Nanocarbons with Different Dimensions as Noble-Metal-Free Co-Catalysts for Photocatalysts
Abstract
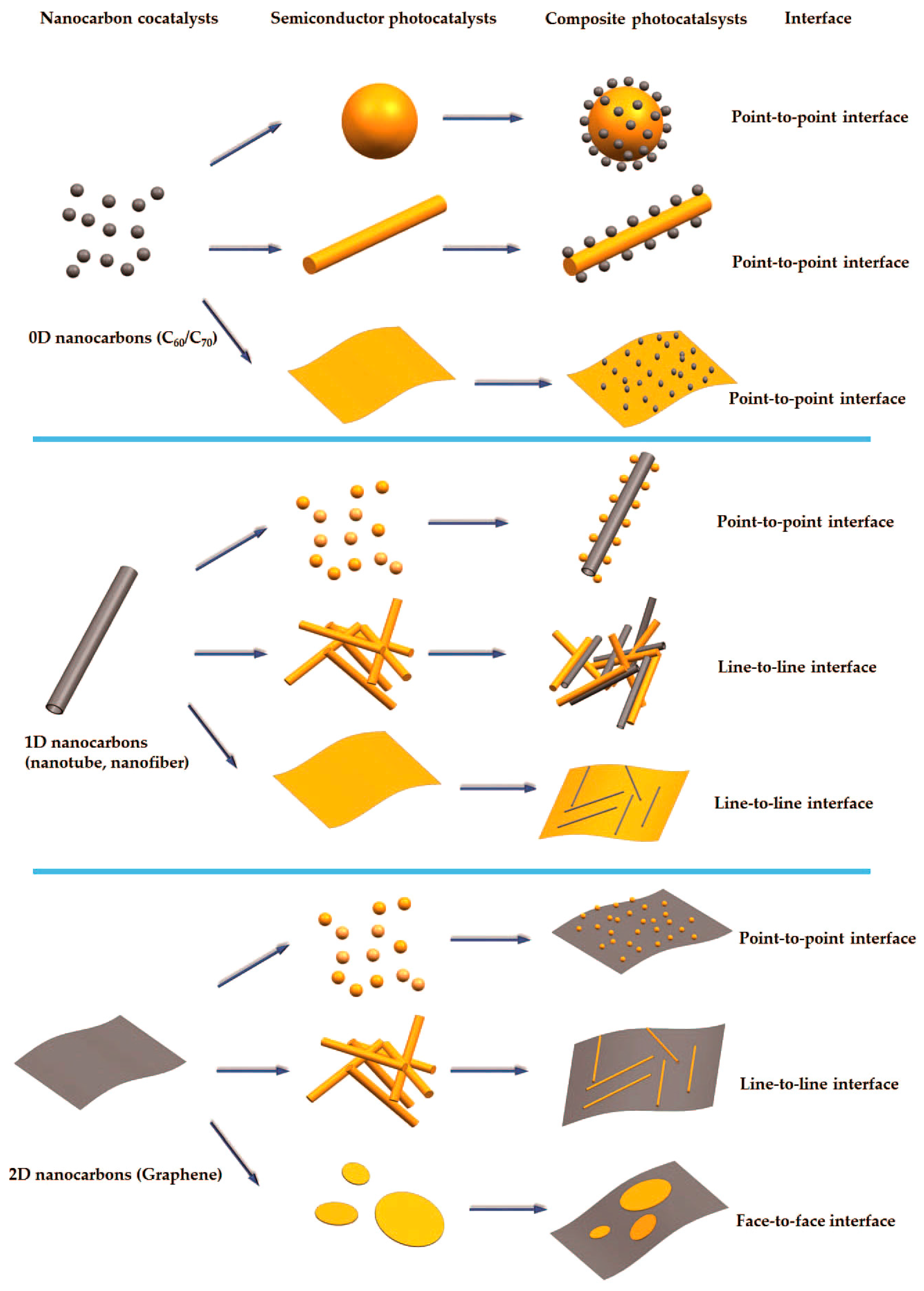
:1. Introduction
2. 0D Carbon Materials as Cocatalysts
2.1. 0D/0D
2.2. 0D/1D
2.3. 0D/2D
3. 1D Nanocarbon Materials as Co-Catalysts
3.1. 1D/0D
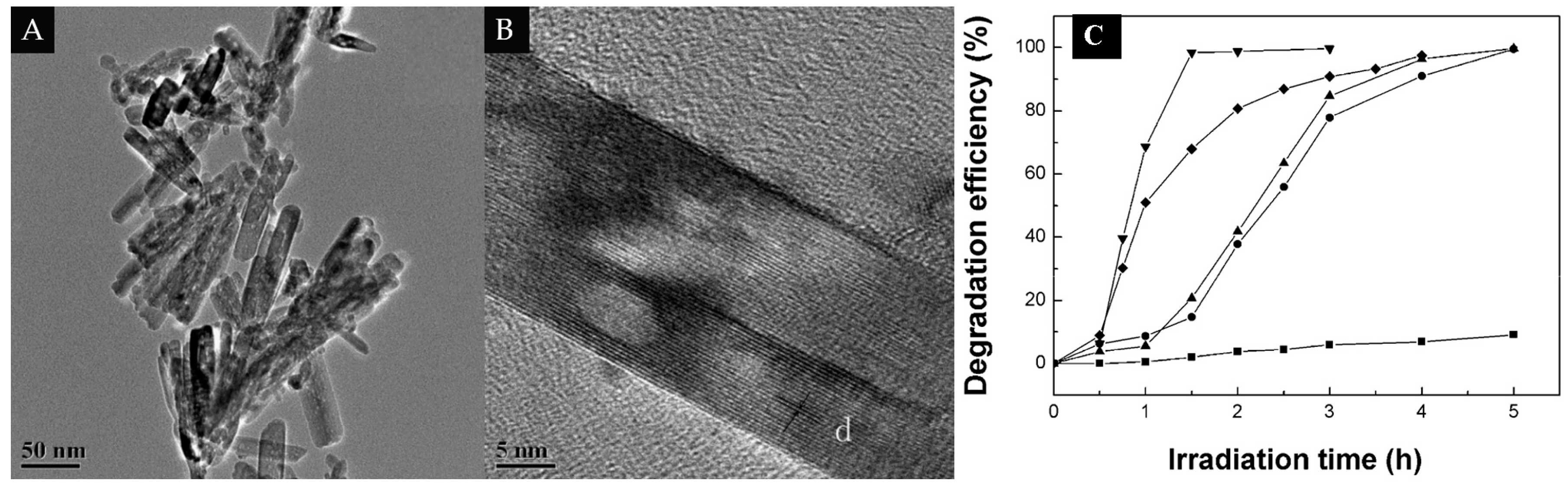
3.2. 1D/1D
3.3. 1D/2D
4. 2D Nanocarbon Materials as Co-Catalysts
4.1. 2D/0D
4.2. 2D/1D
4.3. 2D/2D
5. Summary and Outlook
Acknowledgments
Conflicts of Interest
References
- Fujishima, A. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Halmann, M. Photoelectrochemical reduction of aqueous carbon dioxide on p-type gallium phosphide in liquid junction solar cells. Nature 1978, 275, 115–116. [Google Scholar] [CrossRef]
- Ma, G.; Hisatomi, T.; Domen, K. Semiconductors for photocatalytic and photoelectrochemical solar water splitting. Chem. Soc. Rev. 2014, 43, 7520–7535. [Google Scholar]
- Li, C.; Yuan, J.; Han, B.; Jiang, L.; Shangguan, W. TiO2 nanotubes incorporated with CdS for photocatalytic hydrogen production from splitting water under visible light irradiation. Int. J. Hydrog. Energy 2010, 35, 7073–7079. [Google Scholar] [CrossRef]
- Zhuang, Z.; Peng, Q.; Li, Y. Controlled synthesis of semiconductor nanostructures in the liquid phase. Chem. Soc. Rev. 2011, 40, 5492–5513. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Geng, F.; Di, F.; Guo, L.H.; Wan, B.; Yang, Y.; Zhang, H.; Sun, G. Polyamine-functionalized carbon nanodots: a novel chemiluminescence probe for selective detection of iron(iii) ions. RSC Adv. 2014, 4, 45768–45771. [Google Scholar] [CrossRef]
- Preethi, V.; Kanmani, S. Photocatalytic hydrogen production using Fe2O3-based core shell nano particles with ZnS and CdS. Int. J. Hydrog. Energy 2014, 39, 1613–1622. [Google Scholar] [CrossRef]
- Xiang, Q.; Yu, J.; Jaroniec, M. Graphene-based semiconductor photocatalysts. Chem Soc. Rev. 2012, 41, 782–796. [Google Scholar] [CrossRef] [PubMed]
- Kołacz, K.; Gajewska, M.; Komornicki, S.; Radecka, M. The effect of GO deposition on the photoelectrochemical properties of TiO2 nanotubes. Int. J. Hydrog. Energy 2016, 41, 7538–7547. [Google Scholar] [CrossRef]
- Su, J.; Zhu, L.; Chen, G. Ultrasmall graphitic carbon nitride quantum dots decorated self-organized TiO2 nanotube arrays with highly efficient photoelectrochemical activity. Appl. Catal. Environ. 2016, 186, 127–135. [Google Scholar] [CrossRef]
- Zeng, R.; Sun, Z.; Cao, S.; Shen, R.; Liu, Z.; Xiong, Y.; Long, J.; Zheng, J.; Zhao, Y.; Shen, Y.; Wang, D. Facile synthesis of Ag-doped ZnCdS nanocrystals and transformation into Ag-doped ZnCdSSe nanocrystals with Se treatment. RSC Adv. 2015, 5, 1083–1090. [Google Scholar] [CrossRef]
- Liu, R.; Wang, P.; Wang, X.; Yu, H.; Yu, J. UV- and Visible-Light Photocatalytic Activity of Simultaneously Deposited and Doped Ag/Ag(I)-TiO2 Photocatalyst. J. Phys. Chem. 2012, 116, 17721–17728. [Google Scholar]
- Moniz, S.J.; Shevlin, S.A.; Martin, D.J.; Guo, Z.X.; Tang, J. Visible-light driven heterojunction photocatalysts for water splitting-a critical review. Energy Env. Sci. 2015, 8, 731–759. [Google Scholar] [CrossRef]
- Aazam, E.S. Photocatalytic oxidation of cyanide under visible light by Pt doped AgInS2 nanoparticles. J. Ind. Eng. Chem. 2014, 20, 4008–4013. [Google Scholar] [CrossRef]
- Ampelli, C.; Perathoner, S.; Centi, G. Carbon-based catalysts: opening new scenario to develop next-generation nano-engineered catalytic materials. Chin. J. Catal. 2014, 35, 783–791. [Google Scholar] [CrossRef]
- Zhang, F.; Li, C. Semiconductor-Based Photocatalytic Water Splitting. In Solar to Chemical Energy Conversion; Masakazu, S., Katsushi, F., Shinichiro, N., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 299–317. [Google Scholar]
- Tan, L.L.; Chai, S.P.; Mohamed, A.R. Synthesis and applications of graphene-based TiO2 photocatalysts. ChemSusChem. 2012, 5, 1868–1882. [Google Scholar] [CrossRef] [PubMed]
