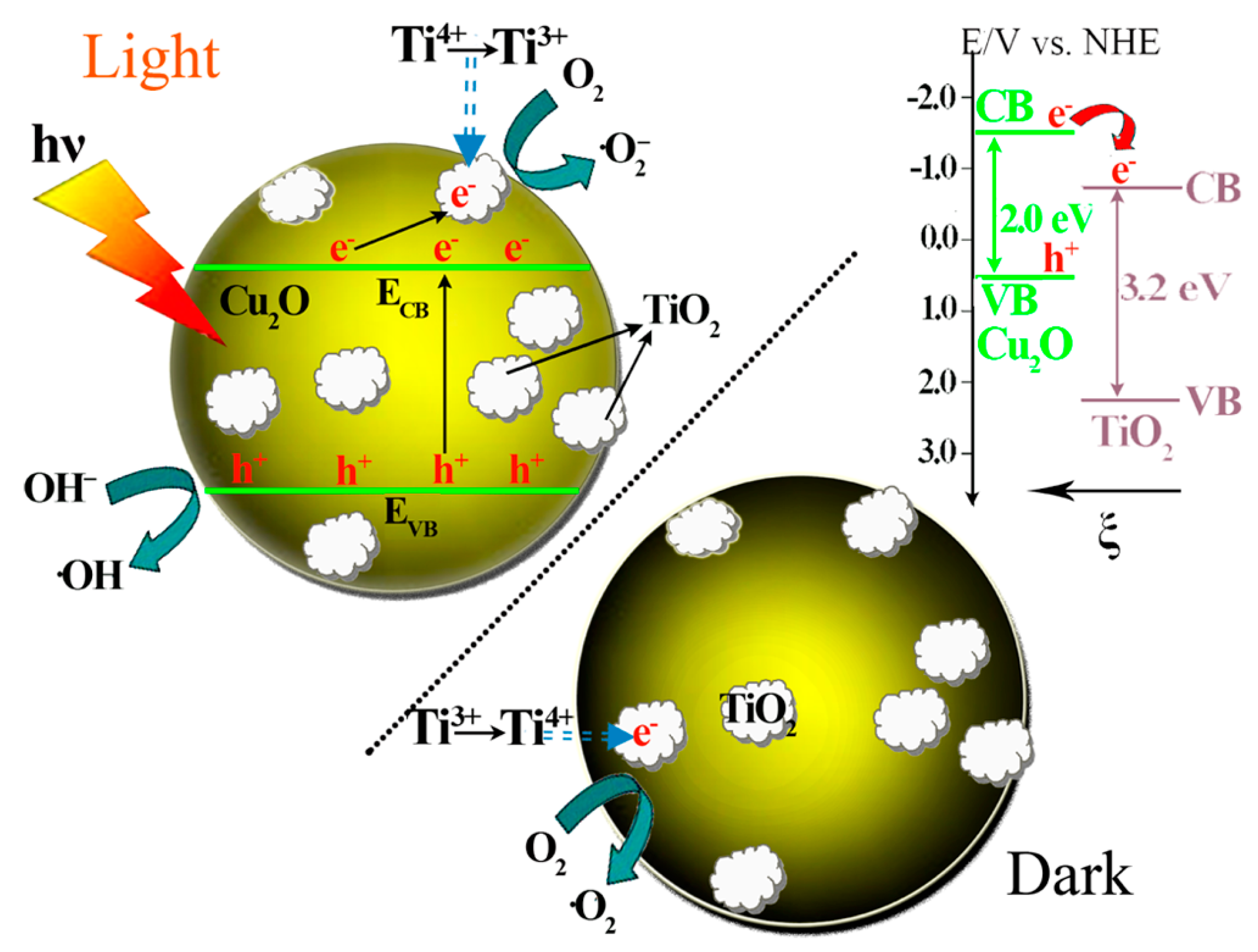
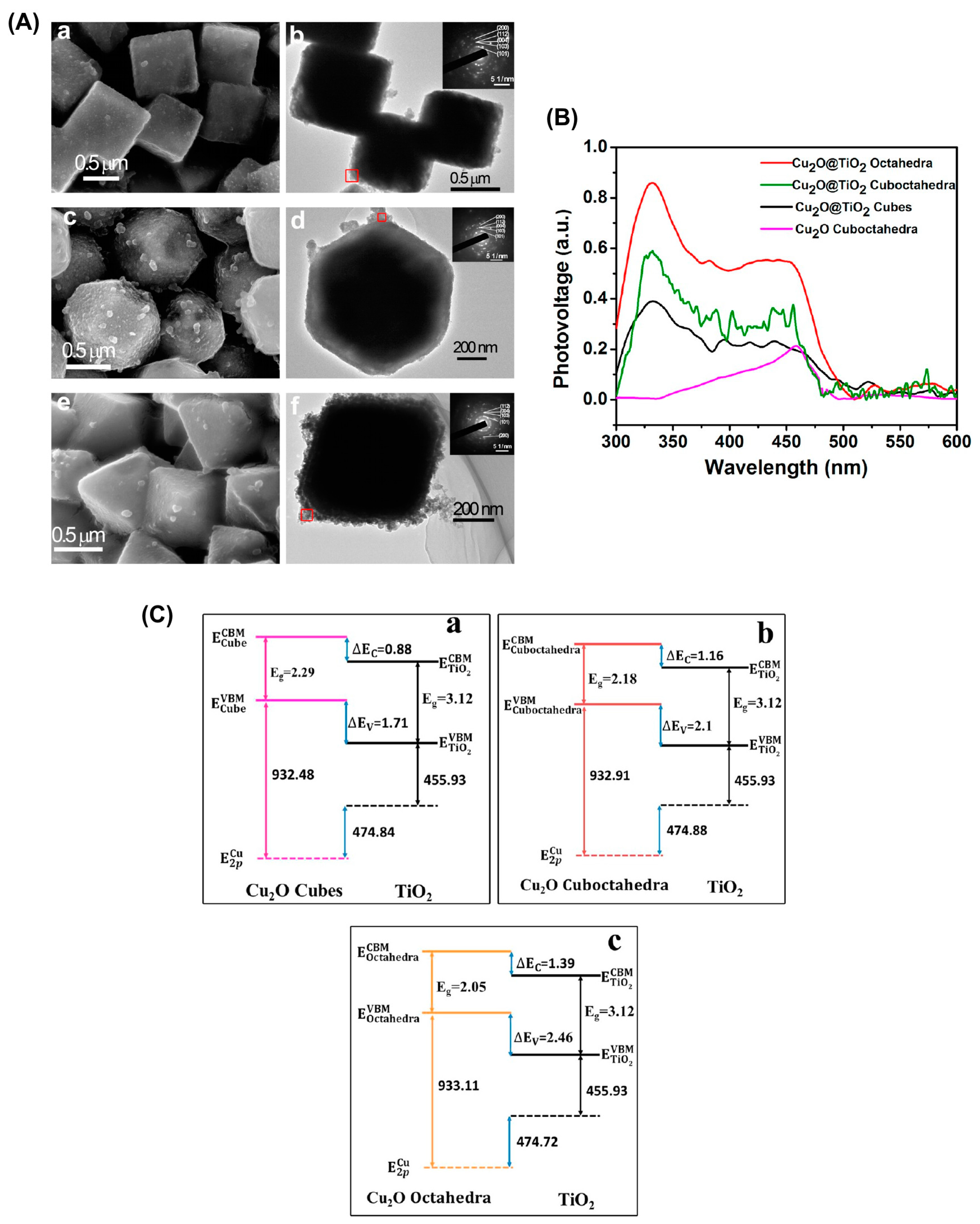
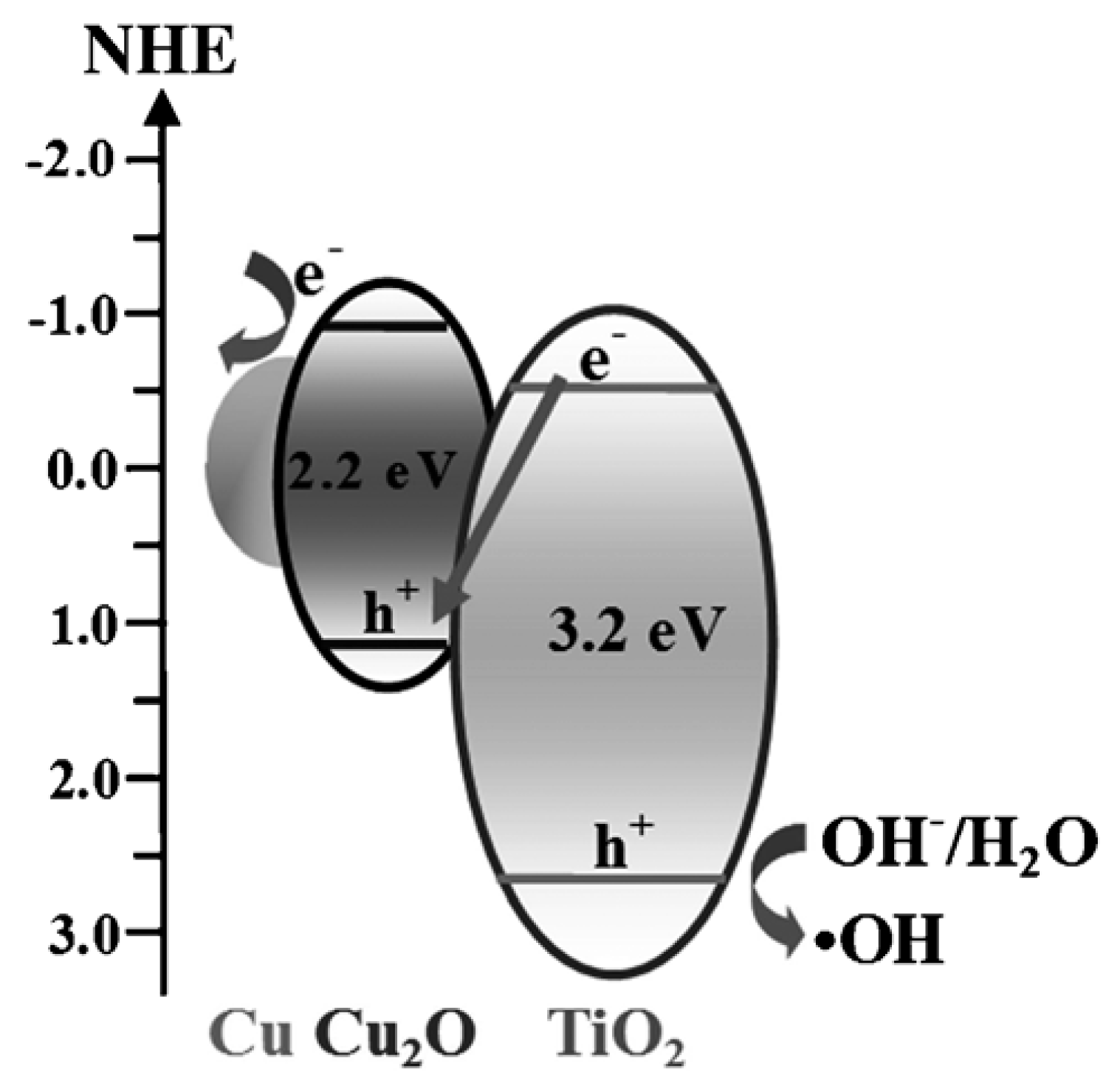
Cu2O-TiO2 Heterojunction
Mixed Copper Species Deposited on Titania Surface
3. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 photocatalysis: Mechanisms and materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef] [PubMed]
- Bahnemann, D.W. Photocatalytic water treatment: Solar energy applications. Sol. Energy 2004, 77, 445–459. [Google Scholar] [CrossRef]
- Lee, S.A.; Choo, K.H.; Lee, H.I.; Hyeon, T.; Choi, W.; Kwon, H.H. Use of ultrafiltration membranes for the separation of TiO2 photocatalysts in drinking water treatment. Ind. Eng. Chem. Res. 2001, 40, 1712–1719. [Google Scholar] [CrossRef]
- Politano, A.; Cupolillo, A.; Di Profio, G.; Arafat, H.A.; Chiarello, G.; Curcio, E. When plasmonics meets membrane technology. J. Phys. Condens. Matter 2016, 28, 363003. [Google Scholar] [CrossRef] [PubMed]
- Hay, S.O.; Obee, T.; Luo, Z.; Jiang, T.; Meng, Y.T.; He, J.K.; Murphy, S.C.; Suib, S. The viability of photocatalysis for air purification. Molecules 2015, 20, 1319–1356. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.B.; Shen, S.H.; Guo, L.J.; Mao, S.S. Semiconductor-based photocatalytic hydrogen generation. Chem. Rev. 2010, 110, 6503–6570. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Peng, B.S.; Peng, T.Y. Recent advances in heterogeneous photocatalytic CO2 conversion to solar fuels. ACS Catal. 2016, 6, 7485–7527. [Google Scholar] [CrossRef]
- Roose, B.; Pathak, S.; Steiner, U. Doping of TiO2 for sensitized solar cells. Chem. Soc. Rev. 2015, 44, 8326–8349. [Google Scholar] [CrossRef] [PubMed]
- Parkin, I.P.; Palgrave, R.G. Self-cleaning coatings. J. Mater. Chem. 2005, 15, 1689–1695. [Google Scholar] [CrossRef]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.M.; Hamilton, J.W.J.; Byrne, J.A.; O’Shea, K.; et al. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B Environ. 2012, 125, 331–349. [Google Scholar] [CrossRef]
- Ohtani, B. Titania photocatalysis beyond recombination: A critical review. Catalysts 2013, 3, 942–953. [Google Scholar] [CrossRef]
- Emeline, A.V.; Ryabchuk, V.K.; Serpone, N. Dogmas and misconceptions in heterogeneous photocatalysis. Some enlightened reflections. J. Phys. Chem. B 2005, 109, 18515–18521. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, J.M. Heterogeneous photocatalysis: State of the art and present applications. Top. Catal. 2005, 34, 49–65. [Google Scholar] [CrossRef]
- Choi, W.Y.; Termin, A.; Hoffmann, M.R. The role of metal-ion dopants in quantum-sized TiO2—Correlation between photoreactivity and charge-carrier recombination dynamics. J. Phys. Chem. 1994, 98, 13669–13679. [Google Scholar] [CrossRef]
- Yu, J.C.; Yu, J.G.; Ho, W.K.; Jiang, Z.T.; Zhang, L.Z. Effects of F-doping on the photocatalytic activity and microstructures of nanocrystalline TiO2 powders. Chem. Mater. 2002, 14, 3808–3816. [Google Scholar] [CrossRef]
- Ohtani, B.; Kakimoto, M.; Nishimoto, S.; Kagiya, T. Photocatalytic reaction of neat alcohols by metal-loaded titanium(IV) oxide particles. J. Photochem. Photobiol. A Chem. 1993, 70, 265–272. [Google Scholar] [CrossRef]
