1. Introduction
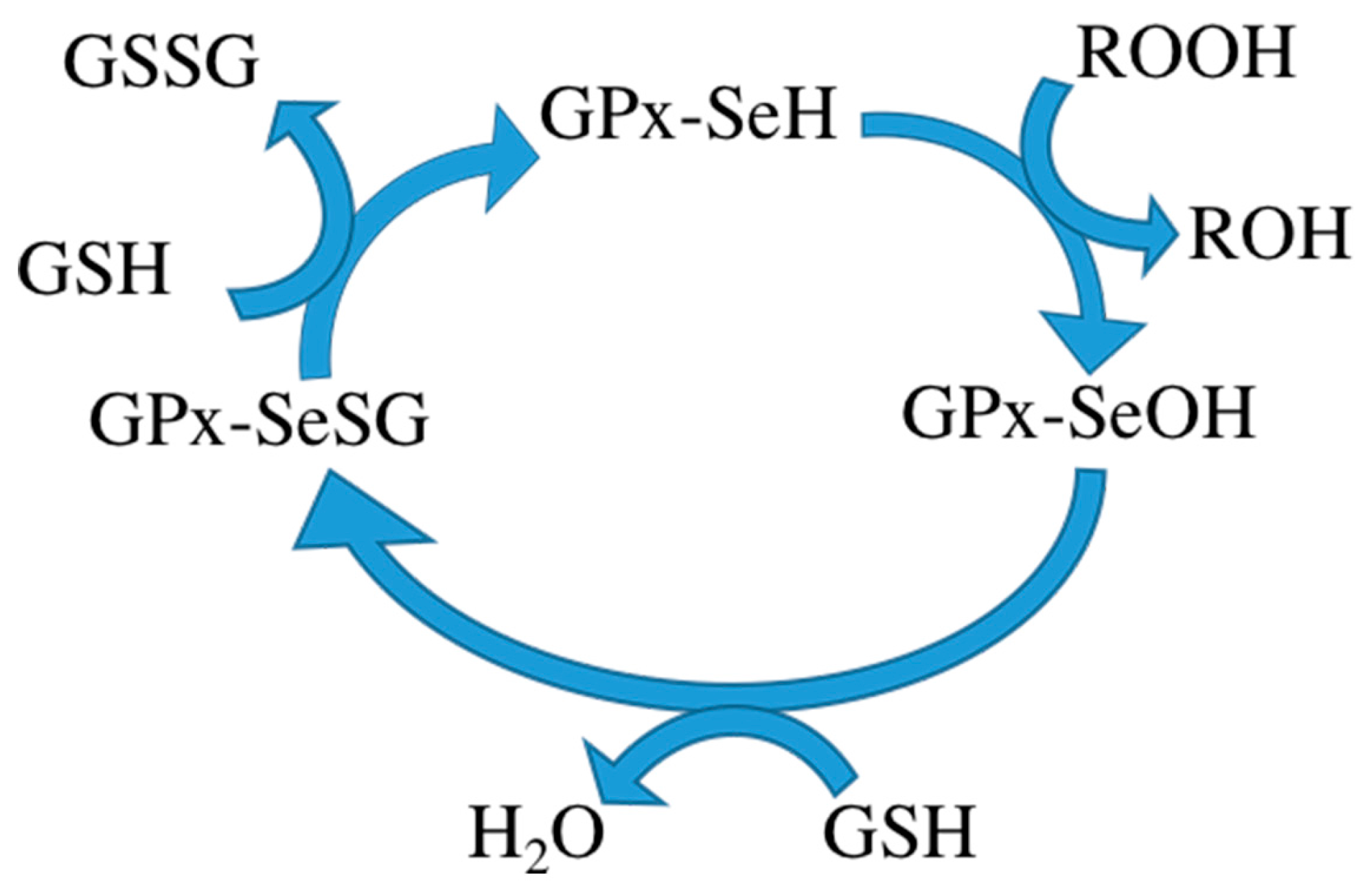
During metabolism, toxic reactive oxygen species (ROS) are produced in the cell. Some ROS can be hydrolyzed by enzymes in the body [
1,
2]. However, excessive ROS can cause oxidative stress, resulting in damage to many important biological macromolecules, potentially inflicting disease. Mammalian cells have a defense mechanism that prevents excessive accumulation of ROS by maintaining the balance between ROS production and degradation. Glutathione peroxidase (GPx) is one of the most important antioxidants in this defense mechanism [
3]. It consists of four subunits, each subunit containing a glutathione binding site and a selenocysteine. Glutathione binding sites are responsible for binding oxidized glutathione and selenocysteine is the catalytic activity center, converting oxidized glutathione to its reduced form. Reduced glutathione in turn interacts with intracellular free radicals, thereby becoming oxidized again but detoxifying the radicals and thus protecting the body from harm of free radicals. Some researchers therefore want to use GPx to treat certain diseases. The mechanism of the GPx contains three steps: (1) oxidation of the selenol form of Sec (GPx-SeH) into a selenenic intermediate (GPx-SeOH), with simultaneous reduction of one equivalent of hydroperoxide to produce water/alcohol; (2) reduction of GPx-SeOH upon reaction with one equivalent of glutathione (GSH), thus producing water and a selenenyl sulfide intermediate (GPx-SeSG); and (3) reduction of GPx-SeSG by reaction with a second equivalent of GSH to produce oxidized glutathione (GSSG), thus resulting in the regeneration of the initial configuration of the enzyme (
Scheme 1) [
4,
5,
6].
However, GPx is unstable, rare, and has a high molecular weight, making it unfavorable for therapeutic use. In order to retain the GPx antioxidant capabilities while overcoming these shortcomings, scientists have produced active selenocysteine GPx mimics by using other macromolecules [
7,
8,
9,
10].