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Graphene-based composite materials. Nature 2006, 442, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Haddon, R. Chemistry of the fullerenes: The manifestation of strain in a class of continuous aromatic molecules. Science 1993, 261, 1545–1550. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.D.; Zhu, L.; Choi, J.G.; Chen, M.L.; Oh, W.C. Effect of Pt treated fullerene/TiO2 on the photocatalytic degradation of MO under visible light. J. Mater. Chem. 2011, 21, 7596. [Google Scholar] [CrossRef]
- Virovska, D.; Paneva, D.; Manolova, N.; Rashkov, I.; Karashanova, D. Photocatalytic self-cleaning poly (l-lactide) materials based on a hybrid between nanosized zinc oxide and expanded graphite or fullerene. Mater. Sci. Eng. C 2016, 60, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Wakimoto, R.; Kitamura, T.; Ito, F.; Usami, H.; Moriwaki, H. Decomposition of methyl orange using C60 fullerene adsorbed on silica gel as a photocatalyst via visible-light induced electron transfer. Appl. Catal. Environ. 2015, 166, 544–550. [Google Scholar] [CrossRef]
- Cho, E.C.; Ciou, J.H.; Zheng, J.H.; Pan, J.; Hsiao, Y.S.; Lee, K.C.; Huang, J.H. Fullerene C70 decorated TiO2 nanowires for visible-light-responsive photocatalyst. Appl. Surf. Sci. 2015, 355, 536–546. [Google Scholar] [CrossRef]
- Kim, K.H.; Ko, J.W.; Ko, W.B. Preparation and kinetics of nanocomposites using WO3 with carbon nanomaterials for photocatalytic degradation of organic dyes. Asian J. Chem. 2016, 28, 194. [Google Scholar] [CrossRef]
- Qi, K.; Selvaraj, R.; Al Fahdi, T.; Al-Kindy, S.; Kim, Y.; Wang, G.C.; Tai, C.W.; Sillanpää, M. Enhanced photocatalytic activity of anatase-TiO2 nanoparticles by fullerene modification: A theoretical and experimental study. Appl. Surf. Sci. 2016, 22, 1498–1504. [Google Scholar]
- Zhang, X.; Wang, Q.; Zou, L.H.; You, J.-W. Facile fabrication of titanium dioxide/fullerene nanocomposite and its enhanced visible photocatalytic activity. J. Colloid Interface Sci. 2016, 466, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Liu, E.; Kang, L.; Yang, Y.; Sun, T.; Hu, X.; Zhu, C.; Liu, H.; Wang, Q.; Li, X.; Fan, J. Plasmonic Ag deposited TiO2 nano-sheet film for enhanced photocatalytic hydrogen production by water splitting. Nanotechnology 2014, 25, 165401. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yan, J.; Wang, T.; Zhao, Z.J.; Zhang, J.; Gong, J.; Guan, N. Sub-10 nm rutile titanium dioxide nanoparticles for efficient visible-light-driven photocatalytic hydrogen production. Nat. Commun. 2015, 6, 5881. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Hsueh, Y.C.; Su, C.Y.; Kei, C.C.; Perng, T.P. Deposition of uniform Pt nanoparticles with controllable size on TiO2-based nanowires by atomic layer deposition and their photocatalytic properties. Nanotechnology 2015, 26, 254002. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Pan, L.; Song, J.J.; Mi, W.; Zou, J.J.; Wang, L.; Zhang, X. Titanium-defected undoped anatase TiO2 with p-type conductivity, room-temperature ferromagnetism, and remarkable photocatalytic performance. J. Am. Chem. Soc. 2015, 137, 2975–2983. [Google Scholar] [CrossRef] [PubMed]
- Oh, W.C.; Jung, A.R.; Ko, W.-B. Preparation of fullerene/TiO2 composite and its photocatalytic effect. J. Ind. Eng. Chem. 2007, 13, 1208–1214. [Google Scholar]
- Huang, X.; Li, Z.; Wang, S.; Chi, D.; Chua, S.J. Solution-grown ZnO films towards transparent and smart dual-color light-emitting diode. ACS Appl. Mater. Interfaces 2016, 8, 15482–15488. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Zhang, H.; Lu, S.; Li, X.; Xu, M.; Zhang, Y. High performance indium-doped ZnO gas sensor. J. Nanomater. 2015, 2015, 74. [Google Scholar] [CrossRef]
- Ghosh, M.; Ghosh, S.; Seibt, M.; Rao, K.Y.; Peretzki, P.; Rao, G.M. Ferroelectric origin in one-dimensional undoped ZnO towards high electromechanical response. Crystengcomm 2015, 18, 622–630. [Google Scholar] [CrossRef]
