Improved Catalytic Performance of Lipase Supported on Clay/Chitosan Composite Beads
Abstract
:1. Introduction
2. Results and Discussion
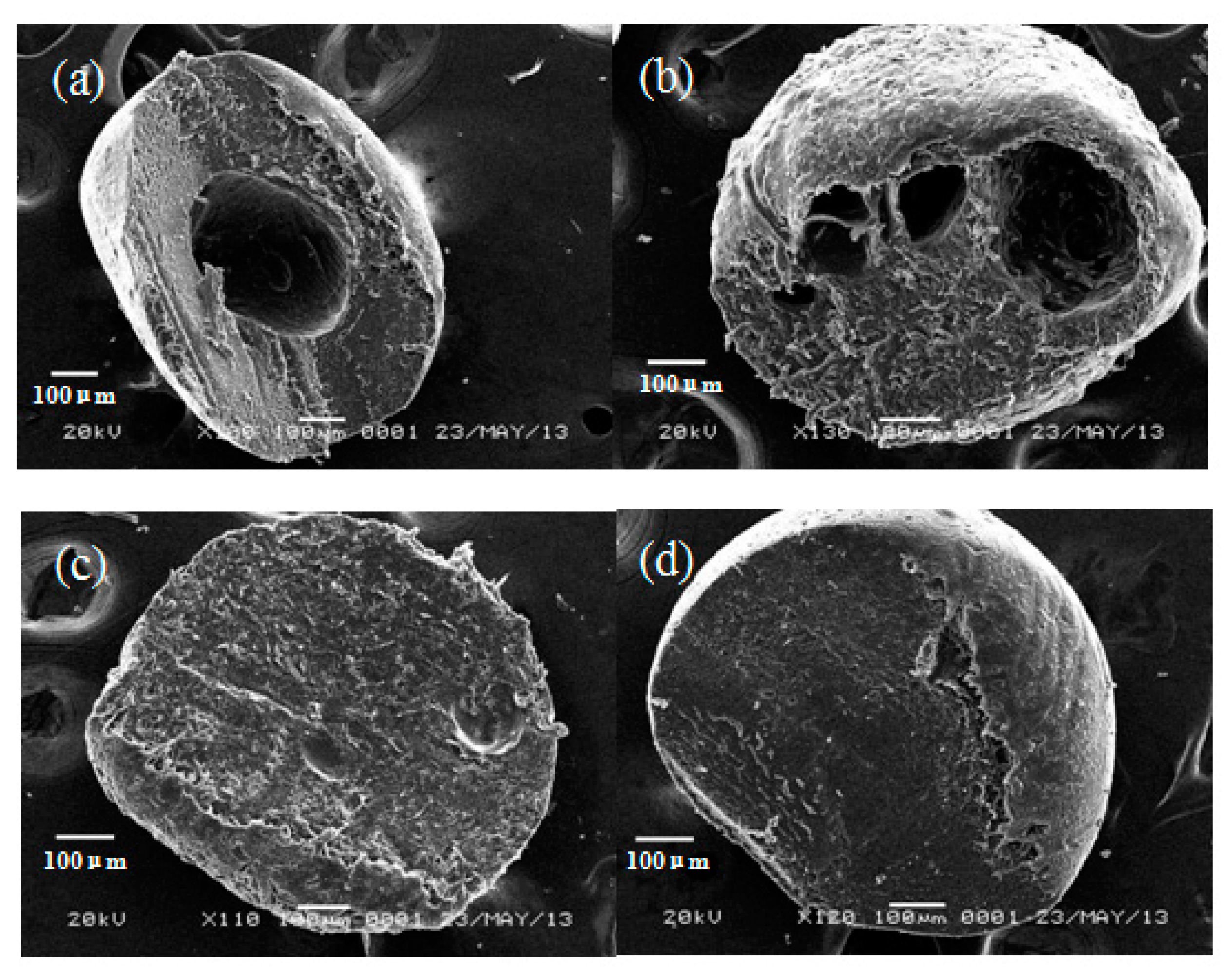
2.1. Characterization of Clay/Chitosan Composite Beads and Chitosan Bead
2.2. Lipase Immobilization and Enzyme Leakage from Supports
2.2.1. Effect of pH and Adsorption Time on Lipase Immobilization
2.2.2. Enzyme Leakage from Supports
2.3. Activities of Immobilized Lipases in Hydrolysis of Olive Oil under Different pHs and Temperatures
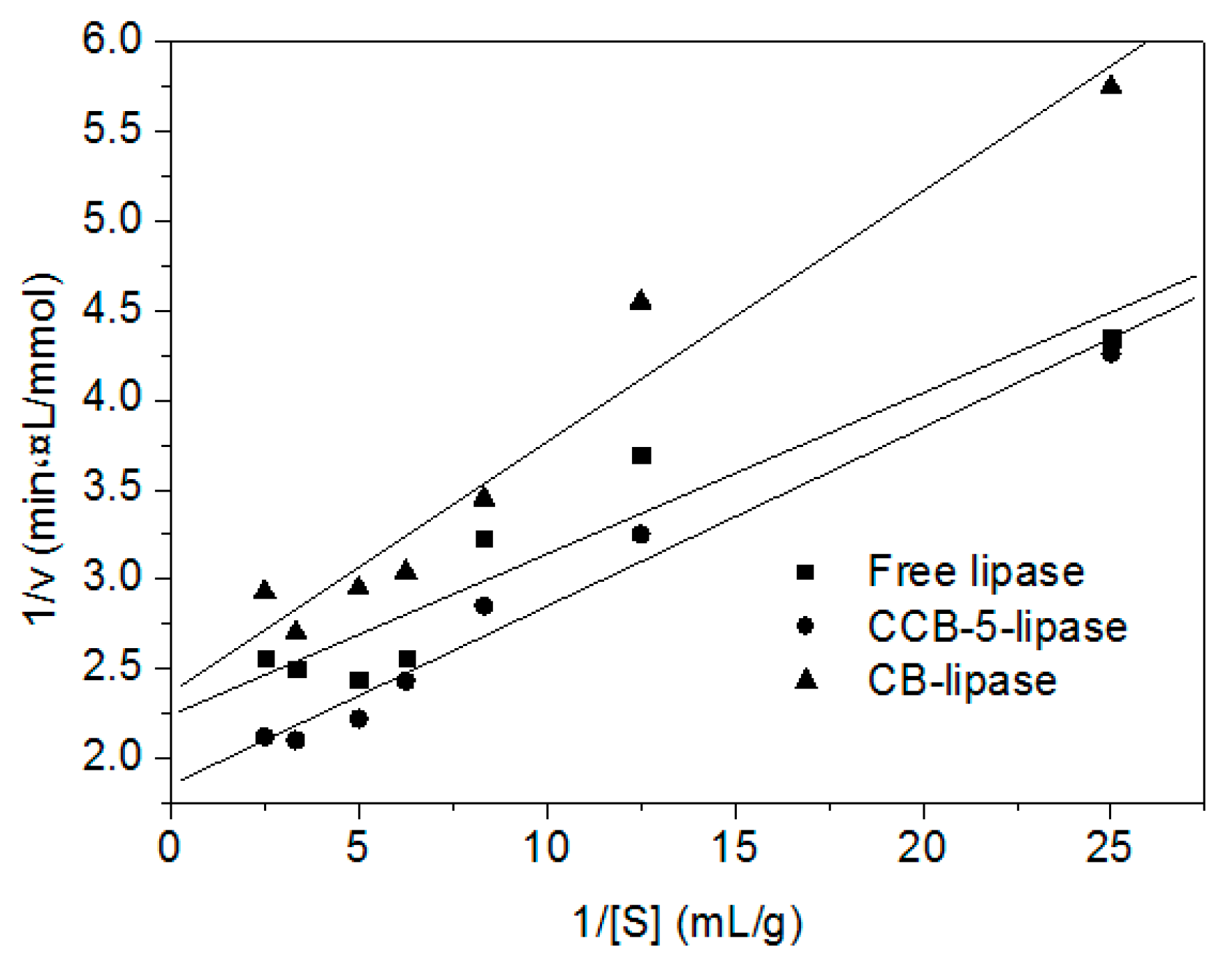
2.4. Catalytic Kinetics of Free and Immobilized Lipase
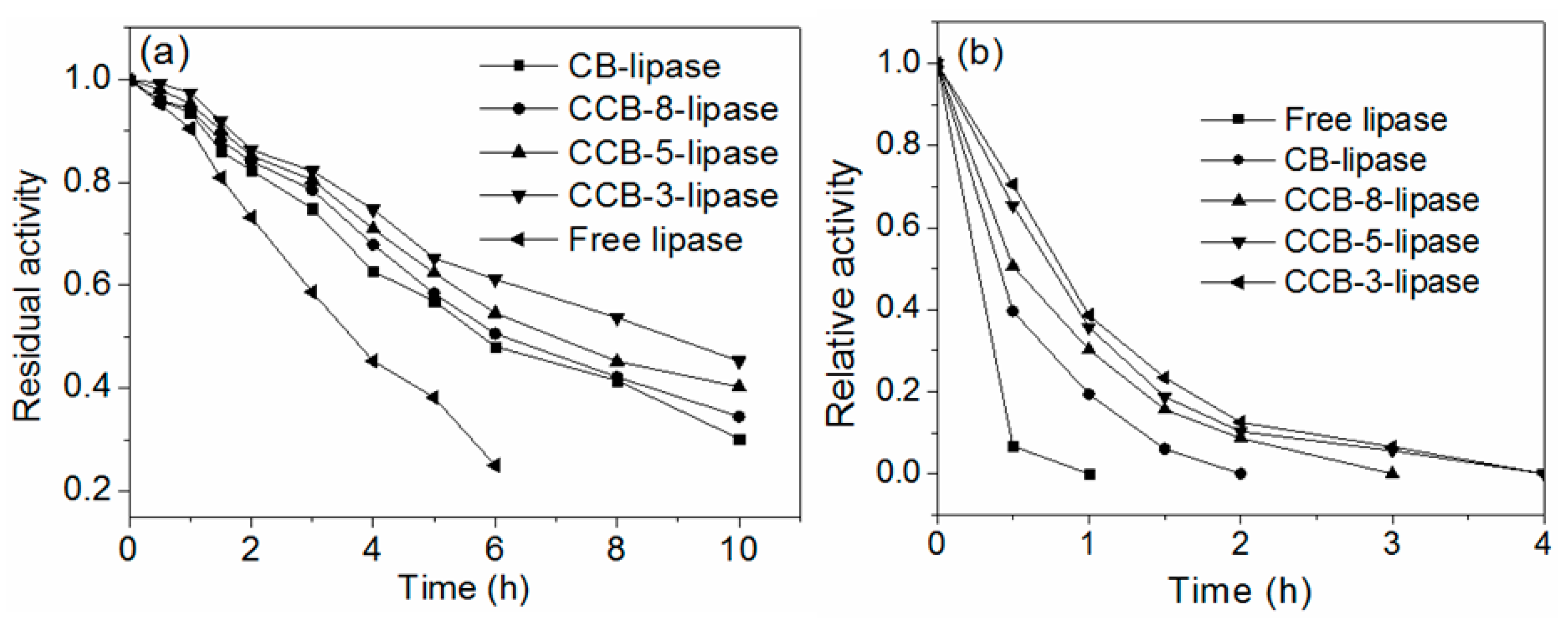
2.5. Thermal Stability of Immobilized Lipases
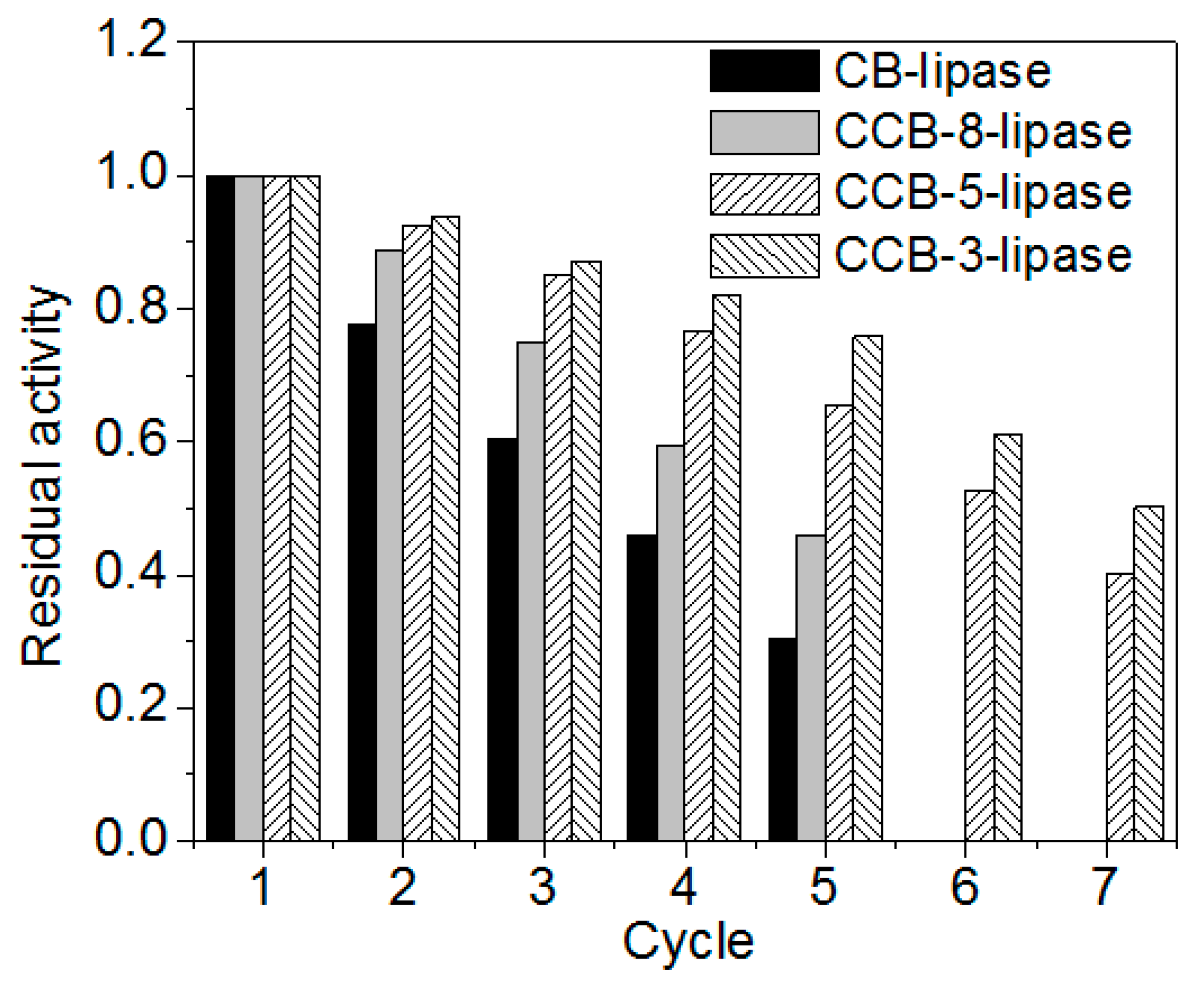
2.6. Reusability of Immobilized Lipases
3. Materials and Methods
3.1. Materials
3.2. Preparation of Clay/Chitosan Composite Beads
3.3. Immobilization of Lipase on Chitosan Bead and Clay/Chitosan Composite Beads
3.4. Enzyme Leaking Test