To date, many approaches have been tested to produce GPx mimics. These include the use of chemical macromolecules as host such as cyclodextrin, dendrimers, and hyperbranched polymers. Natural enzymes have also been used as host such as semi-synthetic selenoenzymes. Here, genetic engineering technology is used to produce the selenoenzymes. Some researchers also produce GPx mimics using monoclonal antibody, nano-micelles, macrocyclic compound scaffolds, and molecular imprinted polymers [
11]. Molecular imprinting is one of the most popular methods to synthesize GPx mimics. Prior to molecular imprinting, chemical mutation [
3] (chemical mutation is the method that enables the substrate to obtain specific function through engineering the specificity of existing enzymes) was commonly used. Hilvert et al. (1989) used chemical mutation for the first time, converting serine to selenocysteine [
12]. Most researchers used chemical mutation to transform macromolecules (cyclodextrins, dendrimers, hyperbranched polymers, etc.) and subsequently introduced catalytic groups to obtain GPx activity mimics. Although widely used, there are still some shortcomings. Ren et al.’s study found that chemical methods provided a means for introducing into enzymes diverse functions that did not occur naturally and could not be easily incorporated by genetic engineering [
13]. Some researchers therefore tried to synthesize GPx mimics by screening for antibodies or single-chain variable fragments with high substrate specificity, and then used chemical mutation. Similarly, others used the substrate binding sites of natural enzymes followed by chemical mutation to obtain GPx mimics. Although these synthetic approaches give rise to GPx mimics with high substrate binding capacity, the locations of their active sites are not clear and therefore impede detailed studies of enzymatic reaction kinetics. Ren et al. introduced selenium by chemical mutagenesis in the experiment, and the selenium content of Se-OVA (egg albumin) (OVA containing 38 serine residues) and Se-BSA (bovine serum albumin) (BSA containing 28 serine residues) was 5.6 mol, 5.2 mol, respectively. So it is not clear which selenium sites were introduced selenium. Zheng et al. [
14] utilized chemical mutation to make a GPx mimic from human glutathione transferase Zeta (hGSTZ1-1). Subsequent analysis of its crystal structure showed that there were two serine (Ser 14 and Ser 15) in its active site and they played an important role in the binding and orientation of glutathione (GSH). Hence, they had to compare the activity between Ser 14 and Ser 15 in order to understand the enzymatic kinetics. It is clear that chemical mutation, used to create GPx mimics, can make research more cumbersome due to the ambiguities of the catalytic sites. Advances in the research meant that GPx mimics could now be produced using either one of two methods that rely on bio-imprinting and chemical mutation.
