Fabrication of Hierarchically Porous Reduced Graphene Oxide/SnIn4S8 Composites by a Low-Temperature Co-Precipitation Strategy and Their Excellent Visible-Light Photocatalytic Mineralization Performance
Abstract
:1. Introduction
2. Results and Discussion
2.1. Morphology Analysis
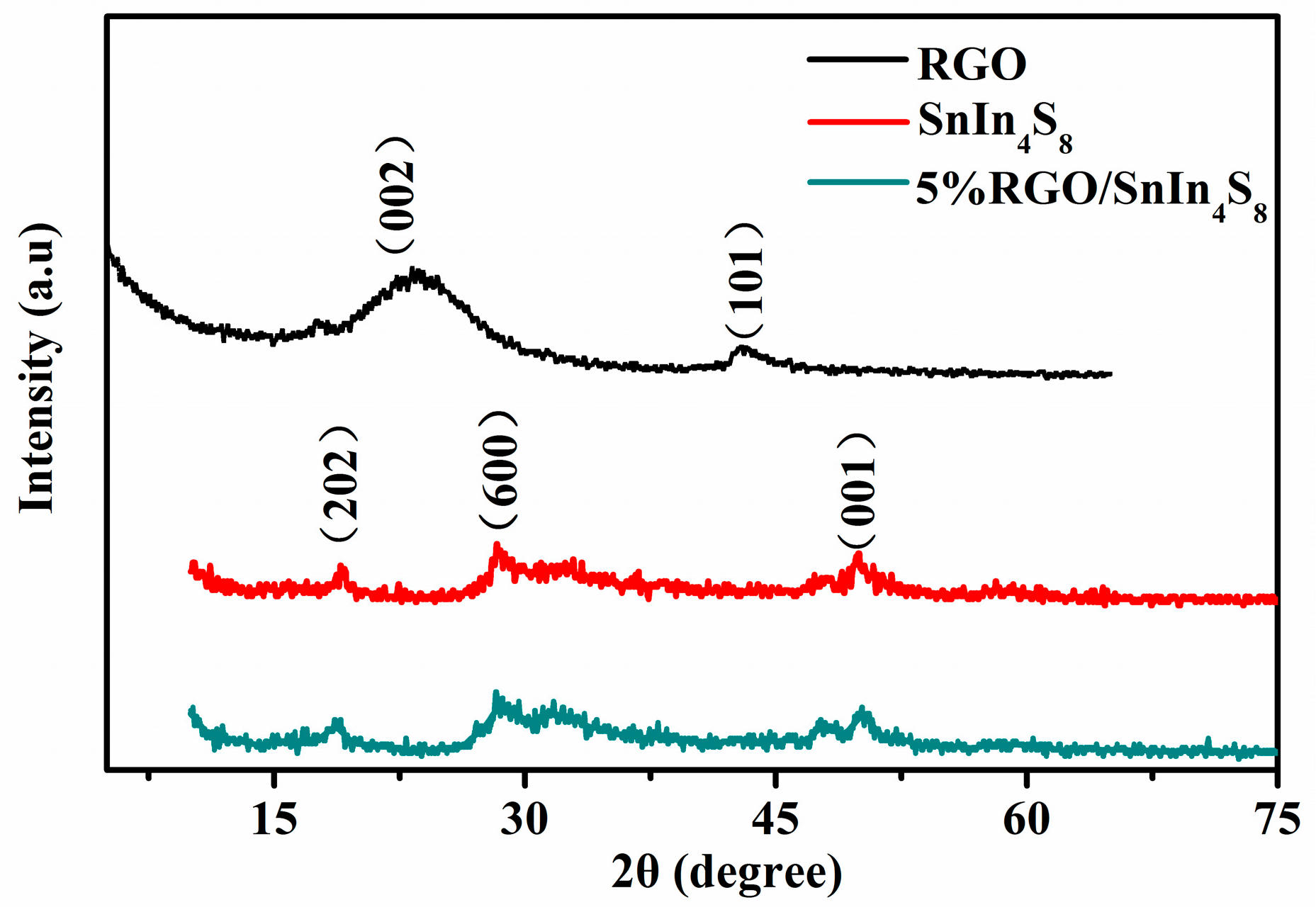
2.2. XRD Analysis
2.3. Raman Spectroscopy
2.4. BET Surface Areas and Pore Structures
2.5. Optical Properties
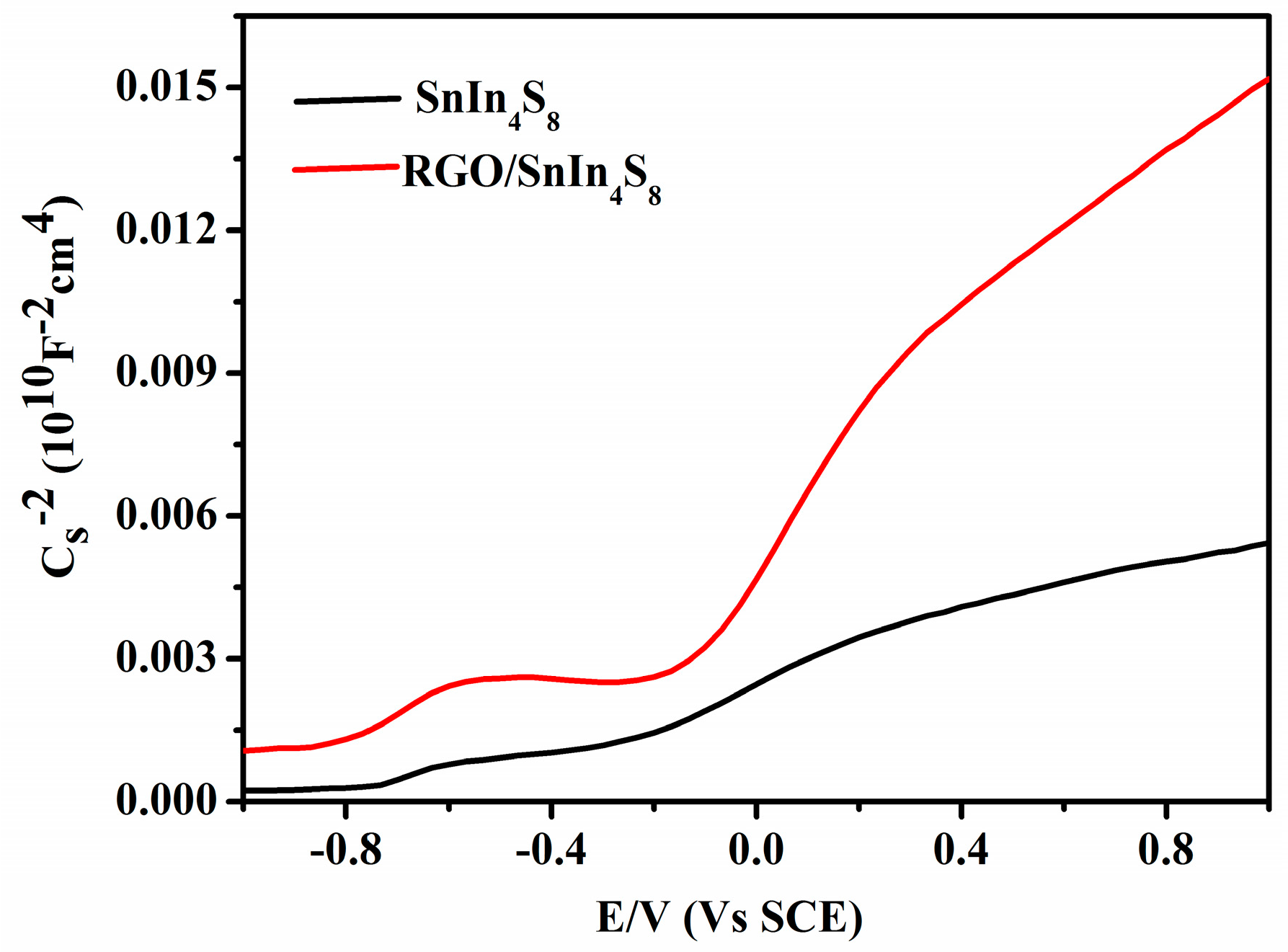
2.6. Mott-Schottky Analysis
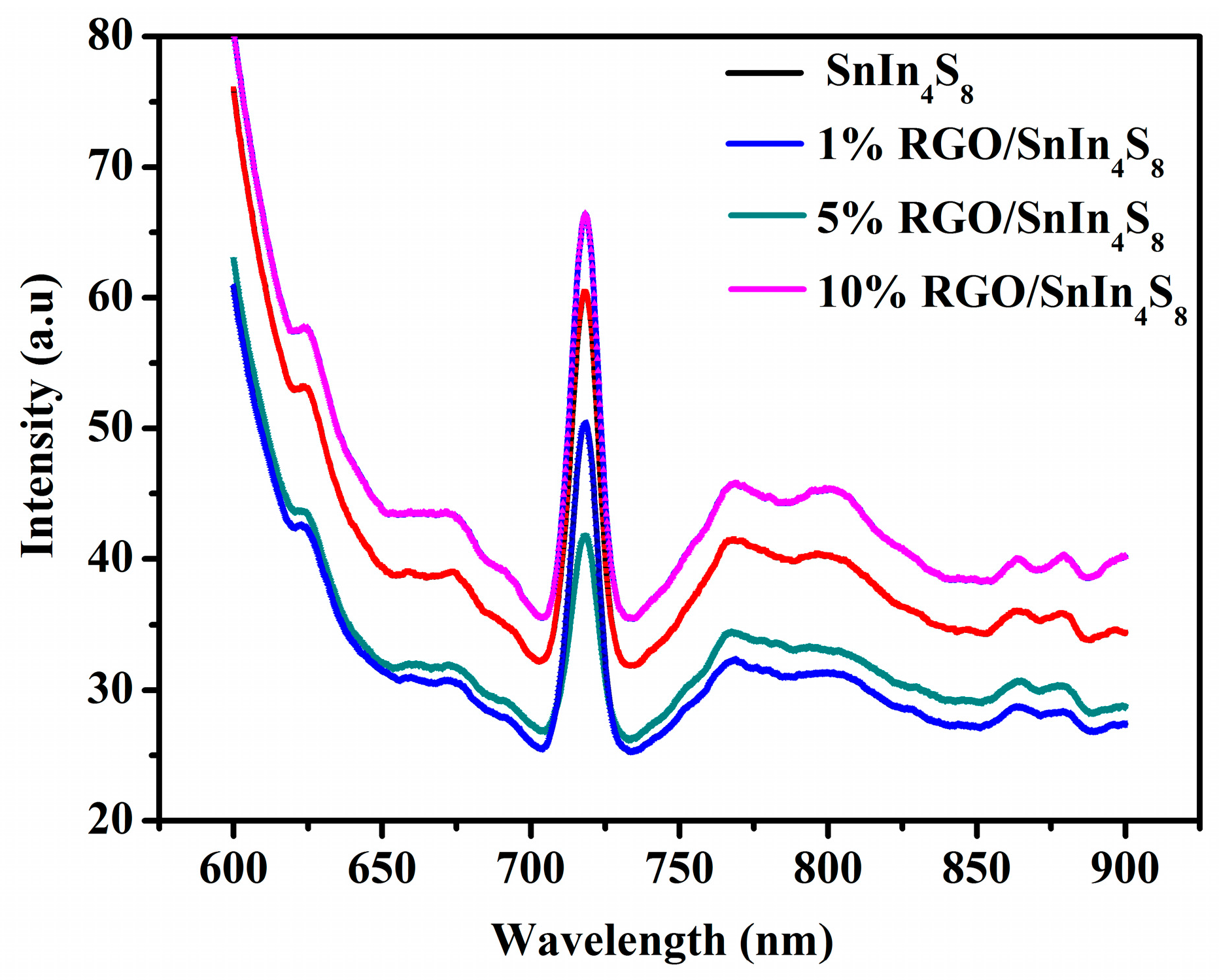
2.7. PL Emission Spectra
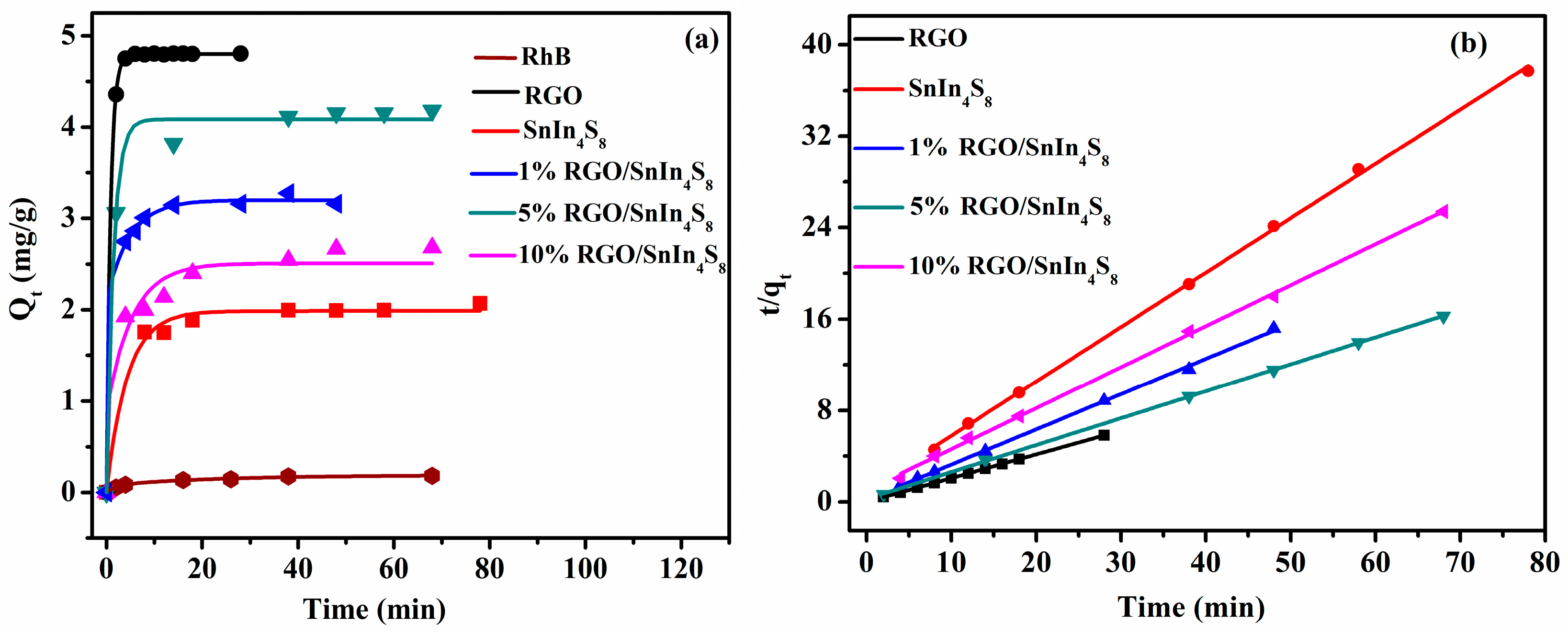
2.8. Adsorption Kinetics
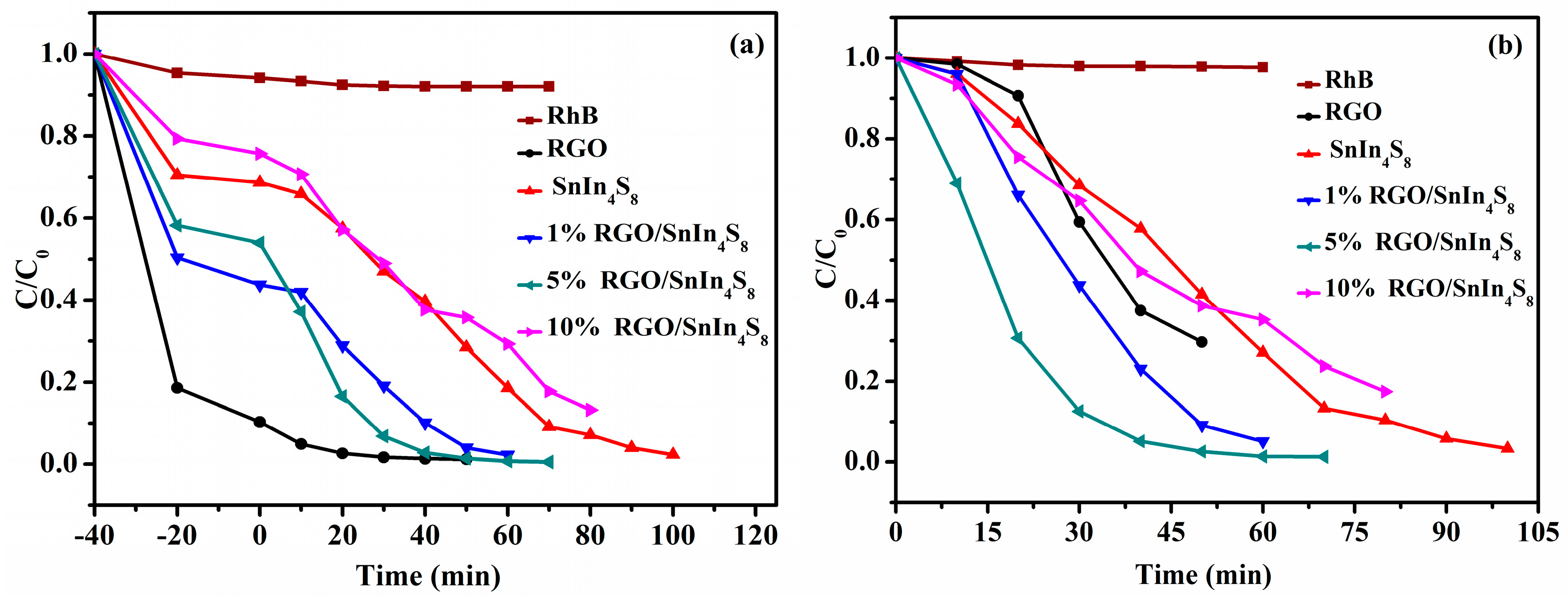
2.9. Photocatalytic Activities
2.9.1. The Temporal Change of UV-Visible Spectra during the Photocatalytic Degradation
2.9.2. Adsorption and Photocatalytic Degradation of RhB
2.9.3. Mineralization of Aqueous RhB
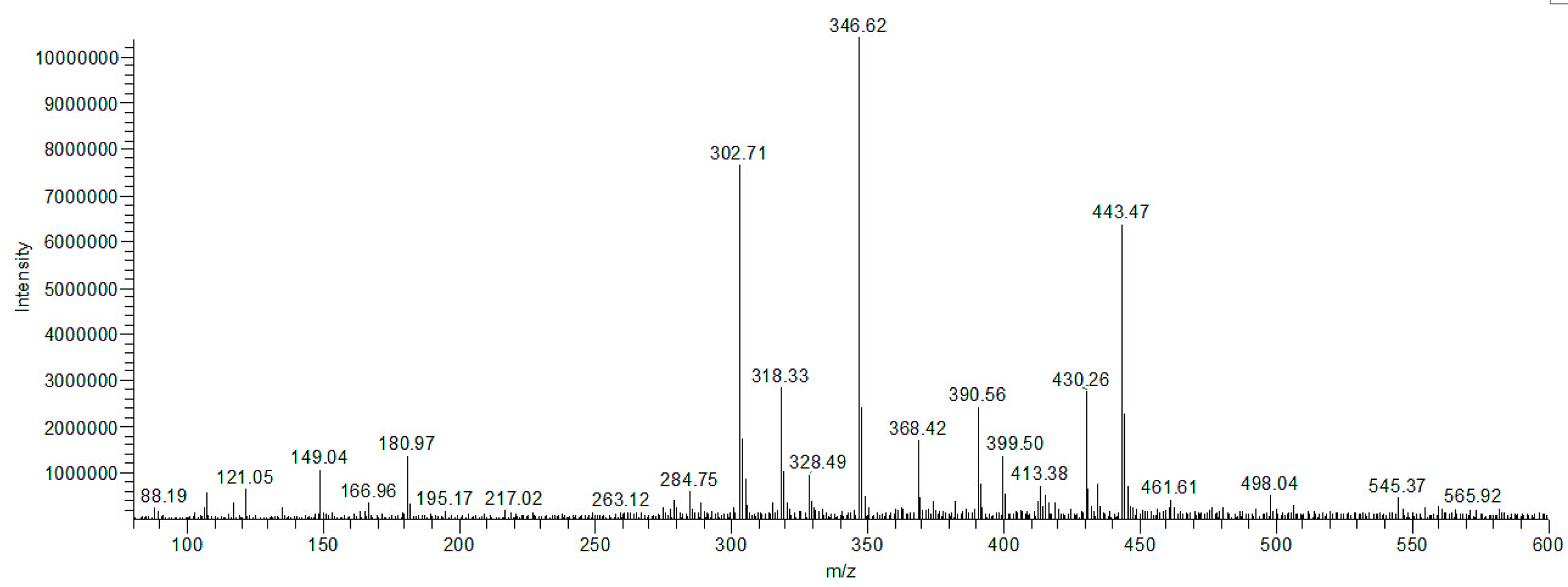
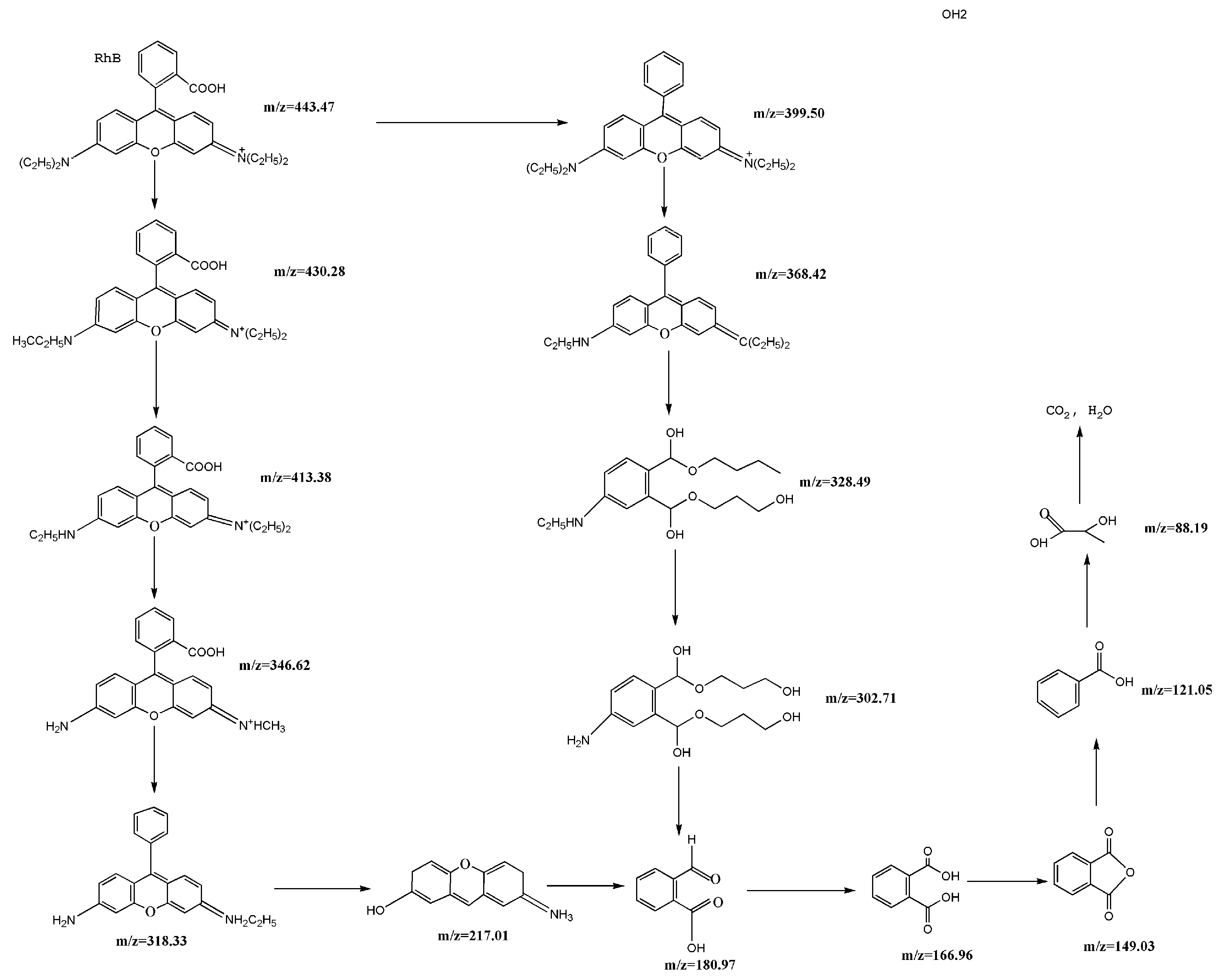
2.10. Separation and Identification of Intermediates
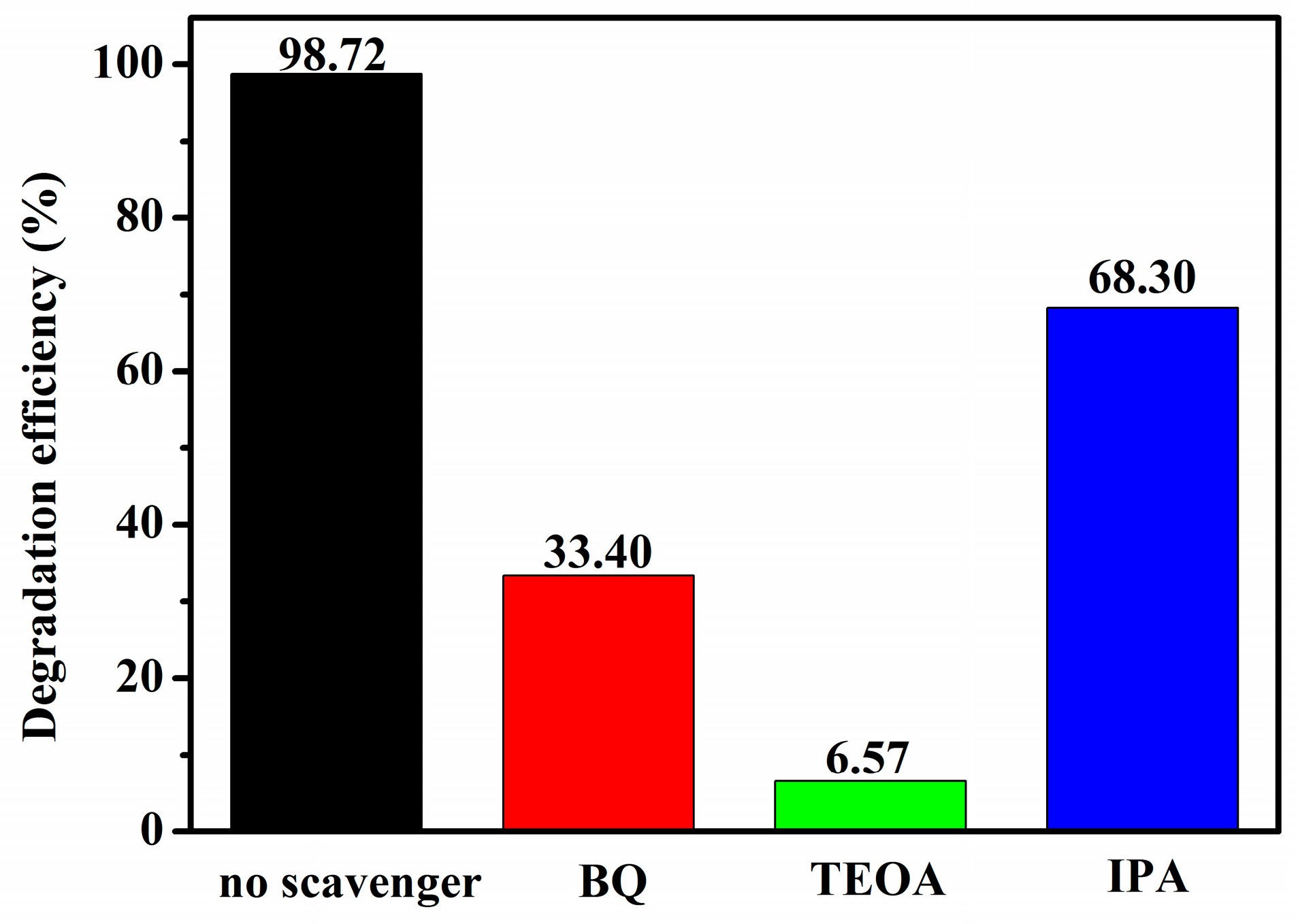
2.11. Photocatalytic Mechanism
2.12. Regeneration and Reuse of Spent RGO/SnIn4S8 Composites
3. Experimental Section
3.1. Materials and Chemicals
3.2. The Low-Temperature Co-Precipitation Synthesis of Pure SnIn4S8 and RGO/SnIn4S8 Composite
3.3. Characterization
3.4. Electrochemical Measurement
3.5. Adsorption Dynamics in the Dark
3.6. Visible-Light Photocatalytic Activity Measurement
3.7. Regeneration and Reuse of Spent RGO/SnIn4S8 Composite Catalyst
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Elimelech, M.; Phillip, W.A. The future of seawater desalination: Energy, technology, and the environment. Science 2011, 333, 712–717. [Google Scholar] [CrossRef] [PubMed]
- Chao, J.; Xie, Z.; Duan, X.B.; Dong, Y.; Wang, Z.; Xu, J.; Liang, B.; Shan, B.; Ye, J.; Chen, D.; et al. Visible-light-driven photocatalytic and photoelectrochemical properties of porous SnSx(x = 1,2) architectures. CrystEngComm. 2012, 14, 3163–3168. [Google Scholar] [CrossRef]