- Sclafani, A.; Herrmann, J.M. Influence of metallic silver and of platinum-silver bimetallic deposits on the photocatalytic activity of titania (anatase and rutile) in organic and aqueous media. J. Photochem. Photobiol. A Chem. 1998, 113, 181–188. [Google Scholar] [CrossRef]
- Engweiler, J.; Harf, J.; Baiker, A. WOx/TiO2 catalysts prepared by grafting of tungsten alkoxides: Morphological properties and catalytic behavior in the selective reduction of NO by NH3. J. Catal. 1996, 159, 259–269. [Google Scholar] [CrossRef]
- Vinodgopal, K.; Kamat, P.V. Enhanced rates of photocatalytic degradation of an azo-dye using SnO2/TiO2 coupled semiconductor thin-films. Environ. Sci. Technol. 1995, 29, 841–845. [Google Scholar] [CrossRef] [PubMed]
- Allen, S.E.; Walvoord, R.R.; Padilla-Salinas, R.; Kozlowski, M.C. Aerobic copper-catalyzed organic reactions. Chem. Rev. 2013, 113, 6234–6458. [Google Scholar] [CrossRef] [PubMed]
- Bhanushali, S.; Ghosh, P.; Ganesh, A.; Cheng, W.L. 1D copper nanostructures: Progress, challenges and opportunities. Small 2015, 11, 1232–1252. [Google Scholar] [CrossRef] [PubMed]
- Clarizia, L.; Spasiano, D.; Di Somma, I.; Marotta, R.; Andreozzi, R.; Dionysiou, D.D. Copper-modified-TiO2 catalysts for hydrogen generation through photoreforming of organics. A short review. Int. J. Hydrogen Energy 2014, 39, 16812–16831. [Google Scholar] [CrossRef]
- Yan, H.H.; Zhao, T.J.; Li, X.J.; Hun, C.H. Synthesis of Cu-doped nano-TiO2 by detonation method. Ceram. Int. 2015, 41, 14204–14211. [Google Scholar] [CrossRef]
- Sreekantan, S.; Zaki, S.M.; Lai, C.W.; Tzu, T.W. Copper-incorporated titania nanotubes for effective lead ion removal. Mater. Sci. Semicond. Process. 2014, 26, 620–631. [Google Scholar] [CrossRef]
- Janczarek, M.; Zielińska-Jurek, A.; Markowska, I.; Hupka, J. Transparent thin films of Cu–TiO2 with visible light photocatalytic activity. Photochem. Photobiol. Sci. 2014, 14, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Xin, B.; Wang, P.; Ding, D.; Liu, J.; Ren, Z.; Fu, H. Effect of surface species on Cu-TiO2 photocatalytic activity. Appl. Surf. Sci. 2008, 254, 2569–2574. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, Y.; Peng, X.; Li, Y.; Liu, Z.; Liu, C.; Ya, J.; Huang, Y. Photoinduced superhydrophilicity of TiO2 thin film with hierarchical Cu doping. Sci. Technol. Adv. Mater. 2012, 13, 025001. [Google Scholar] [CrossRef] [PubMed]
- Gawande, M.B.; Goswami, A.; Felpin, F.X.; Asefa, T.; Huang, X.; Silva, R.; Zou, X.; Zboril, R.; Varma, R.S. Cu and Cu-based nanoparticles: Synthesis and applications in catalysis. Chem. Rev. 2016, 116, 3722–3811. [Google Scholar] [CrossRef] [PubMed]
- Reiche, H.; Dunn, W.W.; Bard, A.J. Heterogeneous photocatalytic and photosynthetic deposition of copper on TiO2 and WO3 powders. J. Phys. Chem. 1979, 83, 2248–2251. [Google Scholar] [CrossRef]
- Foster, N.S.; Noble, R.D.; Koval, C.A. Reversible photoreductive deposition and oxidative dissolution of copper ions in titanium dioxide aqueous suspensions. Environ. Sci. Technol. 1993, 27, 350–356. [Google Scholar] [CrossRef]
- Foster, N.S.; Lancaster, A.N.; Noble, R.D.; Koval, C.A. Effect of Organics on the Photodeposition of Copper in Titanium-Dioxide Aqueous Suspensions. Ind. Eng. Chem. Res. 1995, 34, 3865–3871. [Google Scholar] [CrossRef]