3.5. Characterizations
3.6. Activity Assay of Immobilized Lipases
3.7. Determination of Kinetic Parameters
3.8. Thermal Stability of Free and Immobilized Lipases
3.9. Reusability of Immobilized Lipases
4. Conclusions
Reference
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sharma, R.; Chisti, Y.; Banerjee, U.C. Production, purification, characterization, and applications of lipases. Biotechnol. Adv. 2001, 19, 627–662. [Google Scholar] [CrossRef]
- Acosta, A.; Filice, M.; Fernandez-lorente, G.; Palomo, J.M.; Guisan, J.M. Kinetically controlled synthesis of monoglyceryl esters from chiral and prochiral acids methyl esters catalyzed by immobilized Rhizomucor miehei lipase. Bioresour. Technol. 2011, 102, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Ziegler-Borowska, M.; Chelminiak-Dudkiewicz, D.; Siódmiak, T.; Sikora, A.; Wegrzynowska-Drzymalska, K.; Skopinska-Wisniewska, J.; Kaczmarek, H.; Marszałł, M.P. Chitosan-collagen coated magnetic nanoparticles for lipase immobilization-new type of “enzyme friendly” polymer shell crosslinking with squaric acid. Catalysts 2017, 7, 26. [Google Scholar] [CrossRef]
- Sheldon, R.A.; van Pelt, S. Enzyme immobilisation in biocatalysis: Why, what and how. Chem. Soc. Rev. 2013, 42, 6223–6235. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Li, Y.; Li, J.; Sheng, G.; Chen, H. Comparative study on lipases immobilized onto bentonite and modified bentonites and their catalytic properties. Ind. Eng. Chem. Res. 2013, 52, 9030–9037. [Google Scholar] [CrossRef]
- Zhao, D.; Peng, C.; Zhou, J. Lipase adsorption on different nanomaterials: A multi-scale simulation study. Phys. Chem. Chem. Phys. 2015, 17, 840–850. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, J.; Mori, Y.; Kobayashi, S. Novel immobilization method of enzymes using a hydrophilic polymer support. Chem. Commun. 2006, 4227–4229. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Ke, C.; Huang, Y.; Yan, Y. Immobilized Aspergillus niger lipase with SiO2 nanoparticles in sol-gel materials. Catalysts 2016, 6, 149. [Google Scholar] [CrossRef]
- Dong, H.; Li, J.; Li, Y.; Hu, L.; Luo, D. Improvement of catalytic activity and stability of lipase by immobilization on organobentonite. Chem. Eng. J. 2012, 181–182, 590–596. [Google Scholar] [CrossRef]
- Kang, Y.; He, J.; Guo, X.D.; Guo, X.; Song, Z.H. Influence of pore diameters on the immobilization of lipase in SBA-15. Ind. Eng. Chem. Res. 2007, 46, 4474–4479. [Google Scholar] [CrossRef]
- Zdarta, J.; Klapiszewski, L.; Jedrzak, A.; Nowicki, M.; Moszynski, D.; Jesionowski, T. Lipase B from Candida antarctica immobilized on a silica-lignin matrix as a stable and reusable biocatalytic system. Catalysts 2017, 7, 14. [Google Scholar] [CrossRef]
- Kim, H.J.; Jin, J.N.; Kan, E.; Kim, K.J.; Lee, S.H. Bacterial cellulose-chitosan composite hydrogel beads for enzyme immobilization. Biotechnol. Bioprocess Eng. 2017, 22, 89–94. [Google Scholar] [CrossRef]