- Elfghi, F.M.; Zaidi, S.A.; Abdullah, E.C.; Ali, K.M. Applications of graphene and its derivatives as an adsorbent for heavy metal and dye removal: A systematic and comprehensive overview. RSC Adv. 2015, 5, 50392–50420. [Google Scholar]
- Marahel, F.; Khan, M.A.; Marahelac, E.; Bayestiac, I.; Desalin, S. Kinetics, thermodynamics, and isotherm studies for the adsorption of BR2 dye onto avocado integument. Water Treat. 2015, 53, 826–835. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, C.; Lin, P.; Hou, A.; Niu, Z.; Wang, J. Emergency drinking water treatment during source water pollution accidents in China: Origin analysis, framework and technologies. Environ. Sci. Technol. 2011, 45, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.; Zhang, L.L.; Ma, J.Z.; Zhao, X.S. Photocatalytic degration of dyes over graphene-gold nanocomposites under visible light irradiation. Chem. Commun. 2010, 46, 6099–6101. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Xing, Y.; Fan, W.; Song, S.; Zhang, H. Synthesis characterization and optical property of flower-like indium tin sulfide nanostructures. Dalton Trans. 2009, 162, 1620–1623. [Google Scholar] [CrossRef] [PubMed]
- Ge, M.; Zhu, N.; Zhao, Y.; Li, J.; Liu, L. Sunlight-assisted degradation of dye pollutants in Ag3PO4 suspension. Ind. Eng.Chem. Res. 2012, 51, 5167–5173. [Google Scholar] [CrossRef]
- Wang, J.Q.; Liu, Y.H.; Chen, M.W. Excellent capability in degrading azo dyes by MgZn-based metallic glass powders. Nature 2012, 2, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Dukkancl, M.; Vinatoru, M.; Mason, T.J. The sonochemical decolourisation of textile azo dye Orange II: Effects of Fenton type reagents and UV light. Ultrason. Sonochem. 2014, 21, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Zhang, Y.; Zhang, F.; Wei, J.; Wang, C.; Du, Y.; Ye, W. Investigation of photoelectrocatalytic activity of Cu2O nanoparticles for p-nitrophenol using rotating ring-disk electrode and application for electrocatalytic determination. Electrochim. Acta 2010, 56, 953–958. [Google Scholar] [CrossRef]