- Jacobs, J.W.M.; Kampers, F.W.H.; Rikken, J.M.G.; Bullelieuwma, C.W.T.; Koningsberger, D.C. Copper photodeposition on TiO2 studied with HREM and EXAFS. J. Electrochem. Soc. 1989, 136, 2914–2923. [Google Scholar] [CrossRef]
- Herrmann, J.M.; Disdier, J.; Pichat, P. Photoassisted platinum deposition on TiO2 powder using various platinum complexes. J. Phys. Chem. 1986, 90, 6028–6034. [Google Scholar] [CrossRef]
- Okamoto, K.; Yamamoto, Y.; Tanaka, H.; Tanaka, M.; Itaya, A. Heterogeneous photocatalytic decomposition of phenol over TiO2 powder. Bull. Chem. Soc. Jpn. 1985, 58, 2015–2022. [Google Scholar] [CrossRef]
- Bideau, M.; Claudel, B.; Faure, L.; Rachimoellah, M. Photo-oxidation of formic acid by oxygen in the presence of titanium dioxde and dissolved copper ions: Oxygen transfer and reaction kinetics. Chem. Eng. Commun. 1990, 93, 167–179. [Google Scholar] [CrossRef]
- Bideau, M.; Claudel, B.; Faure, L.; Kazouan, H. The photo-oxidation of acetic acid by oxygen in the presence of titanium dioxide and dissolved copper ions. J. Photochem. Photobiol. A Chem. 1991, 61, 269–280. [Google Scholar] [CrossRef]
- Bideau, M.; Claudel, B.; Faure, L.; Kazouan, H. The photo-oxidation of propionic acid by oxygen in the presence of TiO2 and dissolved copper ions. J. Photochem. Photobiol. A Chem. 1992, 67, 337–348. [Google Scholar] [CrossRef]
- Brezova, V.; Blazkova, A.; Borosova, E.; Ceppan, M.; Fiala, R. The influence of dissolved metal ions on the photocatalytic degradation of phenol in aqueous TiO2 suspensions. J. Mol. Catal. A Chem. 1995, 98, 109–116. [Google Scholar] [CrossRef]
- Butler, E.C.; Davis, A.P. Photocatalytic oxidation in aqueous titanium dioxide suspensions: The influence of dissolved transition metals. J. Photochem. Photobiol. A Chem. 1993, 70, 273–283. [Google Scholar] [CrossRef]
- Sykora, J. Photochemistry of copper complexes and their environmental aspects. Coord. Chem. Rev. 1997, 159, 95–108. [Google Scholar] [CrossRef]
- Bideau, M.; Claudel, B.; Faure, L.; Kazouan, H. Metallic complexes as intermediates in homogeneously and heterogeneously photocatalysed reactions. J. Photochem. Photobiol. A Chem. 1994, 84, 57–67. [Google Scholar] [CrossRef]
- Kraeutler, B.; Baard, A.J. Heterogeneous photocatalytic preparation of supported catalysts. Photodeposition of platinum on titanium dioxide powder and other substrates. J. Am. Chem. Soc. 1978, 100, 4317–4318. [Google Scholar] [CrossRef]
- Koudelka, M.; Sanchez, J.; Augustynski, J. Electrochemical and surface characteristics of the photocatalytic platinum deposits on titania. J. Phys. Chem. 1982, 86, 4277–4280. [Google Scholar] [CrossRef]
- Ueno, K.; Misawa, H. Surface plasmon-enhanced photochemical reactions. J. Photochem. Photobiol. C Photochem. Rev. 2013, 15, 31–52. [Google Scholar] [CrossRef]
- Kowalska, E.; Prieto Mahaney, O.O.; Abe, R.; Ohtani, B. Visible-light-induced photocatalysis through surface plasmon excitation of gold on titania surfaces. Phys. Chem. Chem. Phys. 2010, 12, 2344–2355. [Google Scholar] [CrossRef] [PubMed]