- Fu, H.; Xu, T.; Zhu, S.; Zhu, Y. Photocorrosion Inhibition and Enhancement of Photocatalytic Activity for ZnO via Hybridization with C60. Environ. Sci. Technol. 2008, 42, 8064–8069. [Google Scholar] [CrossRef]
- Song, T.; Huo, J.; Liao, T.; Zeng, J.; Qin, J.; Zeng, H. Fullerene [C60] modified Cr2−xFexO3 nanocomposites for enhanced photocatalytic activity under visible light irradiation. Chem. Eng. J. 2016, 287, 359–366. [Google Scholar] [CrossRef]
- Scuseria, G.E. The equilibrium structure of C70. An ab initio Hartree-Fock study. Chem. Phys. Lett. 1991, 180, 451–456. [Google Scholar] [CrossRef]
- Wang, S.; Liu, C.; Dai, K.; Cai, P.; Chen, H.; Yang, C.; Huang, Q. Fullerene C70-TiO2 hybrids with enhanced photocatalytic activity under visible light irradiation. J. Mater. Chem. A 2015, 3, 21090–21098. [Google Scholar] [CrossRef]
- Long, Y.; Lu, Y.; Huang, Y.; Peng, Y.; Lu, Y.; Kang, S.Z.; Mu, J. Effect of C60 on the photocatalytic activity of TiO2 nanorods. J. Phys. Chem. C 2009, 113, 13899–13905. [Google Scholar] [CrossRef]
- Grandcolas, M.; Ye, J.; Miyazawa, K. Titania nanotubes and fullerenes C60 assemblies and their photocatalytic activity under visible light. Ceram. Int. 2014, 40, 1297–1302. [Google Scholar] [CrossRef]
- Li, G.; Jiang, B.; Li, X.; Lian, Z.; Xiao, S.; Zhu, J.; Zhang, D.; Li, H. C60/Bi2TiO4F2 heterojunction photocatalysts with enhanced visible-light activity for environmental remediation. ACS Appl. Mater. Interfaces 2013, 5, 7190–7197. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Zhao, Q.; Zhu, Z.; Hu, Y.; Tao, Z.; Chen, J. The enhanced hydrogen storage of micro-nanostructured hybrids of Mg(BH4)2-carbon nanotubes. Nanoscale 2015, 7, 18305–18311. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Lai, G.; Zhang, H.; Yu, A. One-pot synthesis of multipod ZnO-carbon nanotube-reduced graphene oxide composites with high performance in photocatalysis. J. Nanosci. Nanotechnol. 2015, 15, 4325–4331. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Xie, F.; Xu, H.; Liu, J.; Oh, W.C. Characterization of Pd/TiO2 embedded in multi-walled carbon nanotube catalyst with a high photocatalytic activity. Kinet. Catal. 2013, 54, 297–306. [Google Scholar] [CrossRef]
- Koziol, K.; Vilatela, J.; Moisala, A.; Motta, M.; Cunniff, P.; Sennett, M.; Alan, W. High-performance carbon nanotube fiber. Science 2007, 318, 1892–1895. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.G.; Zhang, Q.; Yang, D.J.; Sellin, P.J.; Zhong, G.F. Multi-walled carbon nanotube-based gas sensors for NH3 detection. Diamond Related Mater. 2004, 13, 1327–1332. [Google Scholar] [CrossRef]
- Filleter, T.; Espinosa, H.D. Multi-scale mechanical improvement produced in carbon nanotube fibers by irradiation cross-linking. Carbon 2013, 56, 1–11. [Google Scholar] [CrossRef]
- Zhang, L.L.; Zhao, X. Carbon-based materials as supercapacitor electrodes. Chem. Soc. Rev. 2009, 38, 2520–2531. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Wang, L.; Wang, P.; Kostka, A.; Wark, M.; Muhler, M.; Beranek, R. CNT-TiO2-δ composites for improved co-catalyst dispersion and stabilized photocatalytic hydrogen production. Catalysts 2015, 5, 270–285. [Google Scholar] [CrossRef]
- Woan, K.; Pyrgiotakis, G.; Sigmund, W. Photocatalytic carbon-nanotube-TiO2 composites. Adv. Mater. 2009, 21, 2233–2239. [Google Scholar] [CrossRef]
- Peng, T.; Zeng, P.; Ke, D.; Liu, X.; Zhang, X. Hydrothermal preparation of multiwalled carbon nanotubes (MWCNTs)/CdS nanocomposite and its efficient photocatalytic hydrogen production under visible light irradiation. Energy Fuels 2011, 25, 2203–2210. [Google Scholar] [CrossRef]
- Wang, X.; Yao, S.; Li, X. Sol-gel Preparation of CNT/ZnO nanocomposite and its photocatalytic property. Chin. J. Chem. 2009, 27, 1317–1320. [Google Scholar] [CrossRef]