- Cheng, Z.; Liao, J.; He, B.; Zhang, F.; Zhang, F.; Huang, X.; Zhou, L. One-Step fabrication of graphene oxide enhanced magnetic composite gel for highly efficient dye adsorption and catalysis. ACS Sustain. Chem. Eng. 2015, 3, 1677–1685. [Google Scholar] [CrossRef]
- Mondal, T.; Bhowmick, A.K. Synthesis and characterization of bi-functionalized graphene and expanded graphite using n-butyl lithium and their use for efficient water soluble dye adsorption. J. Mater. Chem. A 2013, 1, 8144–8153. [Google Scholar] [CrossRef]
- Zhou, L.; He, B.; Huang, J. One-step synthesis of robust amine-and vinyl-capped magnetic iron oxide nanoparticles for polymer grafting, dye adsorption, and catalysis. ACS Appl. Mater. Inter. 2013, 5, 8678–8685. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liao, H.; Tong, Z.; Wang, C. Porous Ag/polymer composite microspheres for adsorption and catalytic degradation of organic dyes in aqueous solutions. Compos. Sci. Technol. 2015, 107, 137–144. [Google Scholar] [CrossRef]
- Nishikawa, M.; Mitani, Y.; Nosaka, Y. Photocatalytic reaction mechanism of Fe(III)-grafted TiO2 studied by means of ESR spectroscopy and chemi-luminescence photometry. J. Phys. Chem. C 2012, 116, 14900–14907. [Google Scholar] [CrossRef]
- Dinh, C.T.; Yen, H.; Kleitz, F.; Do, T.O. Three-dimensional ordered assembly of thin-shell Au/TiO2 hollow nanospheres for enhanced visible-light-driven photocatalysis. Angew. Chem. Int. Ed. 2014, 53, 6618–6623. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Ma, T.; Liu, G.; Cheng, B. Enhanced photocatalytic activity of bimodal meso-porous titania powders by C60 modification. Dalton Trans. 2011, 40, 6635–6644. [Google Scholar] [CrossRef] [PubMed]