- Ingram, D.B.; Linic, S. Water splitting on composite plasmonic-metal/semiconductor photoelectrodes: Evidence for selective plasmon-induced formation of charge carriers near the semiconductor surface. J. Am. Chem. Soc. 2011, 133, 5202–5205. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Kazuma, E.; Sakai, N.; Tatsuma, T. Photoelectrochemical responses from polymer-coated plasmonic copper nanoparticles on TiO2. Chem. Lett. 2012, 41, 1340–1342. [Google Scholar] [CrossRef]
- Zhang, S.; Peng, B.; Yang, S.; Wang, H.; Yu, H.; Fang, Y.; Peng, F. Non-noble metal copper nanoparticles-decorated TiO2 nanotube arrays with plasmon-enhanced photocatalytic hydrogen evolution under visible light. Int. J. Hydrogen Energy 2015, 40, 303–310. [Google Scholar] [CrossRef]
- DeSario, P.A.; Pietron, J.J.; Brintlinger, T.H.; McEntee, M.; Parker, J.F.; Baturina, O.; Stroud, R.M.; Rolison, D.R. Oxidation-stable plasmonic copper nanoparticles in photocatalytic TiO2 nanoarchitectures. Nanoscale 2017, 9, 11720–11729. [Google Scholar] [CrossRef] [PubMed]
- Chan, G.H.; Zhao, J.; Hicks, E.M.; Schatz, G.C.; Van Duyne, R.P. Plasmonic properties of copper nanoparticles fabricated by nanosphere lithography. Nano Lett. 2007, 7, 1947–1952. [Google Scholar] [CrossRef]
- Kowalska, E. Plasmon-assisted catalysis. In Gold Nanoparticles for Physics, Chemistry and Biology; Pluchery, C.L.O., Ed.; World Scientific: Singapore, 2017; pp. 319–364. [Google Scholar]
- Kaur, R.; Pal, B. Plasmonic coinage metal—TiO2 hybrid nanocatalysts for highly efficient photocatalytic oxidation under sunlight irradiation. New J. Chem. 2015, 39, 5966–5976. [Google Scholar] [CrossRef]
- Hou, W.; Cronin, S.B. A review of surface plasmon resonance-enhanced photocatalysis. Adv. Funct. Mater. 2012, 23, 1612–1619. [Google Scholar] [CrossRef]
- Kanninen, P.; Johans, C.; Merta, J.; Kontturi, K. Influence of ligand structure on the stability and oxidation of copper nanoparticles. J. Colloid Interface Sci. 2008, 318, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Pastoriza-Santos, I.; Sanchez-Iglesias, A.; Rodriguez-Gonzales, B.; Liz-Marzan, L.M. Aerobic synthesis of Cu nanoplates with intense plasmon resonances. Small 2009, 5, 440–443. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Sinha, I.; Premkumar, M.; Singh, A.K.; Mandal, R.K. Structural and surface plasmon behavior of Cu nanoparticles using different stabilizers. Colloids Surf. A 2010, 359, 88–94. [Google Scholar] [CrossRef]
- Mendez-Medrano, M.G.; Kowalska, E.; Lehoux, A.; Herissan, A.; Ohtani, B.; Bahena, D.; Briois, V.; Colbeau-Justin, C.; Rodrigues-Lopez, J.L.; Remita, H. Surface modification of TiO2 with Ag nanoparticles and CuO nanoclusters for application in photocatalysis. J. Phys. Chem. C 2016, 120, 5143–5154. [Google Scholar] [CrossRef]
- Janczarek, M.; Wei, Z.; Endo, M.; Ohtani, B.; Kowalska, E. Silver- and copper-modified decahedral anatase titania particles as visible light-responsive plasmonic photocatalyst. J. Photon. Energy 2017, 7, 012008. [Google Scholar] [CrossRef]