- Xia, X.H.; Jia, Z.J.; Yu, Y.; Liang, Y.; Wang, Z.; Ma, L.L. Preparation of multi-walled carbon nanotube supported TiO2 and its photocatalytic activity in the reduction of CO2 with H2O. Carbon 2007, 45, 717–721. [Google Scholar] [CrossRef]
- Tanaka, A.; Sakaguchi, S.; Hashimoto, K.; Kominami, H. Preparation of Au/TiO2 with Metal Cocatalysts Exhibiting Strong Surface Plasmon Resonance Effective for Photoinduced Hydrogen Formation under Irradiation of Visible Light. ACS Catal. 2013, 3, 79–85. [Google Scholar] [CrossRef]
- Seery, M.K.; George, R.; Floris, P.; Pillai, S.C. Silver doped titanium dioxide nanomaterials for enhanced visible light photocatalysis. J. Photochem. Photobiol. Chem. 2007, 189, 258–263. [Google Scholar] [CrossRef]
- Wang, X.; Ling, D.; Wang, Y.; Long, H.; Sun, Y.; Shi, Y.; Chen, Y.; Jing, Y.; Sun, Y.; Dai, Y. N-doped graphene quantum dots-functionalized titanium dioxide nanofibers and their highly efficient photocurrent response. J. Mater. Res. 2014, 29, 1408–1416. [Google Scholar] [CrossRef]
- Yu, K.; Song, M.; Gao, X.; Hou, C.; Liang, J. Preparation and Photocatalytic Property of Nickel-Doped Titanium Dioxide Nanotubes. Synth. React. Inorg. Metal.-Org. Nano-Metal. Chem. 2014, 45, 1576–1579. [Google Scholar] [CrossRef]
- Venditti, F.; Cuomo, F.; Ceglie, A.; Avino, P.; Russo, M.V.; Lopez, F. Visible light caffeic acid degradation by carbon-doped titanium dioxide. Langmuir 2015, 31, 3627–3634. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.J.; Oh, W.C. Visible light photocatalytic properties of novel molybdenum treated carbon nanotube/titania composites. Bull. Mater. Sci. 2011, 34, 543–549. [Google Scholar] [CrossRef]
- Liu, B.; Xu, Y.; Cui, J.; Wang, S.; Wang, T. Carbon nanotubes-dispersed TiO2, nanoparticles with their enhanced photocatalytic activity. Mater. Res. Bull. 2014, 59, 278–282. [Google Scholar] [CrossRef]
- Natarajan, T.S.; Lee, J.Y.; Bajaj, H.C.; Jo, W.K.; Tayade, R.J. Synthesis of multiwall carbon nanotubes/TiO2 nanotube composites with enhanced photocatalytic decomposition efficiency. Catal. Today 2016. [Google Scholar] [CrossRef]
- Chen, Y.; Tian, G.; Ren, Z.; Pan, K.; Shi, Y.; Wang, J.; Fu, H. Hierarchical core-shell carbon nanofiber@ZnIn2S4 composites for enhanced hydrogen evolution performance. ACS Appl. Mater. Interfaces 2014, 6, 13841–13849. [Google Scholar] [CrossRef] [PubMed]
- Bonaccorso, F.; Colombo, L.; Yu, G.; Stoller, M.; Tozzini, V.; Ferrari, A.C.; Ruoff, R.S.; Pellegrini, V. Graphene, related two-dimensional crystals, and hybrid systems for energy conversion and storage. Science 2015, 347, 1246501. [Google Scholar] [CrossRef] [PubMed]
- Perreault, F.; de Faria, A.F.; Elimelech, M. Environmental applications of graphene-based nanomaterials. Chem. Soc. Rev. 2015, 44, 5861–5896. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Q.; Lang, D.; Shen, T.; Liu, F. Graphene-modified nanosized Ag3PO4 photocatalysts for enhanced visible-light photocatalytic activity and stability. Appl. Catal. B Environ. 2015, 162, 196–203. [Google Scholar] [CrossRef]
- Liu, J.; Xue, Y.; Zhang, M.; Dai, L. Graphene-based materials for energy applications. MRS Bull. 2012, 37, 1265–1272. [Google Scholar] [CrossRef]
- Huang, X.; Qi, X.; Boey, F.; Zhang, H. Graphene-based composites. Chem. Soc. Rev. 2012, 41, 666–686. [Google Scholar] [CrossRef] [PubMed]
- An, X.; Yu, J.C. Graphene-based photocatalytic composites. RSC Adv. 2011, 1, 1426. [Google Scholar] [CrossRef]