- Deng, F.; Liu, Y.; Luo, X.B.; Wu, S.L.; Luo, S.L.; Au, C.; Qi, R. Sol-hydrothermal synthesis of inorganic-framework molecularly imprinted TiO2/SiO2 nanocomposite and its preferential photocatalytic degradation towards target contaminant. J. Hazard. Mater. 2014, 278, 108–115. [Google Scholar]
- Sackmann, E.K.; Fulton, A.; Beebe, D.J. The present and future role of microfluidics in biomedical research. Nature 2014, 507, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liu, C.; Wang, W.; Cheng, B.; Yu, J. Unique photocatalytic oxidation reactivity and selectivity of TiO2-graphene nanocomposites. Nanoscale 2012, 4, 3193–3200. [Google Scholar] [PubMed]
- Yang, X.; Chen, W.; Huang, J.; Zhou, Y.; Zhu, Y.; Li, C. Rapid degradation of methylene blue in a novel heterogeneous Fe3O4@rGO@TiO2-catalyzed photo-Fenton system. Nature 2015, 5, 10632–10642. [Google Scholar] [CrossRef] [PubMed]
- Niu, M.; Cheng, D.; Cao, D. SiH/TiO2 and GeH/TiO2 heterojunctions: Promising TiO2-based photocatalysts under visible light. Nature 2014, 4, 4810–4815. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Li, L.; Li, G.; Wang, Y.; Hu, W.; Guan, X. Porous SnIn4S8 microspheres in a new polymorph that promotes dyes degradation under visible light irradiation. J. Hazard. Mater. 2011, 186, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.Q.; Wang, G.H.; Zhou, L.; Hu, W.; Song, S.Y.; Fan, W.Q.; Zhang, H.J. Cubic spinel In4SnS8: Electrical transport properties and electrochemical hydrogen storage properties. Dalton Trans. 2010, 39, 7021–7024. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, X.; Teng, W.; Zhao, Q.; Shi, Y.; Yue, R.; Chen, Y. Efficient photocatalytic reduction of aqueous Cr(VI) over flower-like SnIn4S8 microspheres under visible light illumination. J. Hazard. Mater. 