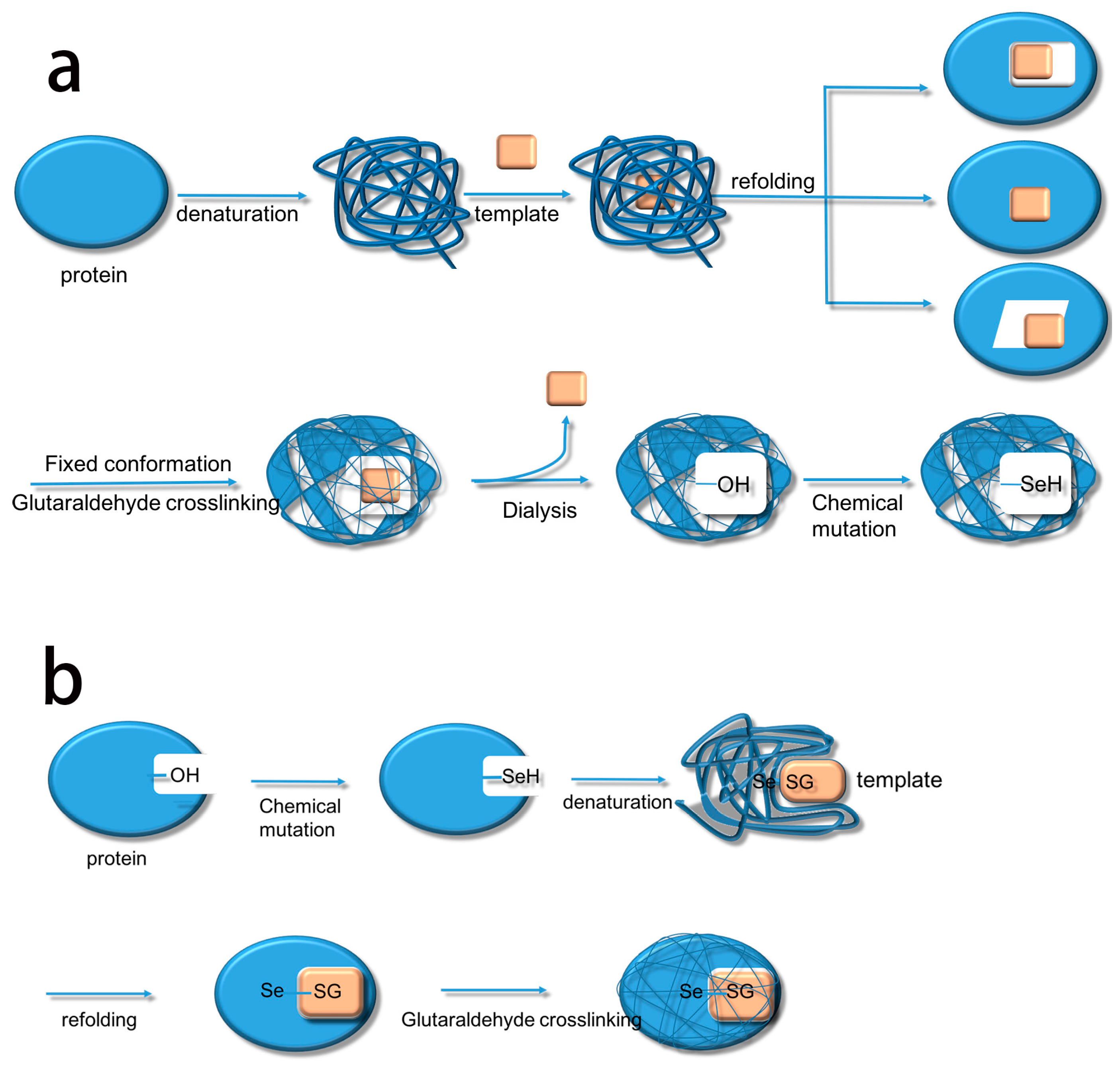
Based on this, we summarize the two methods. The first method uses biological imprinting to produce substrate binding sites and then produces a catalytic site by chemical mutation (BICM) (
Scheme 2a) [
15,
16]. The second method first produces a catalytic site through chemical mutation and then introduces the substrate binding sites by biological imprinting (CMBI) (
Scheme 2b). After several experiments, it became clear that BICM has some disadvantages: the location of the binding sites was not always clear at the time of chemical mutation, and the locations did not necessarily have a suitable hydroxyl group, meaning that the catalytic activity did not always increase. For instance, Liu et al. [
15] used BICM to synthesize a GPx mimic using albumin as the imprinted molecule. The selenium content of the active site was quantitatively determined by 2-nitrobenzoic acid. The results showed that the ratio of the mimic containing selenium to the selenium content was not the same, which implied that BICM did not allow selenium to specifically connect to the active site, resulting in a waste of selenium. At the same time, it also made the active center of the mimic structure unclear and affected the reaction rate [
15]. The CMBI method was also used by Liu et al. [
1], this time used subtilisin as the imprinted molecule. Quantitative determination of selenium in the active site of the enzyme showed that the ratio of the imprinted selenosubtilisin in the active site to the selenium content of the system was 1:1. CMBI thus greatly improved the utilization of selenium compared with BICM, and made the active center structure clearer to improve the catalytic efficiency. CMBI therefore overcame the drawbacks of BICM but, since the method was reported [
17,
18], there have been no further reports using CMBI to produce GPx mimics. This was unexpected given the improvements and suggested that there may have been further complications. In this study, we therefore used the CMBI method to introduce selenium sites with trypsin as the imprinted molecule for biological imprinting and produced GPx mimics. Previously, we already determined that introducing the catalytic group of selenated trypsin through chemical mutation made a special kind of GPx mimic with a clear catalytic site. Herein, we elucidate the activity and kinetic parameters of TSeO
2H (Trypsin-SeO
2H), TSeSG (Trypsin-Se-SG), ITSeSG, and CITSeSG to further support our previous research.