- Wei, Z.; Endo, M.; Wang, K.; Charbit, E.; Markowska-Szczupak, A.; Ohtani, B.; Kowalska, E. Noble metal-modified octahedral anatase titania particles with enhanced activity for decomposition of chemical and microbiological pollutants. Chem. Eng. J. 2017, 318, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Muniz-Miranda, M.; Gellini, C.; Simonelli, A.; Tiberi, M.; Giammanco, F.; Giorgetti, E. Characterization of copper nanoparticles obtained by laser ablation in liquids. Appl. Phys. A 2013, 110, 829–833. [Google Scholar] [CrossRef]
- Guo, X.; Hao, C.; Jin, G.; Zhu, H.Y.; Guo, X.Y. Copper Nanoparticles on Graphene Support: An Efficient Photocatalyst for Coupling of Nitroaromatics in Visible Light. Angew. Chem. Int. Ed. 2014, 53, 1973–1977. [Google Scholar] [CrossRef] [PubMed]
- Gonzales-Posada, F.; Sellapan, R.; Vanpoucke, B.; Charakov, D. Oxidation of copper nanoparticles in water monitored in situ by localized surface plasmon resonance spectroscopy. RSC Adv. 2014, 4, 20659–20664. [Google Scholar] [CrossRef]
- Rice, K.P.; Walker, E.J.; Stoykovich, M.P.; Saunders, A.E. Solvent-Dependent Surface Plasmon Response and Oxidation of Copper Nanocrystals. J. Phys. Chem. C 2011, 115, 1793–1799. [Google Scholar] [CrossRef]
- Susman, M.D.; Feldman, Y.; Vaskevich, A.; Rubinstein, I. Chemical deposition and stabilization of plasmonic copper nanoparticle films on transparent substrates. Chem. Mater. 2012, 24, 2501–2508. [Google Scholar] [CrossRef]
- Jimenez, J.A. Carbon as reducing agent for the precipitation of plasmonic Cu particles in glass. J. Alloys Compd. 2016, 656, 685–688. [Google Scholar] [CrossRef]
- Fu, Q.; Wagner, T. Interaction of nanostructured metal overlayers with oxide surfaces. Surf. Sci. Rep. 2007, 62, 431–498. [Google Scholar] [CrossRef]
- Kum, J.M.; Park, Y.J.; Kim, H.J.; Cho, S.O. Plasmon-enhanced photocatalytic hydrogen production over visible-light responsive Cu/TiO2. Nanotechnology 2015, 26, 125402. [Google Scholar] [CrossRef] [PubMed]
- Bessekhouad, Y.; Robert, D.; Weber, J.V. Bi2S3/TiO2 and CdS/TiO2 heterojunctions as an available configuration for photocatalytic degradation of organic pollutant. J. Photochem. Photobiol. A Chem. 2004, 163, 569–580. [Google Scholar] [CrossRef]
- Serpone, N.; Maruthamuthu, P.; Pichat, P.; Pelizzetti, E.; Hidaka, H. Exploiting the interparticle electron transfer process in the photocatalysed oxidation of phenol, 2-chlorophenol and pentachlorophenol: Chemical evidence for electron and hole transfer between coupled semiconductors. J. Photochem. Photobiol. A Chem. 1995, 85, 247–255. [Google Scholar] [CrossRef]
- Bessekhouad, Y.R.D.; Weber, J.-V. Photocatalytic activity of Cu2O/TiO2, Bi2O3/TiO2 and ZnMn2O4/TiO2 heterojunctions. Catal. Today 2005, 101, 315–321. [Google Scholar] [CrossRef]
- Marschall, R. Semiconductor composites: Strategies for enhancing charge carrier separation to improve photocatalytic activity. Adv. Funct. Mater. 2014, 24, 2421–2440. [Google Scholar] [CrossRef]
- Barreca, D.; Fornasiero, P.; Gasparotto, A.; Gombac, V.; Maccato, C.; Montini, T.; Tondello, E. The potential of supported Cu2O and CuO nanosystems in photocatalytic H2 production. ChemSusChem 2009, 2, 230–233. [Google Scholar] [CrossRef] [PubMed]
- Hara, M.; Kondo, T.; Komoda, M.; Ikeda, S.; Shinohara, K.; Tanaka, A.; Kondo, J.N.; Domen, K. Cu2O as a photocatalyst for overall water splitting under visible light irradiation. Chem. Commun. 1998, 357–358. [Google Scholar] [CrossRef]