2013, 244–245, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Feng, H.; Li, J. Graphene oxide: Preparation, functionalization and electrochemical applications. Chem. Rev. 2012, 112, 6027–6053. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Q.; Yu, J.; Jaroniec, M. Graphene-based semiconductor photocatalysts. Chem. Soc. Rev. 2012, 41, 782–796. [Google Scholar] [CrossRef] [PubMed]
- Tu, W.; Zhou, Y.; Zou, Z. Versatile graphene-promoting photocatalytic performance of semiconductors: Basic principles, synthesis, solar Energy Conversion, and environmental applications. Adv. Funct. Mater. 2013, 23, 4996–5008. [Google Scholar] [CrossRef]
- Ong, W.L.; Gao, M.; Ho, G.W. Hybrid organic PVDF–inorganic M–rGO–TiO2 (M = Ag, Pt) nanocomposites for multifunctional volatile organic compound sensing and photocatalytic degradation–H2 production. Nanoscale 2013, 5, 11283–11290. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Peh, C.K.N.; Ong, W.L.; Ho, G.W. Green chemistry synthesis of a nanocomposite grapheme hydrogel with three-dimensional nano-mesopores for photocatalytic H2 production. RSC Adv. 2013, 3, 13169–13177. [Google Scholar] [CrossRef]
- Liu, X.; Pan, L.; Lv, T.; Zhu, G.; Sun, Z.; Sun, C. Microwave-assisted synthesis of CdS-reduced graphene oxide composites for photocatalytic reduction of Cr(VI). Chem. Commun. 2011, 47, 11984–11986. [Google Scholar] [CrossRef] [PubMed]
- Yavari, F.; Kritzinger, C.; Gaire, C.; Song, L.; Gulapalli, H.; Tasciuc1, T.B.; Ajayan, P.M.; Koratkar, N. Tunable bandgap in graphene by the controlled adsorption of water molecules. Small 2010, 6, 2535–2538. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.Y.; Tang, Y.; Star, A. Understanding interfaces in metal–graphitic hybrid nanostructures. J. Phys. Chem. Lett. 2013, 4, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Perera, S.D.; Mariano, R.G.; Vu, K.; Nour, N.; Seitz, O.; Chabal, Y.; Balkus, K.J. Hydrothermal synthesis of graphene-TiO2 nanotube composites with enhanced photocatalytic activity. ACS Catal. 2012, 2, 949–956. [Google Scholar] [CrossRef]