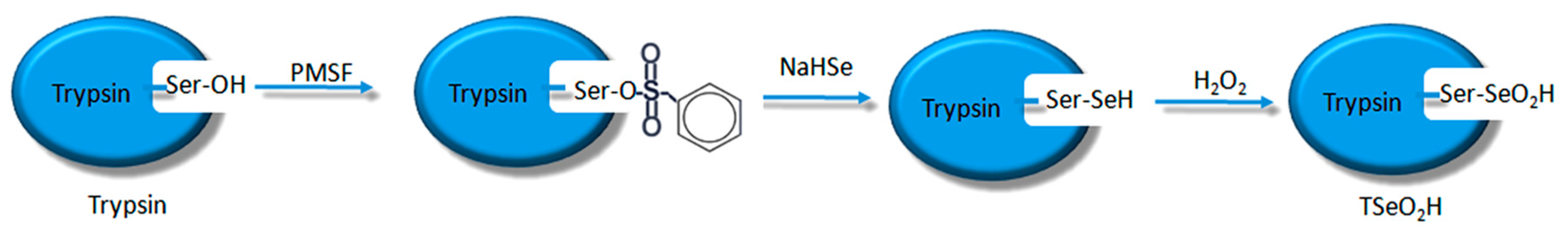
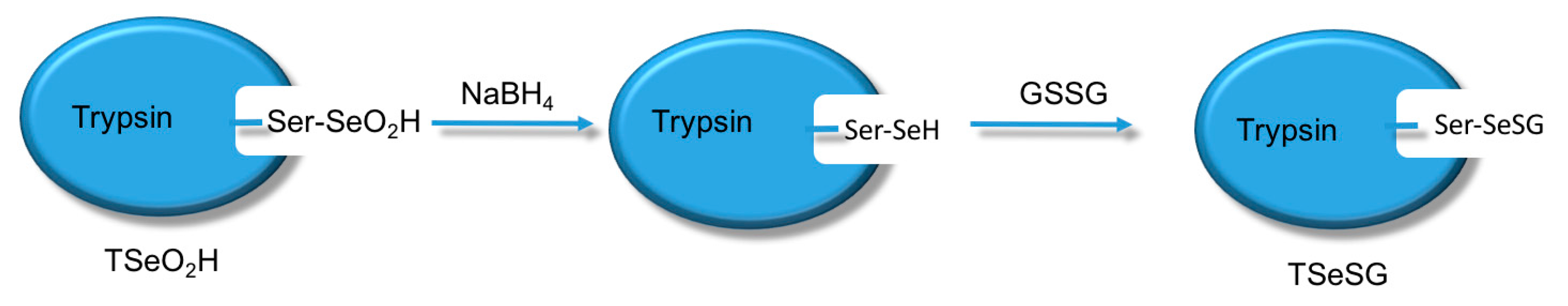
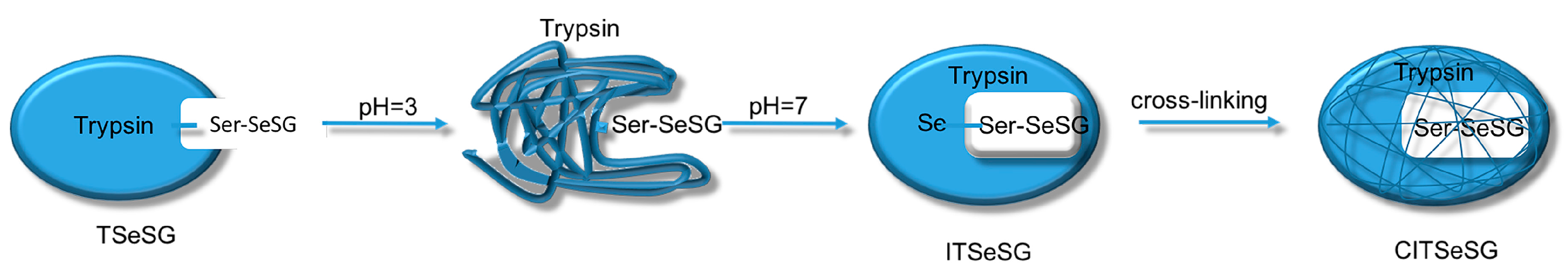
The process of synthesizing molecular imprinted polymers can be divided into three steps: (1) template molecules and functional monomers are combined; (2) imprinting molecules are polymerized with complexes formed by template molecules and functional monomers; (3) template molecules are removed from the polymer [
19]. Scientists have already demonstrated that bio-imprinting, an integral part of molecular imprinting, is an effective means to introduce binding sites into proteins or other biomolecules. In this study, imprinting molecules were synthesized by bio-imprinting, and the GPx mimic with high catalytic activity and a clear catalytic site was prepared using the above three steps. In our paper, we synthesized GPx mimic through the following route: the hydroxyl group of Ser was site-specifically activated by the addition of phenylmethanesulfonyl fluoride (PMSF) to form a sulfonylated trypsin. Then selenium was introduced into the active site by reaction of the sulfonated trypsin with NaHSe. Adding H
2O
2 subsequently to the resulting selenol form of the trypsin yields the seleninic acid form of TSeO
2H. Then through adding NaBH
4 and GSSG (template molecule) TSeO
2H changes into TSeSG, the process is that the S–S of GSSG combines with the SeH group of trypsin and synthesizes a Se–S bond. This process leads GSH to combine with trypsin in the form of Se–S bond. Finally through the change of pH, imprinted molecules stretch and refold to let GSH wrap in the trypsin. The product of this process called ‘ITSeSG’. After crosslinking of glutaraldehyde, ITSeSG changes into CITSeSG.
2. Results and Discussion
2.1. Preparation and Characterization of Mimics
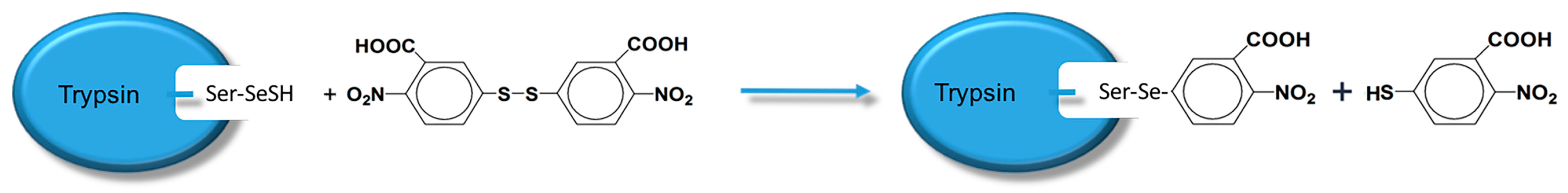
According to Luo [
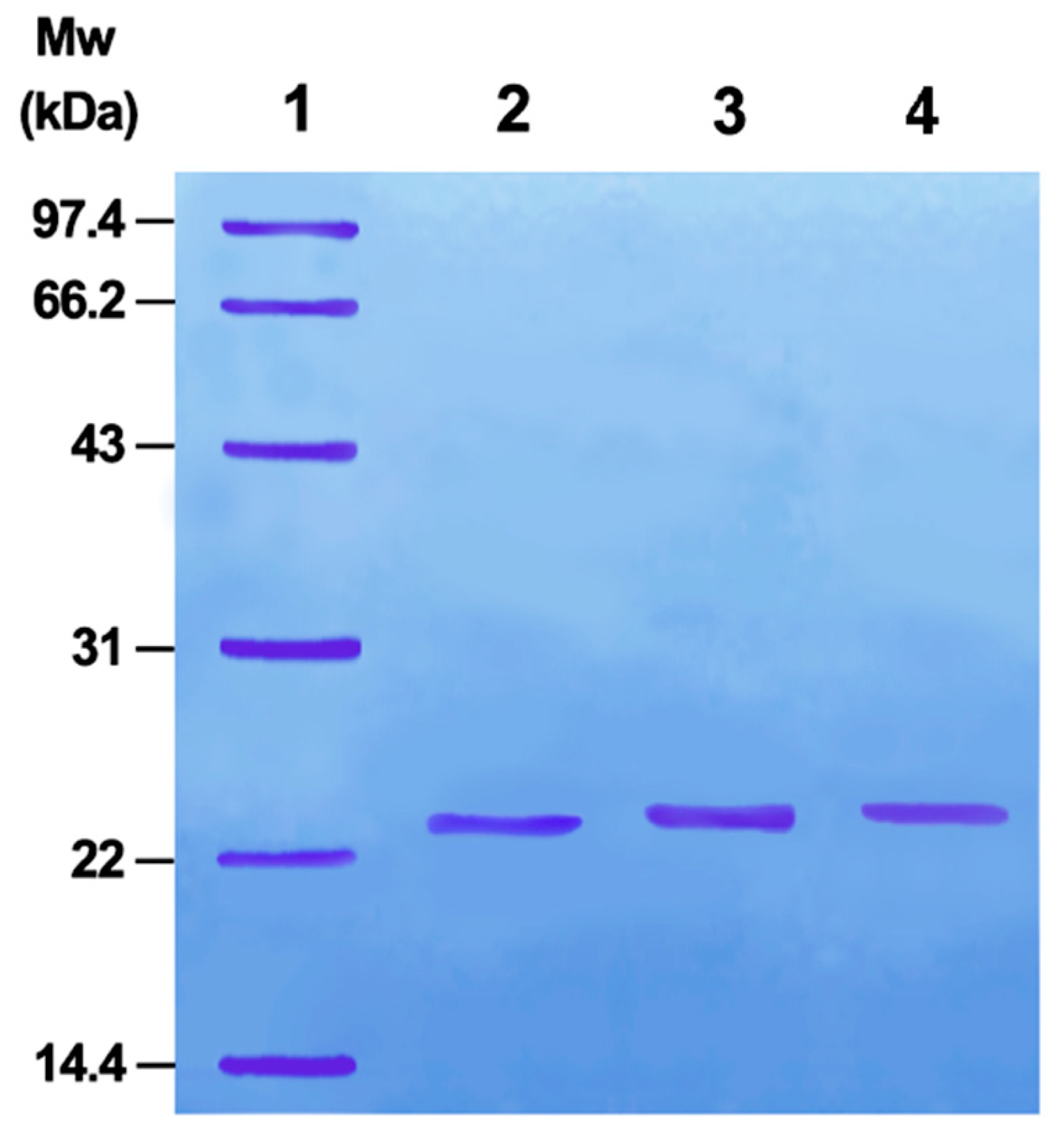
20], PMSF addition can activate the serine side chain -OH in the trypsin active site. Further, activated byproducts do not affect the following selenide substitution reaction. Therefore, once activated, the selenide substitution reaction can directly occur without purification. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) indicated that the product is a single protein (
Figure 1). Selenoprotein (TSeO
2H) interacted with DTNB and the absorbance of the new product was measured, indicating 0.96 SeH per TSeO
2H. Trypsin activity of TSeO
2H was determined using
N-benzoyl-
l-tyrosine ethyl ester as substrate (PBS, pH 7.4). TSeO
2H completely abolished trypsin activity, illustrating that the serine side chain -OH in the active site of trypsin had essentially translated into SeO
2H with GPx catalytic activity.