- Cabana, H.; Ahamed, A.; Leduc, R. Conjugation of laccase from the white rot fungus Trametes versicolor to chitosan and its utilization for the elimination of triclosan. Bioresour. Technol. 2011, 102, 1656–1662. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.-Y.; Juang, R.S. Use of chitosan-clay composite as immobilization support for improved activity and stability of β-glucosidase. Biochem. Eng. J. 2007, 35, 93–98. [Google Scholar] [CrossRef]
- De Mello, M.D.; Cordeiro, D.; Costa, L.T.; Follmer, C. Catalytic properties of lipases immobilized onto ultrasound-treated chitosan supports. Biotechnol. Bioprocess Eng. 2013, 18, 1090–1100. [Google Scholar] [CrossRef]
- Stefanescu, C.; Daly, H.W.; Negulescu, I.I. Biocomposite films prepared from ionic liquid solutions of chitosan and cellulose. Carbohydr. Polym. 2012, 87, 435–443. [Google Scholar] [CrossRef]
- Alarcón-Payán, D.A.; Koyani, R.D.; Vazquez-Duhalt, R. Chitosan-based biocatalytic nanoparticles for pollutant removal from wastewater. Enzyme Microb. Technol. 2017, 100, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Gilani, S.L.; Najafpour, G.D.; Moghadamnia, A.; Kamaruddin, A.H. Stability of immobilized porcine pancreas lipase on mesoporous chitosan beads: A comparative study. J. Mol. Catal. B Enzym. 2016, 133, 144–153. [Google Scholar] [CrossRef]
- Metin, A.Ü.; Alver, E. Fibrous polymer-grafted chitosan/clay composite beads as a carrier for immobilization of papain and its usability for mercury elimination. Bioprocess Biosyst. Eng. 2016, 39, 1137–1149. [Google Scholar] [CrossRef] [PubMed]
- Çeklik, A.; Dinçer, A.; Aydemir, T. Characterization of β-glucosidase immobilized on chitosan-multiwalled carbon nanotubes (MWCNTS) and their application on tea extracts for aroma enhancement. Int. J. Biol. Macromol. 