- Leofanti, G.; Padovan, M.; Tozzola, G.; Venturelli, B. Surface area and pore texture of catalysts. Catal. Today 1998, 90, 207–219. [Google Scholar] [CrossRef]
- Walton, K.S.; Snurr, R.Q. Applicability of the BET method for determining surface areas of microporous metal−organic frameworks. J. Am. Chem. Soc. 2007, 129, 8552–8556. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Castelló, D.; Cazorla-Amorós, D.; Linares-Solano, A.; Shiraishi, S.; Kurihara, H.; Oya, A. Influence of pore structure and surface chemistry on electric double layer capacitance in non-aqueous electrolyte. Carbon 2003, 41, 1765–1775. [Google Scholar] [CrossRef]
- Xiang, Q.J.; Lv, K.L.; Yu, J.G. Pivotal role of fluorine in enhanced photocatalytic activity of anatase TiO2 nanosheets with dominant (001) facets for the photocatalytic degradation of acetone in air. Appl. Catal. B Environ. 2010, 96, 557–564. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, J.; Jaroniec, M.; Gong, J.R. Noble metal-free reduced graphene oxide-ZnxCd1−xS nanocomposite with enhanced solar photocatalytic H2-production performance. Nano Lett. 2012, 12, 4584–4589. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Murugan, P.; Lakshminarasimhan, N.; Kim, J.Y.; Lee, J.S.; Lee, S.H.; Choi, W. Synergic photocatalytic effects of nitrogen and niobium co-doping in TiO2 for the redox conversion of aquatic pollutants under visible light. J. Catal. 2014, 310, 91–99. [Google Scholar] [CrossRef]
- Yang, Y.H.; Chen, X.Y.; Feng, Y.; Yang, G.W. Physical mechanism of blue-shift of UV luminescence of a single pencil-like ZnO nanowire. Nano Lett. 2007, 7, 3879–3883. [Google Scholar] [CrossRef] [PubMed]
- Bu, Y.; Chen, Z.; Li, W.; Hou, B. Highly efficient photocatalytic performance of graphene−ZnO quasi-shell-core composite material. ACS Appl. Mater. Inter. 2013, 5, 12361–12368. [Google Scholar] [CrossRef] [PubMed]