Biological imprinting technologies have improved the imprinting capabilities of molecules. By adjusting the solution to pH 3.0, trypsin was first partially stretched and its amino acid structure was unfolded. The solution was then slowly adjusted to pH 7.0 to allow for molecular recognition of the amino acids. This induced the conformation with -Se-SG [
1] so that the protein folded again with a substrate binding site, thereby obtaining ITSeSG.
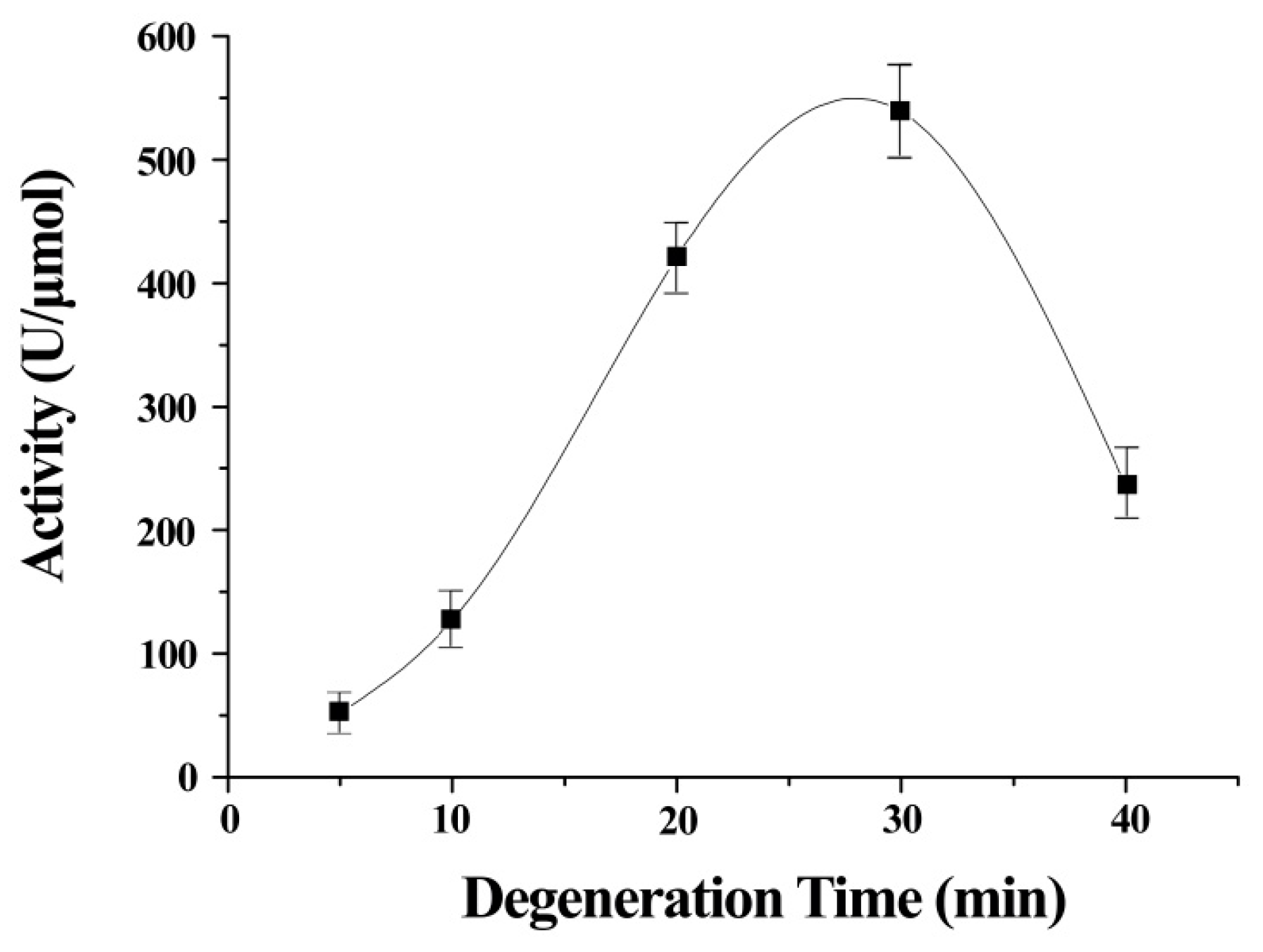
The stretch time was an important contributor to the activity of ITSeSG (
Figure 2). This is possibly because the stretch time is positively correlated with the extent of protein stretch. A shorter stretch time may therefore lead to inadequate protein unfolding and thus less interaction with -Se-SG and omitting the substrate binding site. Decreased GPx activity was indicative of excessive ITSeSG degeneration (
Figure 2). Therefore, we inferred that a partially stretched protein creates a suitable binding site and that this did not affect the formation of catalytic intermediates. The experimental results showed that a stretch time of 20 to 30 min was needed to reach the highest GPx activity.