- Iwashina, K.; Iwase, A.; Ng, Y.H.; Amal, R.; Kudo, A. Z-schematic water splitting into H2 and O2 using metal sulfide as a hydrogen-evolving photocatalyst and reduced graphene oxide as a solid-state electron mediator. J. Am. Chem. Soc. 2015, 137, 604–607. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Li, G.; Wang, X. Surface Modification of Carbon Nitride Polymers by Core-Shell Nickel/Nickel Oxide Cocatalysts for Hydrogen Evolution Photocatalysis. ChemCatChem 2015, 7, 2864–2870. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, S.; Yang, M.Q.; Xu, Y.J. Synthesis of uniform CdS nanospheres/graphene hybrid nanocomposites and their application as visible light photocatalyst for selective reduction of nitro organics in water. ACS Appl. Mater. Interfaces 2013, 5, 4309–4319. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Zhang, Y.; Pan, X.; Fu, X.; Liu, S.; Xu, Y.J. Assembly of CdS nanoparticles on the two-dimensional graphene scaffold as visible-light-driven photocatalyst for selective organic transformation under ambient conditions. J. Phys. Chem. C 2011, 115, 23501–23511. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, N.; Tang, Z.R.; Xu, Y.J. Improving the photocatalytic performance of graphene-TiO2 nanocomposites via a combined strategy of decreasing defects of graphene and increasing interfacial contact. Phys. Chem. Chem. Phys. 2012, 14, 9167–9175. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Li, H.P.; Cui, X.L.; Lin, Y. Graphene/TiO2 nanocomposites: Synthesis, characterization and application in hydrogen evolution from water photocatalytic splitting. J. Mater. Chem. 2010, 20, 2801. [Google Scholar] [CrossRef]
- Peng, T.; Li, K.; Zeng, P.; Zhang, Q.; Zhang, X. Enhanced photocatalytic hydrogen production over graphene oxide–cadmium sulfide nanocomposite under visible light irradiation. J. Phys. Chem. C 2012, 116, 22720–22726. [Google Scholar] [CrossRef]
- Zhu, C.; Guo, S.; Wang, P.; Xing, L.; Fang, Y.; Zhai, Y.; Dong, S. One-pot, water-phase approach to high-quality graphene/TiO2 composite nanosheets. Chem. Commun. 2010, 46, 7148–7150. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.X.; Yang, H.B.; Sheng, Z.M.; Lu, Z.S.; Song, Q.L.; Li, C.M. Layered graphene/quantum dots for photovoltaic devices. Angew. Chem. Int. Ed. 2010, 49, 3014–3017. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Guo, B.; Yu, J.; Ran, J.; Zhang, B.; Yan, H.; Gong, J.R. Highly efficient visible-light-driven photocatalytic hydrogen production of CdS-cluster-decorated graphene nanosheets. J. Am. Chem. Soc. 2011, 133, 10878–10884. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Wang, D.H.; Huang, Y.X.; Xu, A.W.; Yu, H.Q. Highly durable N-doped graphene/CdS nanocomposites with enhanced photocatalytic hydrogen evolution from water under visible light irradiation. J. Phys. Chem. C 2011, 115, 11466–11473. [Google Scholar] [CrossRef]
- Soin, N.; Sinha Roy, S.; Roy, S.; Hazra, K.S.; Misra, D.S.; Lim, T.H.; Hetherington, C.J.; McLaughlin, J.A. Enhanced and stable field emission from in situ nitrogen-doped few-layered graphene nanoflakes. J. Phys. Chem. C 2011, 115, 5366–5372. [Google Scholar] [CrossRef]
- Mou, Z.; Wu, Y.; Sun, J.; Yang, P.; Du, Y.; Lu, C. TiO2 nanoparticles-functionalized N-doped graphene with superior interfacial contact and enhanced charge separation for photocatalytic hydrogen generation. ACS Appl. Mater. Interfaces 2014, 6, 13798–13806. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Cao, C.; Jiang, J.Z. Light non-metallic atom (B, N, O and F)-doped graphene: A first-principles study. Nanotechnology 2010, 21, 505202. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Mo, Y.; Tian, J.; Wang, P.; Yu, H.; Yu, J. The synergistic effect of graphitic N and pyrrolic N for the enhanced photocatalytic performance of nitrogen-doped graphene/TiO2 nanocomposites. Appl. Catal. B Environ. 2016, 181, 810–817. [Google Scholar] [CrossRef]
- Yan, J.; Ye, Q.; Wang, X.; Yu, B.; Zhou, F. CdS/CdSe quantum dot co-sensitized graphene nanocomposites via polymer brush templated synthesis for potential photovoltaic applications. Nanoscale 2012, 4, 2109–2116. [Google Scholar] [CrossRef] [PubMed]
- Park, W.I.; Lee, C.H.; Lee, J.M.; Kim, N.-J.; Yi, G.-C. Inorganic nanostructures grown on graphene layers. Nanoscale 2011, 3, 3522–3533. [Google Scholar] [CrossRef] [PubMed]