2016, 89, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.Y.; Juang, R.S. Activities, stabilities, and reaction kinetics of three free and chitosan-clay composite immobilized enzymes. Enzyme Microb. Technol. 2005, 36, 75–82. [Google Scholar] [CrossRef]
- Dinçer, A.; Becerik, S.; Aydemir, T. Immobilization of tyrosinase on chitosan-clay composite beads. Int. J. Biol. Macromol. 2012, 50, 815–820. [Google Scholar] [CrossRef] [PubMed]
- Li, J.F.; Li, Y.M.; Meng, Q.L. Removal of nitrate by zero-valent iron and pillared bentonite. J. Hazard. Mater. 2010, 174, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.H.; Bai, C.H.; Wang, Y.S.; Jiang, Y.C.; Hu, M.C.; Li, S.N.; Zhai, Q.G. Improvement of chloroperoxidase stability by covalent immobilization on chitosan membranes. Biotechnol. Lett. 2009, 31, 1269–1272. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
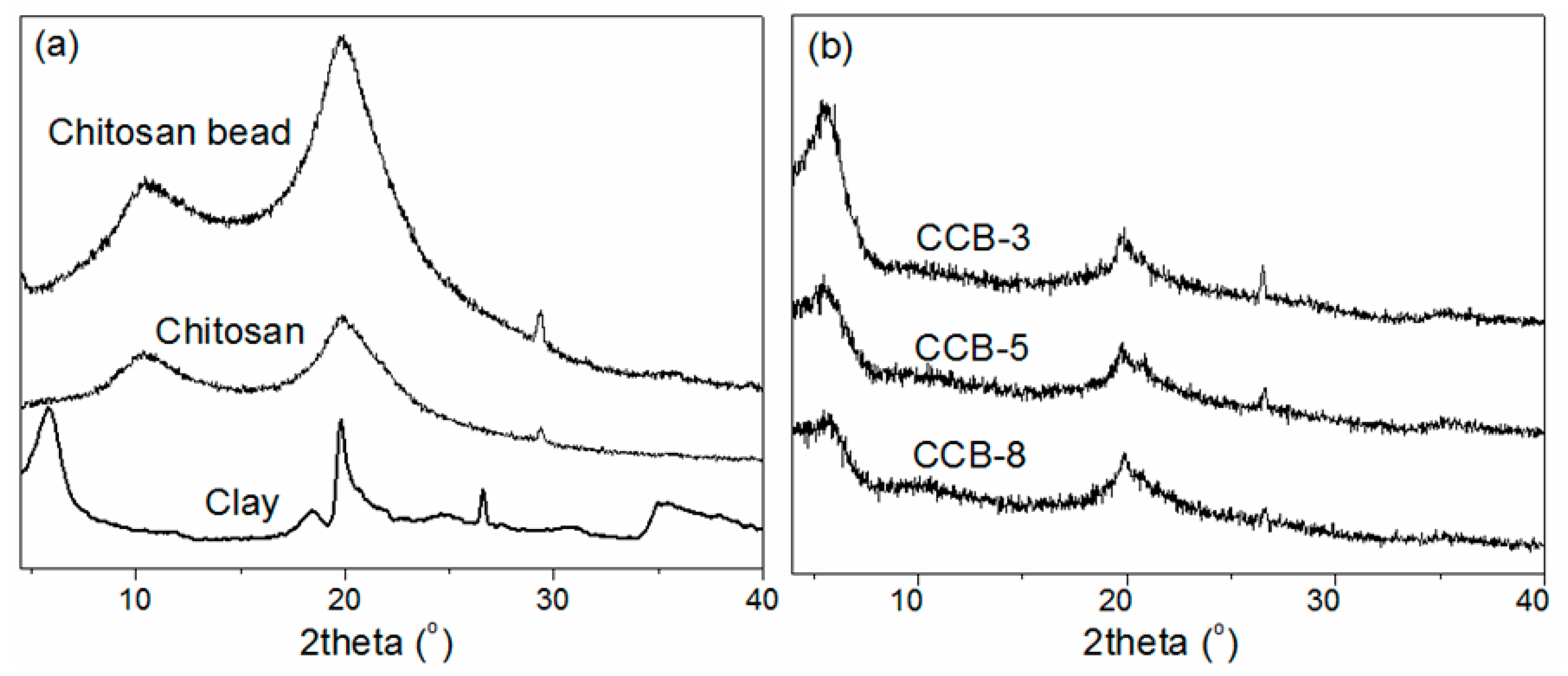
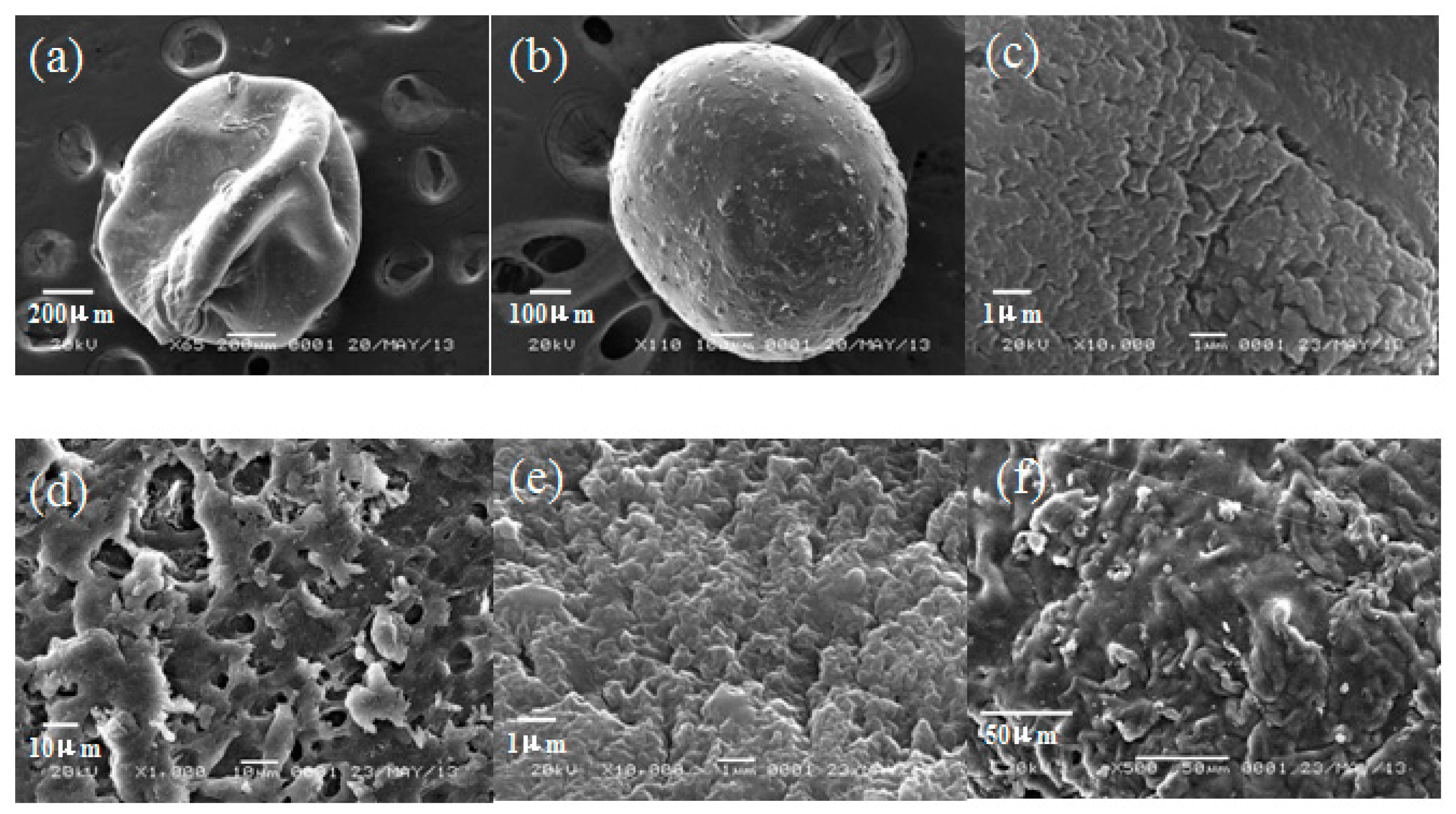
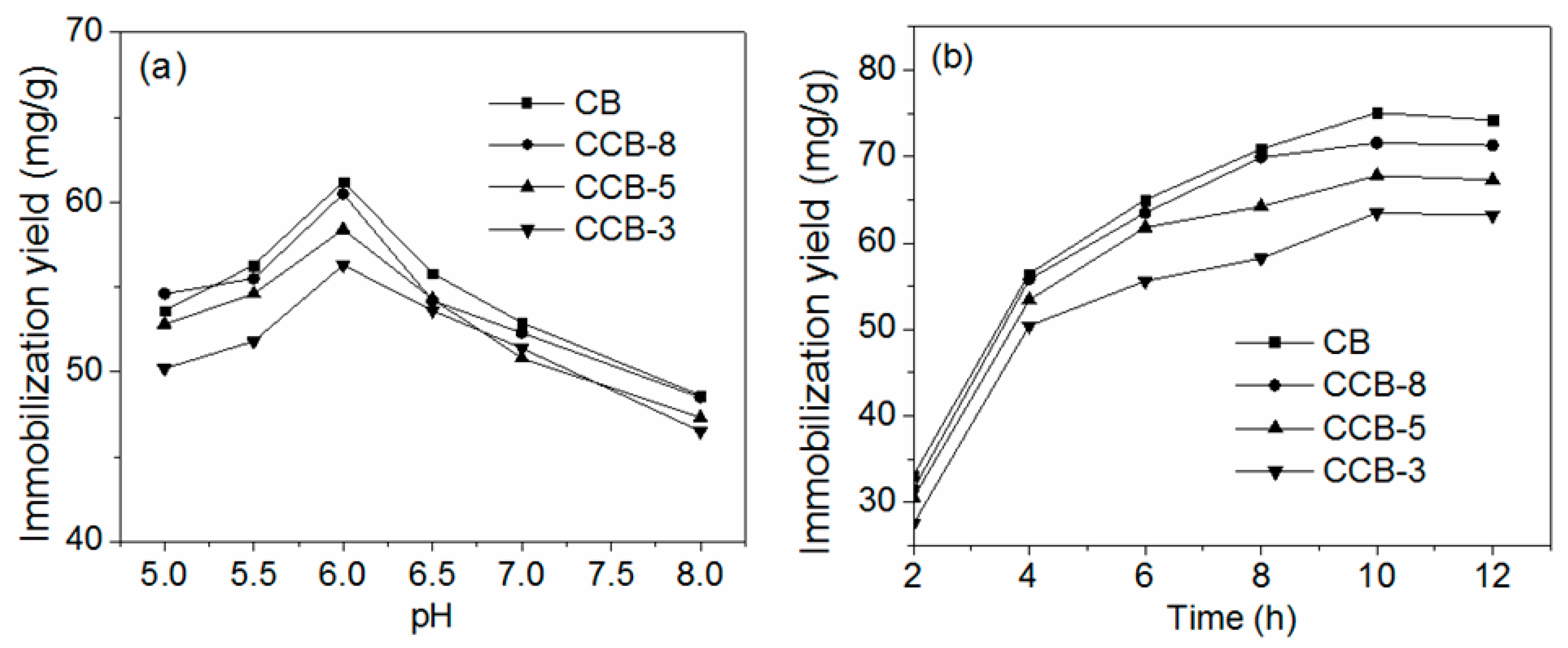

| Support | CB | CCB-8 | CCB-5 | CCB-3 |
|---|---|---|---|---|