Error bars represent the mean ± standard error (n = 4).
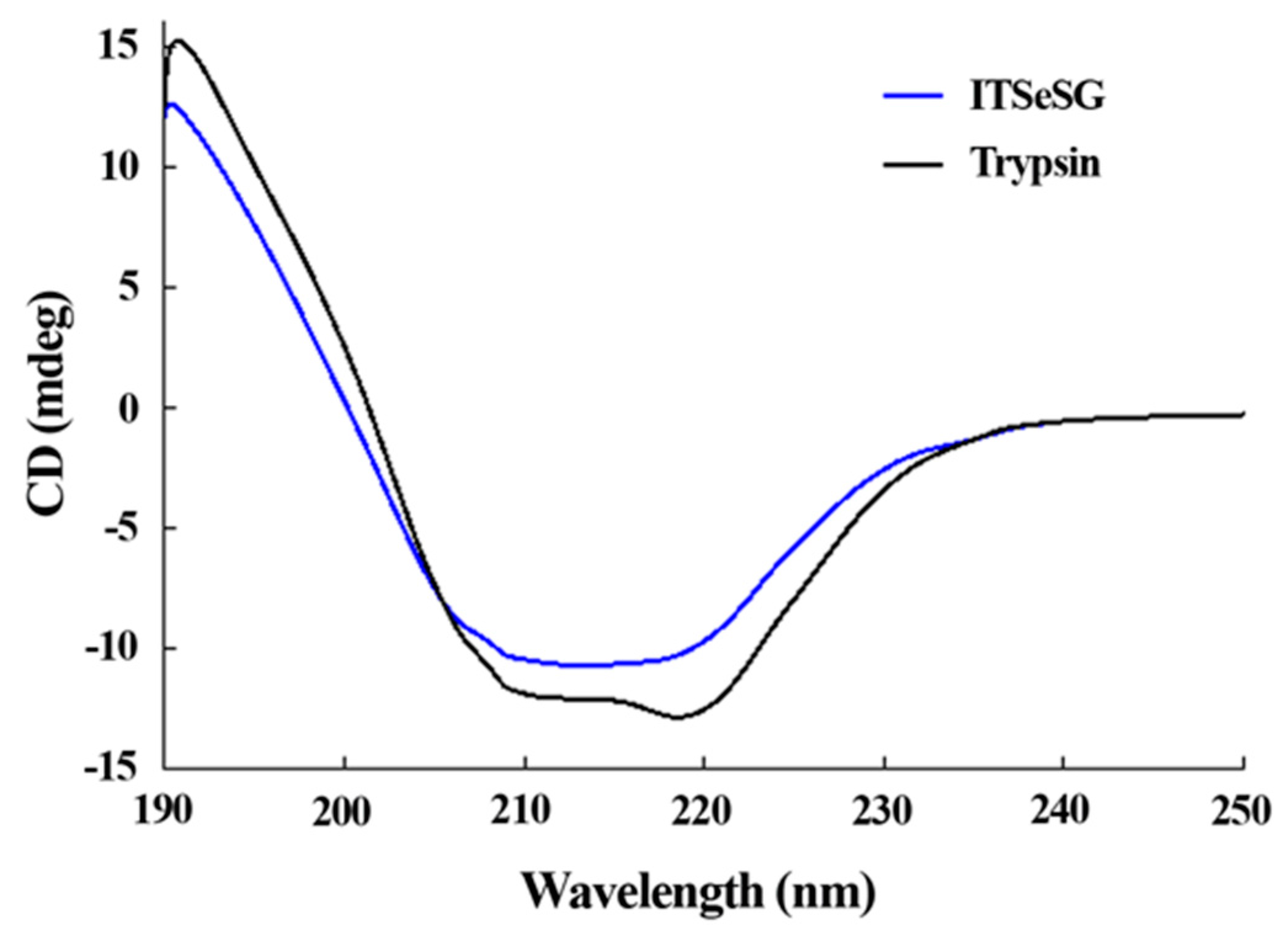
The changing of the secondary structure of trypsin and ITSeSG was detected by circular dichroism spectra (
Figure 3).
The proportion of α-helices and β-folds was 19.2% and 48.8% for trypsin, and 16.3% and 39.39% for ITSeSG, respectively. The proportion of loose structure changed from 27.3% (trypsin) to 36.3% (ITSeSG) (
Table 1).
2.2. The GPx Activity of Mimic
GPx activities of the GPx mimics catalyzed the reduction of H2O2 by GSH and was determined as a change in NADPH absorption at 340 nm (Equations (1) and (2)).
The trypsin active site serine was replaced with selenium cysteine and a catalytic site was introduced to get TSeO
2H by chemical mutation. As expected, because TSeO
2H had no substrate GSH binding sites, it showed low GPx activity. When transformed to ITSeSG, GPx activity increased 10-fold from 51 U μmol
−1 (TSeO
2H) to 536 U μmol
−1 (ITSeSG). Although the activity of ITSeSG has greatly improved using this technique, it is still very different from natural GPx. GPx activity of ITSeSG is 10.8-fold less than that of native GPx (5780 U μmol
−1 from rabbit liver), and is 540 times more active than that of the selenoorganic compound 2-phenyl-1,2-benzoisoselenazol-3(
H)-one (Ebselen). The result showed in the
Table 2.
2.3. Kinetic Properties of Mimic
The steady state kinetics was observed for substrates H
2O
2 and GSH. The initial velocities for reduction of H
2O
2 by GSH were determined as a function of substrate concentration at 37 °C and pH 7.0, varying one substrate concentration while another was fixed. The relevant steady-state equation for the mimic reaction is shown as Equation (3) [
22].
where
v0 is the initial reaction rate, [E]
0 is the initial enzyme mimic concentration,
kmax is a pseudo-first-order rate constant and
KH2O2 and
KGSH are the Michaelis–Menten constants
(Km) for the H
2O
2 and GSH, respectively.