- Khan, K.A. Stability of a Cu2O photoelectrode in an electrochemical cell and the performances of the photoelectrode coated with Au and SiO thin films. Appl. Energy 2000, 65, 59–66. [Google Scholar] [CrossRef]
- Nguyen, M.A.; Bedford, N.M.; Ren, Y.; Zahran, E.M.; Goodin, R.C.; Chagani, F.F.; Bachas, L.G.; Knecht, M.R. Direct synthetic control over the size, composition, and photocatalytic activity of octahedral copper oxide materials: Correlation between surface structure and catalytic functionality. ACS Appl. Mater. Interfaces 2015, 7, 13238–13250. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.L.; Wang, C.G.; Shao, M.H.; Xu, X.J.; Huang, J.Z. Low-temperature solution synthesis of CuO/Cu2O nanostructures for enhanced photocatalytic activity with added H2O2: Synergistic effect and mechanism insight. RSC Adv. 2017, 7, 4329–4338. [Google Scholar] [CrossRef]
- Song, K.Y.; Kwon, Y.T.; Choi, G.J.; Lee, W.I. Photocatalytic activity of Cu/TiO2 with oxidation state of surface-loaded copper. Bull. Korean Chem. Soc. 1999, 20, 957–960. [Google Scholar]
- Chiang, K.; Amal, R.; Tran, T. Photocatalytic degradation of cyanide using titanium dioxide modified with copper oxide. Adv. Environ. Res. 2002, 6, 471–485. [Google Scholar] [CrossRef]
- Wu, S.-X.; Ma, Z.; Qin, Y.-N.; He, F.; Jia, L.-S.; Zhang, Y.-J. XPS study of copper doping TiO2 photocatalyst. Acta Phys.-Chim. Sin. 2003, 19, 967–969. [Google Scholar]
- Arana, J.; Alonso, A.P.; Rodriguez, J.M.D.; Melian, J.A.H.; Diaz, O.G.; Pena, J.P. Comparative study of MTBE photocatalytic degradation with TiO2 and Cu-TiO2. Appl. Catal. B Environ. 2008, 78, 355–363. [Google Scholar] [CrossRef]
- Irie, H.; Miura, S.; Kamiya, K.; Hashimoto, K. Efficient visible light-sensitive photocatalysts: Grafting Cu(II) ions onto TiO2 and WO3 photocatalysts. Chem. Phys. Lett. 2008, 457, 202–205. [Google Scholar] [CrossRef]
- Irie, H.; Kamiya, K.; Shibanuma, T.; Miura, S.; Tryk, D.A.; Yokoyama, T.; Hashimoto, K. Visible light-sensitive Cu(II)-grafted TiO2 photocatalysts: Activities and X-ray absorption fine structure analyses. J. Phys. Chem. C 2009, 113, 10761–10766. [Google Scholar] [CrossRef]
- Yu, H.; Irie, H.; Shimodaira, Y.; Hosogi, Y.; Kuroda, Y.; Miyauchi, M.; Hashimoto, K. An efficient visible-light-sensitive Fe(III)-grafted TiO2 photocatalyst. J. Phys. Chem. C 2010, 114, 16481–16487. [Google Scholar] [CrossRef]
- Arai, T.; Horiguchi, M.; Yanagida, M.; Gunji, T.; Sugihara, H.; Sayama, K. Reaction mechanism and activity of WO3-catalyzed photodegradation of organic substances promoted by a CuO cocatalyst. J. Phys. Chem. C 2009, 113, 6602–6609. [Google Scholar] [CrossRef]
- Liu, M.; Sunada, K.; Hashimoto, K.; Miyauchi, M. Visible-light sensitive Cu(II)–TiO2 with sustained anti-viral activity for efficient indoor environmental remediation. J. Mater. Chem. A 2015, 3, 17312–17319. [Google Scholar] [CrossRef]
- Li, G.; Dimitrijevic, N.M.; Chen, L.; Rajh, T.; Gray, K.A. Role of surface/interfacial Cu2+ sites in the photocatalytic activity of coupled CuO-TiO2 nanocomposites. J. Phys. Chem. C 2008, 112, 19040–19044. [Google Scholar] [CrossRef]