- Ong, W.; Voon, S.; Tan, L.; Goh, B.; Yong, S.; Chai, S. Enhanced daylight-induced photocatalytic activity of solvent exfoliated graphene (SEG)/ZnO hybrid nanocomposites toward degradation of reactive black 5. Ind. Eng. Chem. Res. 2014, 53, 17333–17344. [Google Scholar] [CrossRef]
- Yang, L.; Sun, W.; Luo, S.; Luo, Y. White fungus-like mesoporous Bi2S3 ball/TiO2 heterojunction with high photocatalytic efficiency in purifying 2,4-dichlorophenoxyaceticacid/Cr(VI) contaminated water. Appl. Catal. B Environ. 2014, 156–157, 25–34. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Z.; Liu, S.; Xu, Y. Size effect induced activity enhancement and anti-photocorrosion of reduced graphene oxide/ZnO composites for degradation of organic dyes and reduction of Cr(VI) in water. Appl. Catal. B Environ. 2013, 140–141, 598–607. [Google Scholar] [CrossRef]
- Rajeshwar, K.; Osugi, M.E.; Chanmanee, W.; Chenthamarakshan, C.R.; Zanoni, M.V.B.; Kajitvichyanukul, P.; Krishnan-Ayer, R. Heterogeneous photocatalytic treatment of organic dyes in air and aqueous media. J. Photoch. Photobio. C Photochem. Rev. 2008, 9, 171–192. [Google Scholar] [CrossRef]
- Qu, P.; Zhao, J.C.; Zang, L.; Shen, T.; Hidaka, H.S. Enhancement of the photoinduced electron transfer from cationic dyes to colloidal TiO2 particles by addition of an anionic surfactant in acidic media. Colloids Surfaces A Physicochem. Eng. Aspects 1998, 138, 39–50. [Google Scholar] [CrossRef]
- Avouris, P. Graphene: Electronic and photonic properties and devices. Nano Lett. 2010, 10, 4285–4294. [Google Scholar] [CrossRef] [PubMed]








| Samples | SBET (m2·g−1) | Pore Volume (cm3·g−1) | Average Pore Size (nm) |
|---|---|---|---|
| RGO | 134.31 | 0.029 | 3.402 |
| SnIn4S8 | 23.35 | 0.11 | 19.067 |
| 1% RGO/SnIn4S8 | 35.02 | 0.175 | 20.015 |
| 5% RGO/SnIn4S8 | 35.29 | 0.159 | 18.094 |