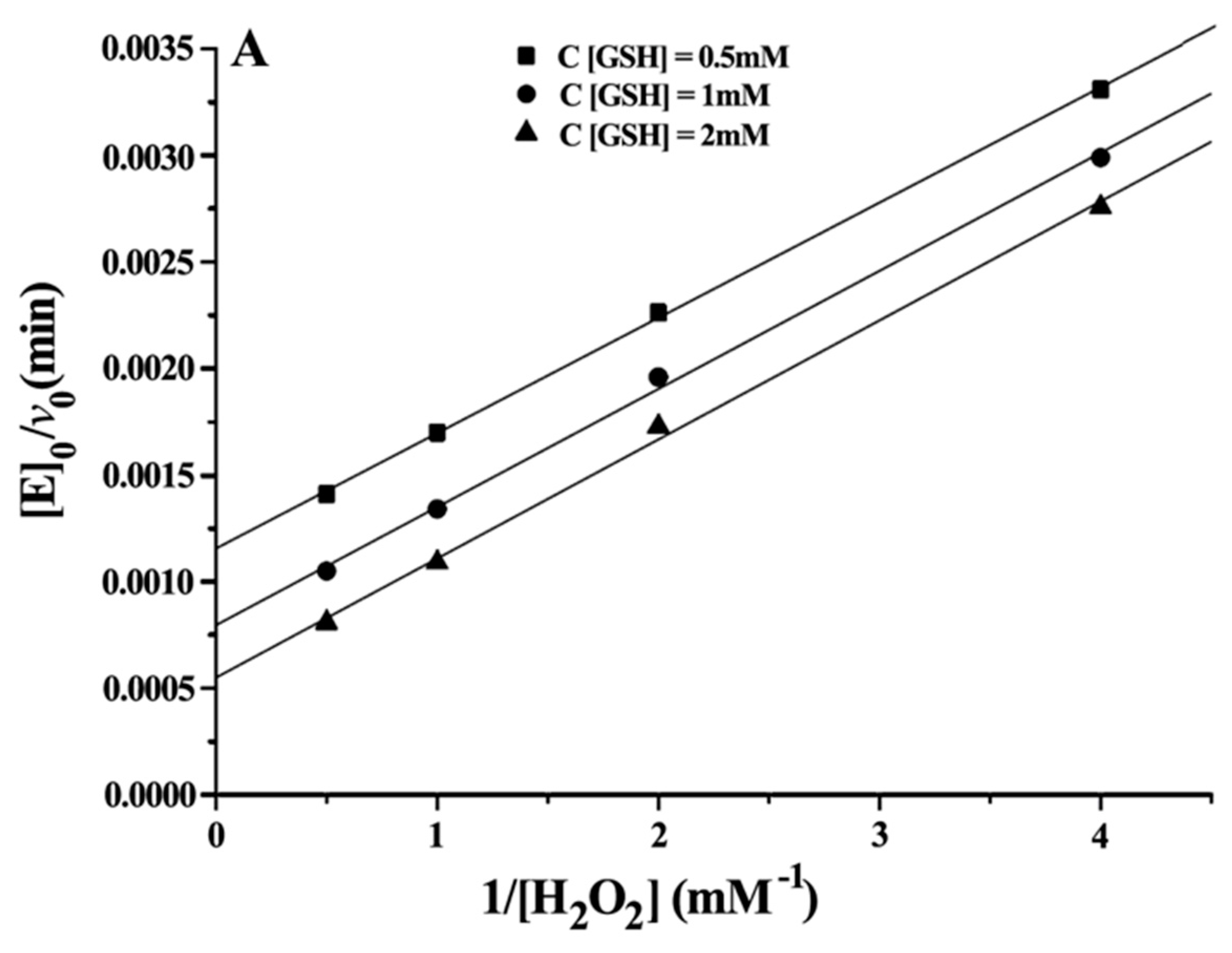
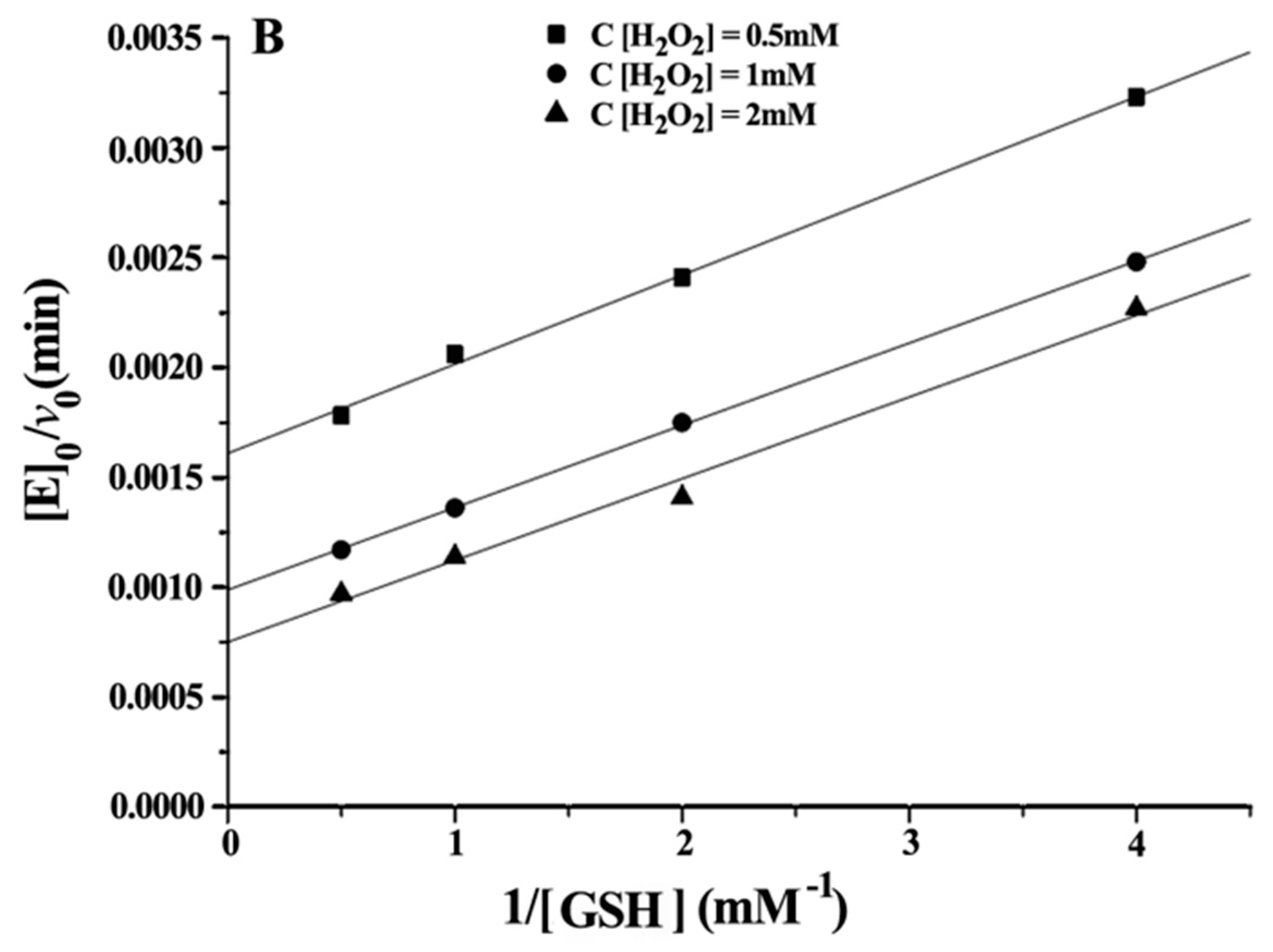
Double reciprocal plots of initial velocity versus the concentration of substrates gave a family of parallel lines (
Figure 4), indicating that the reaction mechanism belongs to the ping-pong mechanism. This result demonstrated that the GPx mimic, has the same catalytic mechanism as that of native GPx. From the steady-state equation, the kinetic parameters were obtained (
Table 3).
With H2O2 as the substrate: kmax·Km−1 H2O2 (ITSeSG) > kmax·Km−1 H2O2 (CITSeSG) > kmax·Km−1 H2O2 (TSeSG) > kmax·Km−1 H2O2 (TSeO2H). ITSeSG had the fastest reaction rate to H2O2, and the reaction rate of TSeO2H to H2O2 was the slowest. The KmH2O2 exhibited by the four mimics is close to that of the substrate H2O2 because the H2O2 molecule is very small; it freely diffuses into the binding site and is less susceptible to the spatial structure of the mimic.
With GSH as the substrate: Km GSH (TSeSG) > Km GSH (TSeO2H) > Km GSH (CITSeSG) > Km GSH (ITSeSG) can be found by comparing the kinetic parameters of the four intermediates. Similarly, kmax·Km−1 GSH (ITSeSG) > kmax·Km−1 GSH (CITSeSG) > kmax·Km−1 GSH (TSeSG) > kmax·Km−1 GSH (TSeO2H). Since the Km value is a coefficient related to substrate affinity, a large Km value indicates low affinity, whereas a small Km value is indicative of high substrate affinity. Therefore, it can be concluded that TSeSG has the lowest affinity for GSH, simply because TSeSG does not have a GSH binding site and can therefore not easily interact with GSH. GSH substrate affinity of TSeSG is similar to TSeO2H. However, due to its -SeSG structure, TSeSG directly enters into the catalytic cycle and the kmax showed a significant increase. The -SeOOH in the TSeO2H molecule must be reduced from GSH to -SeOH or -SeSG to enter the catalytic cycle and thus kmax is the lowest. ITSeSG has the highest GSH substrate affinity. ITSeSG has a GSH binding site and a flexible molecular structure, allowing for the strong binding of GSH. CITSeSG also has a substrate binding site, but due to the glutaraldehyde cross-linking, has a less flexible molecular structure than ITSeSG; moreover, the cross-linked structure will to some extent also affect the release of GSH substrate, and the transformation number was also higher.