- Slamet, N.H.W.; Purnama, E.; Kosela, S.; Gunlazuardi, J. Photocatalytic reduction of CO2 on copper-doped titania catalysts prepared by improved-impregnation method. Catal. Commun. 2005, 6, 313–319. [Google Scholar]
- Kowalska, E.; Rau, S.; Ohtani, B. Plasmonic titania photocatalysts active under UV and visible-light irradiation: Influence of gold amount, size, and shape. J. Nanotechnol. 2012, 2012, 361853. [Google Scholar] [CrossRef]
- Wang, K.; Wei, Z.; Ohtani, B.; Kowalska, E. Interparticle electron transfer in methanol dehydrogenation on platinum-loaded titania particles prepared from P25. Catal Today 2017. [Google Scholar] [CrossRef]
- Moniz, S.J.A.; Tang, J. Charge transfer and photocatalytic activity in CuO/TiO2 nanoparticle heterojunctions synthesised through a rapid, one-pot, microwave solvothermal route. Chem. Cat. Chem. 2015, 7, 1659–1667. [Google Scholar]
- Siah, W.R.; Lintang, H.O.; Shamsuddin, M.; Yoshida, H.; Tuliati, L. Masking effect of copper oxides photodeposited on titanium dioxide: Exploring UV, visible, and solar light activity. Catal. Sci. Technol. 2016, 6, 5079–5087. [Google Scholar] [CrossRef]
- Liu, M.; Qiu, X.; Miyauchi, M.; Hashimoto, K. Cu(II) oxide amorphous nanoclusters grafted Ti3+ self-doped TiO2: An efficient visible light photocatalyst. Chem. Mater. 2011, 23, 5282–5286. [Google Scholar] [CrossRef]
- Siripala, W.I.A.; Jaramillo, T.F.; Baeck, S.-H.; McFarland, E.W. A Cu2O/TiO2 heterojunction thin film cathode for photoelectrocatalysis. Sol. Energy Mater. Sol. C 2003, 77, 229–237. [Google Scholar] [CrossRef]
- Li, J.; Liu, L.; Yu, Y.; Tang, Y.; Li, H.; Du, F. Preparation of highly photocatalytic active nano-size TiO2-Cu2O particle composites with a novel electrochemical method. Electrochem. Commun. 2004, 6, 940–943. [Google Scholar] [CrossRef]
- Huang, L.; Peng, F.; Wang, H.; Yu, H.; Li, Z. Preparation and characterization of Cu2O/TiO2 nano–nano heterostructure photocatalysts. Catal. Commun. 2009, 10, 1839–1843. [Google Scholar] [CrossRef]
- Kisch, H.; Macyk, W. Visible-light photocatalysis by modified titania. Chem. Phys. Chem. 2002, 3, 399–400. [Google Scholar] [CrossRef]
- Yan, X.; Ohno, T.; Nishijima, K.; Abe, R.; Ohtani, B. Is methylene blue an appropriate substrate for a photocatalytic activity test? A study with visible-light responsive titania. Chem. Phys. Lett. 2006, 429, 606–610. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, H.; Zheng, S.; Pan, F.; Wang, T. TiO2 Film/Cu2O microgrid heterojunction with photocatalytic activity under solar light irradiation. Appl. Mater. Interfaces 2009, 1, 2111–2114. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.B.; Yang, F.; Yan, L.L.; Yan, N.N.; Yang, X.; Qiu, M.Q.; Yu, Y. Bifunctional photocatalysis of TiO2/Cu2O composite under visible light: Ti3+ in organic pollutant degradation and water splitting. J. Phys. Chem. Solids. 2011, 72, 1104–1109. [Google Scholar] [CrossRef]
- Wang, M.; Sun, L.; Cai, J.; Xie, K.; Lin, C. p–n Heterojunction photoelectrodes composed of Cu2O-loaded TiO2 nanotube arrays with enhanced photoelectrochemical and photoelectrocatalytic activities. Energy Environ. Sci. 2013, 6, 1211–1220. [Google Scholar] [CrossRef]