- Manga, K.K.; Wang, J.; Lin, M.; Zhang, J.; Nesladek, M.; Nalla, V.; Ji, W.; Loh, K.P. High-performance broadband photodetector using solution-processible PbSe-TiO2-graphene hybrids. Adv. Mater. 2012, 24, 1697–1702. [Google Scholar] [CrossRef] [PubMed]
- Kamegawa, T.; Yamahana, D.; Yamashita, H. Graphene coating of TiO2 nanoparticles loaded on mesoporous silica for enhancement of photocatalytic activity. J. Phys. Chem. C 2010, 114, 15049–15053. [Google Scholar] [CrossRef]
- Xiang, Q.; Yu, J.; Jaroniec, M. Synergetic effect of MoS2 and graphene as cocatalysts for enhanced photocatalytic H2 production activity of TiO2 nanoparticles. J. Am. Chem. Soc. 2012, 134, 6575–6578. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Wang, L.; Chen, X.; Du, J.; Xiong, Y. Chemically exfoliated metallic MoS2 nanosheets: A promising supporting co-catalyst for enhancing the photocatalytic performance of TiO2 nanocrystals. Nano Res. 2015, 8, 175–183. [Google Scholar] [CrossRef]
- Lang, D.; Shen, T.; Xiang, Q. Roles of MoS2 and graphene as cocatalysts in the enhanced visible-light photocatalytic H2 production activity of multiarmed CdS nanorods. ChemCatChem 2015, 7, 943–951. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, Y.; Pan, X.; Yang, M.Q.; Xu, Y.J. Constructing ternary CdS-graphene-TiO2 hybrids on the flatland of graphene oxide with enhanced visible-light photoactivity for selective transformation. J. Phys. Chem. C 2012, 116, 18023–18031. [Google Scholar] [CrossRef]
- Xiao, F.X.; Liu, B. 1D TiO2, Nanotube-Based Photocatalysts. Heterogeneous Photocatalysis. In Heterogeneous PhotoCatal; Colmenares, J.C., Xu, Y.J., Eds.; Springer: Berlin, Germany; Heidelberg, Germany, 2016; pp. 151–173. [Google Scholar]
- Tien, H.N.; Hur, S.H. Fabrication of 3D structured ZnO nanorod/reduced graphene oxide hydrogels and their use for photo-enhanced organic dye removal. J. Colloid Interface Sci. 2015, 437, 181–186. [Google Scholar]
- Agegnehu, A.K.; Pan, C.-J.; Tsai, M.-C.; Rick, J.; Su, W.-N.; Lee, J.-F.; Hwang, B.J. Visible light responsive noble metal-free nanocomposite of V-doped TiO2 nanorod with highly reduced graphene oxide for enhanced solar H2 production. Int. J. Hydrog. Energy 2016, 41, 6752–6762. [Google Scholar] [CrossRef]
- Utterback, J.K.; Wilker, M.B.; Brown, K.A.; King, P.W.; Eaves, J.D.; Dukovic, G. Competition between electron transfer, trapping, and recombination in CdS nanorod-hydrogenase complexes. Phys. Chem. Chem. Phys. 2015, 17, 5538–5542. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, L.; Liu, X.; Li, X.A.; Huang, W. High-performance CdS-ZnS core-shell nanorod array photoelectrode for photoelectrochemical hydrogen generation. J. Mater. Chem. A 2015, 3, 535–541. [Google Scholar] [CrossRef]
- Liu, S.; Chen, Z.; Zhang, N.; Tang, Z.-R.; Xu, Y.J. An efficient self-assembly of CdS nanowires-reduced graphene oxide nanocomposites for selective reduction of nitro organics under visible light irradiation. J. Phys. Chem. C 2013, 117, 8251–8261. [Google Scholar] [CrossRef]
- Yu, J.; Jin, J.; Cheng, B.; Jaroniec, M. A noble metal-free reduced graphene oxide-CdS nanorod composite for the enhanced visible-light photocatalytic reduction of CO2 to solar fuel. J. Mater. Chem. A 2014, 2, 3407. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, X. One step synthesis and characterization of CdS nanorod/graphene nanosheet composite. Appl. Surf. Sci. 2011, 257, 10379–10383. [Google Scholar] [CrossRef]
- Cao, M.; Wang, P.; Ao, Y.; Wang, C.; Hou, J.; Qian, J. Investigation on graphene and Pt co-modified CdS nanowires with enhanced photocatalytic hydrogen evolution activity under visible light irradiation. Dalton Trans. 2015, 44, 1507–1508. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Ma, A.; Tang, Z.; Han, Z.; Wang, M.; Wang, Z.; Zhi, L.; Yang, J. Facile synthesis of Zn0.5Cd0.5S ultrathin nanorods on reduced graphene oxide for enhanced photocatalytic hydrogen evolution under visible light. ChemCatChem 2015, 7, 609–615. [Google Scholar] [CrossRef]