The data confirm that the GPx mimic synthesized by molecular imprinting is very effective. Further, the affinity of CITSeSG was only slightly decreased from ITSeSG, suggesting that cross-linking of glutaraldehyde affects GPx activity.
4. Conclusions
We used CMBI to prepare CITSeSG from trypsin. It has proved that CMBI can be used for other proteins, indicating that CMBI is a versatile technique. Our work explored, for the first time, the use of bio-imprinting for the preparation of efficient GPx mimics from the perspective of reaction kinetics parameters. Bio-imprinting of converted TSeSG to ITSeSG, the
KmGSH value of the mimic decreased from 4.82 ± 0.27 mM (TSeSG) to 0.52 ± 0.05 mM (ITSeSG), proving that we produced a highly efficient substrate recognition cavities. Compared with the non-imprinted mimic (TSeSG), the activity of ITSeSG increased 5.7 times. Importantly, this article focused on the activity of the intermediates at various steps leading to the synthetic GPx. In order to confirmed the advantages of CMBI, we compared our GPx mimic’s activity with the activity of natural GPx, Ebselen [
26] (the well GPx mimic known), and we found that ITSeSG displays a high GPx like activity and is only 10 times less than that of the native GPx (5780 U/μmol). However, when comparing with Ebselen (0.99 U/μmol), the GPx activity of CITSeSG is 382 times that for Ebselen. After crosslinking of glutaraldehyde, the activity of CITSeSG has a little lower. Comparing with GPx mimic produced by Liu [
1] through CMBI which used Subtilisin as imprinted molecular, the activity of ITSeSG is similar to imprinted selenosubtilisin. We believe that this work has laid the foundation for the future design of efficient biological imprinting enzyme.