| Enzyme leakage (%) | 23.5 | 19.1 | 16.5 | 14.3 |
| Biocatalyst | Michaelis–Menten Constant (Km) (g/mL) | Maximum Reaction Rate (Vmax) (mmol/min·L) | r * |
|---|---|---|---|
| Free lipase | 0.040 | 0.447 | 0.950 |
| CCB-5-lipase | 0.052 | 0.541 | 0.988 |
| CB-lipase | 0.060 | 0.423 | 0.978 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tu, N.; Shou, J.; Dong, H.; Huang, J.; Li, Y. Improved Catalytic Performance of Lipase Supported on Clay/Chitosan Composite Beads. Catalysts 2017, 7, 302. https://doi.org/10.3390/catal7100302
Tu N, Shou J, Dong H, Huang J, Li Y. Improved Catalytic Performance of Lipase Supported on Clay/Chitosan Composite Beads. Catalysts. 2017; 7(10):302. https://doi.org/10.3390/catal7100302
Chicago/Turabian StyleTu, Ni, Jianxin Shou, Huaping Dong, Jin Huang, and Yimin Li. 2017. "Improved Catalytic Performance of Lipase Supported on Clay/Chitosan Composite Beads" Catalysts 7, no. 10: 302. https://doi.org/10.3390/catal7100302