- Liu, J.; Bai, H.; Wang, Y.; Liu, Z.; Zhang, X.; Sun, D.D. Self-assembling TiO2, nanorods on large graphene oxide sheets at a two-phase interface and their anti-recombination in photocatalytic applications. Adv. Funct. Mater. 2010, 20, 4175–4181. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, L.; Liu, R.; Gao, Z.; Yang, X.; Tu, Z.; Yang, F.; Ye, Z.; Cui, L.; Xu, C.; Li, Y. Hydrothermal synthesis of N-doped TiO2 nanowires and N-doped graphene heterostructures with enhanced photocatalytic properties. J. Alloys Compd. 2016, 656, 24–32. [Google Scholar] [CrossRef]
- Nurlaela, E.; Ouldchikh, S.; Llorens, I.; Hazemann, J.L.; Takanabe, K. Establishing efficient cobalt-based catalytic sites for oxygen evolution on a Ta3N5 photocatalyst. Chem. Mater. 2015, 27, 5685–5694. [Google Scholar] [CrossRef]
- Akimov, A.V.; Muckerman, J.T.; Prezhdo, O.V. Nonadiabatic dynamics of positive charge during photocatalytic water splitting on GaN (10-10) surface: charge localization governs splitting efficiency. J. Am. Chem. Soc. 2013, 135, 8682–8691. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Teramura, K.; Saito, N.; Inoue, Y.; Domen, K. Photocatalytic overall water splitting on gallium nitride powder. Bull. Chem. Soc. Jpn. 2007, 80, 1004–1010. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, L.; Geng, F.; Guo, L.H.; Wan, B.; Yang, Y. Carbon dots decorated graphitic carbon nitride as an efficient metal-free photocatalyst for phenol degradation. Appl. Catal. B Environ. 2016, 180, 656–662. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Dong, G.; Zhao, K.; Zhang, L. Carbon self-doping induced high electronic conductivity and photoreactivity of g-C3N4. Chem. Commun. 2012, 48, 6178–6180. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Low, J.; Yu, J.; Jaroniec, M. Polymeric photocatalysts based on graphitic carbon nitride. Adv. Mater. 2015, 27, 2150–2176. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhou, F.; Zhan, S.; Yang, Y.; Yin, Y. Significantly enhanced performance of g-C3N4/Bi2MoO6 films for photocatalytic degradation of pollutants under visible-light irradiation. Chem. Res. Chin. Univ. 2016, 32, 284–290. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, B.; Lin, S.; Zhang, Y.; Wang, X. Activation of n→π* transitions in two-dimensional conjugated polymers for visible light photocatalysis. J. Phys. Chem. C 2014, 118, 29981–29989. [Google Scholar] [CrossRef]
- Akhundi, A.; Habibi-Yangjeh, A. A simple large-scale method for preparation of g-C3N4/SnO2 nanocomposite as visible-light-driven photocatalyst for degradation of an organic pollutant. Mater. Express 2015, 5, 309–318. [Google Scholar] [CrossRef]
- Chai, B.; Wang, X. Sonochemical Synthesis of CdS/C3N4 Composites with Efficient Photocatalytic Performance Under Visible Light Irradiat. J. Nanosci. Nanotechnol. 2016, 16, 2032–2041. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Wang, Z.; Yue, D.; Yang, G.; Ren, T.; Ding, H. Synthesis and Visible Photodegradation Enhancement of CdS/mpg-C3N4 Photocatalyst. J. Nanosci. Nanotechnol. 2016, 16, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Vo, V.; Van Kim, N.; Nga, N.T. V.; Trung, N.T.; Van Hanh, P.; Hoang, L.H.; Kim, S.-J. Preparation of g-C3N4/Ta2O5 Composites with Enhanced Visible-Light Photocatalytic Activity. J. Electron. Mater. 2016, 45, 2334–2340. [Google Scholar] [CrossRef]
- Yu, Q.; Guo, S.; Li, X.; Zhang, M. One-step fabrication and high photocatalytic activity of porous graphitic carbon nitride/graphene oxide hybrid by direct polymerization of cyanamide without templates. Russ. J. Phys. Chem. A 2014, 88, 1643–1649. [Google Scholar] [CrossRef]
- Xu, L.; Huang, W.Q.; Wang, L.L.; Tian, Z.A.; Hu, W.; Ma, Y.; Wang, X.; Pan, A.; Huang, G.-F. Insights into Enhanced Visible-Light Photocatalytic Hydrogen Evolution of g-C3N4 and Highly Reduced Graphene Oxide Composite: The Role of Oxygen. Chem. Mater. 2015, 27, 1612–1621. [Google Scholar] [CrossRef]