| 10% RGO/SnIn4S8 | 23.48 | 0.109 | 18.628 |
| Sample | Eg (eV) | Vfb (V vs. SCE) | Vfb ≈ ECB (V vs. NHE) | EVB (V) a |
|---|---|---|---|---|
| SnIn4S8 | 2.24 | −0.614 | −0.372 | 1.868 |
| 5% RGO/SnIn4S8 | 1.99 | −0.294 | −0.052 | 1.938 |
| Sample | qexp (mg·g−1) | Pseudo-First-Order | Pseudo-Second-Order | ||||
|---|---|---|---|---|---|---|---|
| qcal (mg·g−1) | K1 (min−1) | R2 | qcal (mg·g−1) | K2 (g·mg−1·min−1) | R2 | ||
| RGO | 4.805 | 0.453 | 0.323 | 0.518 | 4.826 | 2.452 | 0.999 |
| SnIn4S8 | 2.037 | 0.719 | 0.0476 | 0.712 | 2.102 | 0.218 | 0.999 |
| 1% RGO/SnIn4S8 | 3.157 | 2.837 | 0.3945 | 0.985 | 3.244 | 0.501 | 0.998 |
| 5% RGO/SnIn4S8 | 4.181 | 1.855 | 0.0818 | 0.919 | 4.243 | 0.208 | 0.999 |
| 10% RGO/SnIn4S8 | 2.677 | 1.690 | 0.0923 | 0.883 | 2.789 | 0.124 | 0.998 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, F.; Pei, X.; Luo, Y.; Luo, X.; Dionysiou, D.D.; Wu, S.; Luo, S. Fabrication of Hierarchically Porous Reduced Graphene Oxide/SnIn4S8 Composites by a Low-Temperature Co-Precipitation Strategy and Their Excellent Visible-Light Photocatalytic Mineralization Performance. Catalysts 2016, 6, 113. https://doi.org/10.3390/catal6080113
Deng F, Pei X, Luo Y, Luo X, Dionysiou DD, Wu S, Luo S. Fabrication of Hierarchically Porous Reduced Graphene Oxide/SnIn4S8 Composites by a Low-Temperature Co-Precipitation Strategy and Their Excellent Visible-Light Photocatalytic Mineralization Performance. Catalysts. 2016; 6(8):113. https://doi.org/10.3390/catal6080113
Chicago/Turabian StyleDeng, Fang, Xule Pei, Yiting Luo, Xubiao Luo, Dionysios D. Dionysiou, Shaolin Wu, and Shenglian Luo. 2016. "Fabrication of Hierarchically Porous Reduced Graphene Oxide/SnIn4S8 Composites by a Low-Temperature Co-Precipitation Strategy and Their Excellent Visible-Light Photocatalytic Mineralization Performance" Catalysts 6, no. 8: 113. https://doi.org/10.3390/catal6080113




_Dionysiou.jpg)