- Chu, S.; Zheng, X.M.; Kong, F.; Wu, G.H.; Luo, L.L.; Guo, Y.; Liu, H.L.; Wang, Y.; Yu, H.X.; Zou, Z.G. Architecture of Cu2O@TiO2 core-shell heterojunction and photodegradation for 4-nitrophenol under simulated sunlight irradiation. Mater. Chem. Phys. 2011, 129, 1184–1188. [Google Scholar] [CrossRef]
- Liu, L.M.; Yang, W.Y.; Li, Q.; Gao, S.A.; Shang, J.K. Synthesis of Cu2O nanospheres decorated with TiO2 nanoislands, their enhanced photoactivity and stability under visible light illumination, and their post-illumination catalytic memory. ACS Appl. Mater. Inter. 2014, 6, 5629–5639. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Kowalska, E.; Verret, J.; Colbeau-Justin, C.; Remita, H.; Ohtani, B. Morphology-dependent photocatalytic activity of octahedral anatase particles prepared by ultrasonication-hydrothermal reaction of titanates. Nanoscale 2015, 7, 12392–12404. [Google Scholar] [CrossRef] [PubMed]
- Janczarek, M.; Kowalska, E.; Ohtani, B. Decahedral-shaped anatase titania photocatalyst particles: Synthesis in a newly developed coaxial-flow gas-phase reactor. Chem. Eng. J. 2016, 289, 502–512. [Google Scholar] [CrossRef]
- Liu, L.; Yang, W.; Sun, W.; Li, Q.; Shang, J.K. Creation of Cu2O@TiO2 composite photocatalysts with p–n heterojunctions formed on exposed Cu2O facets, their energy band alignment study, and their enhanced photocatalytic activity under illumination with visible light. ACS Appl. Mater. Inter. 2015, 7, 1465–1476. [Google Scholar] [CrossRef] [PubMed]
- Helaili, N.; Bessekhouad, Y.; Buouguelia, A.; Trari, M. Visible light degradation of Orange II using xCuyOz/TiO2 heterojunctions. J. Hazard. Mater. 2009, 168, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.; Chen, Z.P.; Xiao, F.Y.; Ma, X.Y.; Wen, C.Z.; Li, Z.; Yang, H.G. Cu-Cu2O-TiO2 nanojunction systems with an unusual electron-hole transportation pathway and enhanced photocatalytic properties. Chem. Asian J. 2013, 8, 1265–1270. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Miyauchi, M.; Sunada, K.; Minoshima, M.; Liu, M.; Lu, Y.; Li, D.; Shimodaira, Y.; Hosogi, Y.; Kuroda, Y.; et al. Hybrid CuxO/TiO2 nanocomposites as risk-reduction materials in indoor environments. ACS Nano 2012, 6, 1609–1618. [Google Scholar] [CrossRef] [PubMed]
- Luna, A.L.; Valenzuela, M.A.; Colbeau-Justin, C.; Vazquez, P.; Rodriguez, J.; Avendano, J.R.; Alfaro, S.; Tirado, S.; Garduno, A.; De la Rosa, J.M. Photocatalytic degradation of gallic acid over CuO-TiO2 composites under UV/Vis LEDs irradiation. Appl. Catal. A Gen. 2016, 521, 140–148. [Google Scholar] [CrossRef]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janczarek, M.; Kowalska, E. On the Origin of Enhanced Photocatalytic Activity of Copper-Modified Titania in the Oxidative Reaction Systems. Catalysts 2017, 7, 317. https://doi.org/10.3390/catal7110317
Janczarek M, Kowalska E. On the Origin of Enhanced Photocatalytic Activity of Copper-Modified Titania in the Oxidative Reaction Systems. Catalysts. 2017; 7(11):317. https://doi.org/10.3390/catal7110317
Chicago/Turabian StyleJanczarek, Marcin, and Ewa Kowalska. 2017. "On the Origin of Enhanced Photocatalytic Activity of Copper-Modified Titania in the Oxidative Reaction Systems" Catalysts 7, no. 11: 317. https://doi.org/10.3390/catal7110317