- Xiang, Q.; Yu, J.; Jaroniec, M. Preparation and Enhanced Visible-Light Photocatalytic H2-Production Activity of Graphene/C3N4 Composites. J. Phys. Chem. C 2011, 115, 7355–7363. [Google Scholar] [CrossRef]
- Tiong, P.; Lintang, H.O.; Endud, S.; Yuliati, L. Improved interfacial charge transfer and visible light activity of reduced graphene oxide–graphitic carbon nitride photocatalysts. RSC Adv. 2015, 5, 94029–94039. [Google Scholar] [CrossRef]
- Lee, J.S.; You, K.H.; Park, C.B. Highly photoactive, low bandgap TiO2 nanoparticles wrapped by graphene. Adv. Mater. 2012, 24, 1084–1088. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, H.; Liu, P.; Wang, D.; Li, Y.; Zhao, H. Cross-linked g-C3N4/rGO nanocomposites with tunable band structure and enhanced visible light photocatalytic activity. Small 2013, 9, 3336–3344. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Ruoff, R.S. Chemical methods for the production of graphenes. Nat. Nanotechnol. 2009, 4, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Dai, K.; Lu, L.; Liu, Q.; Zhu, G.; Wei, X.; Bai, J.; Xuan, L.; Wang, H. Sonication assisted preparation of graphene oxide/graphitic-C3N4 nanosheet hybrid with reinforced photocurrent for photocatalyst applications. Dalton Trans. 2014, 43, 6295–6299. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Q.; Yu, J.; Jaroniec, M. Enhanced photocatalytic H2-production activity of graphene-modified titania nanosheets. Nanoscale 2011, 3, 3670–3678. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.S.; Huang, W.Q.; Yang, Y.C.; Zhou, B.X.; Hu, W.Y.; Long, M.Q.; Peng, P.; Huang, G.-F. Dual role of monolayer MoS2 in enhanced photocatalytic performance of hybrid MoS2/SnO2 nanocomposite. J. Appl. Phys. 2016, 119, 205704. [Google Scholar] [CrossRef]
- Weng, B.; Zhang, X.; Zhang, N.; Tang, Z.R.; Xu, Y.J. Two-dimensional MoS2 nanosheet-coated Bi2S3 discoids: synthesis, formation mechanism, and photocatalytic application. Langmuir 2015, 31, 4314–4322. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Wen, Z.; Cui, S.; Guo, X.; Chen, J. Constructing 2D porous graphitic C3N4 nanosheets/nitrogen-doped graphene/layered MoS2 ternary nanojunction with enhanced photoelectrochemical activity. Adv. Mater. 2013, 25, 6291–6297. [Google Scholar] [CrossRef] [PubMed]




| Dimension of Nanocarbons/Photocatalyst | Nanocarbon Co-Catalysts | Semiconductor Photocatalysts | Content of Co-Catalysts (wt%) | Evaluation | Reference |
|---|---|---|---|---|---|
| 0D/0D | C60 | TiO2 | - | Degrade of MB | [31] |
| C60 | ZnO | 1.5% | Degrade of MB | [35] | |
| C60 | Cr1.3Fe0.3O3 | 3% | H2 evolution rate of 220.5 μmol·h−1·g−1 (Xe lamp, λ ≥ 420 nm, 10 vol% triethanolamine as sacrificial reagent) | [36] | |
| C70 | TiO2 | 18% | Degrade of sulfathiazole | [38] | |
| 0D/1D | C60 | TiO2 nanorods | 0.5% | Degrade of RhB | [39] |
| C60 | TiO2 nanotubes | 5% | Degrade of organic molecule | [40] | |
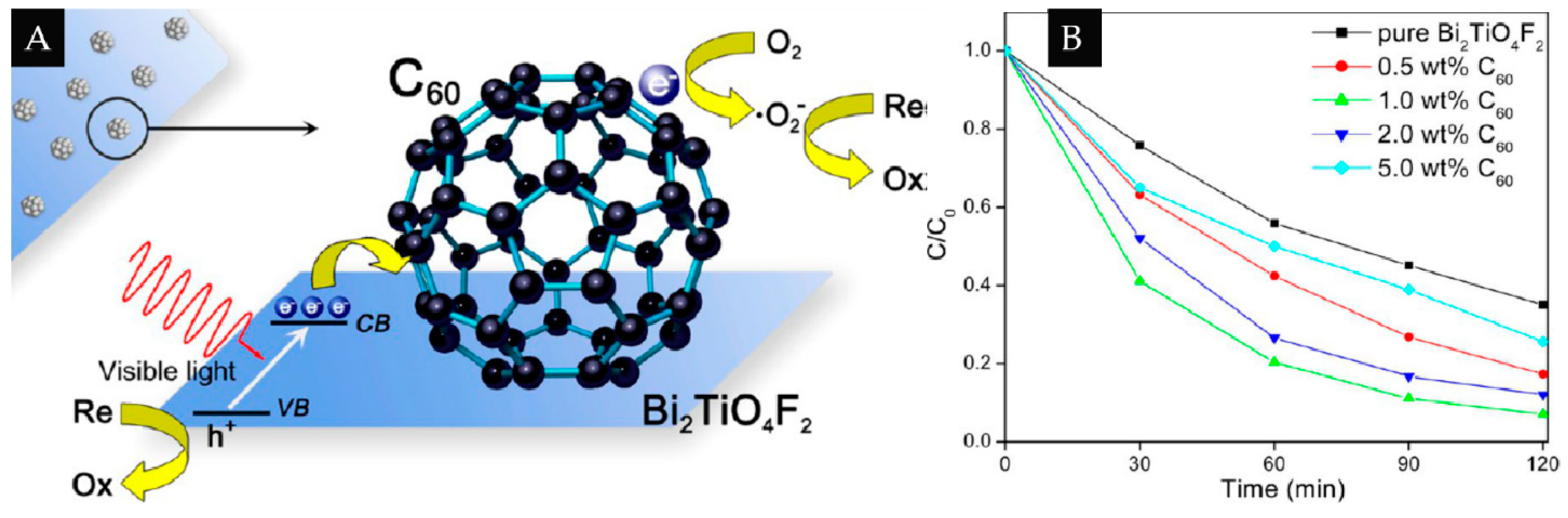
| 0D/2D | C60 | Bi2TiO4F2 | 1% | Degrade of RhB and EY | [41] |
| 1D/0D | MWCNTs | CdS | 10% | H2 evolution rate of 174.2 μmol·h−1, (Xe lamp, λ ≥ 420 nm, 0.35 M Na2S and 0.25 M Na2SO3 as sacrificial reagent) | [51] |
| CNT | ZnO | 60% | Degrade of methyl red | [52] | |
| MWCNTs | TiO2 | - | Reduction of CO2 | [53] | |
| CNT | Mo+TiO2 | - | Degrade of MB | [59] | |
| 1D/1D | MWCNTs | TiO2 nanotubes | 10% | Degrade of RhB-6G dye | [61] |
| 1D/2D | CNFs | ZnIn2S4 nanosheets | 15% | H2 evolution rate of 95 μmol·h−1. (Xe lamp, λ ≥ 420 nm, 0.35 M Na2S and 0.25 M Na2SO3 as sacrificial reagent) | [62] |
| 2D/0D | Graphene | CdS | 5% | Selective oxidation of alcohols | [72] |
| RGO | CdS | 5% | Reduction of aromatic nitro organics | [71] | |
| GO | CdS | 5% | H2 evolution rate of 314 μmol·h−1. (Xe lamp, λ ≥ 420 nm, 0.35 M Na2S and 0.25 M Na2SO3 as sacrificial reagent) | [75] | |
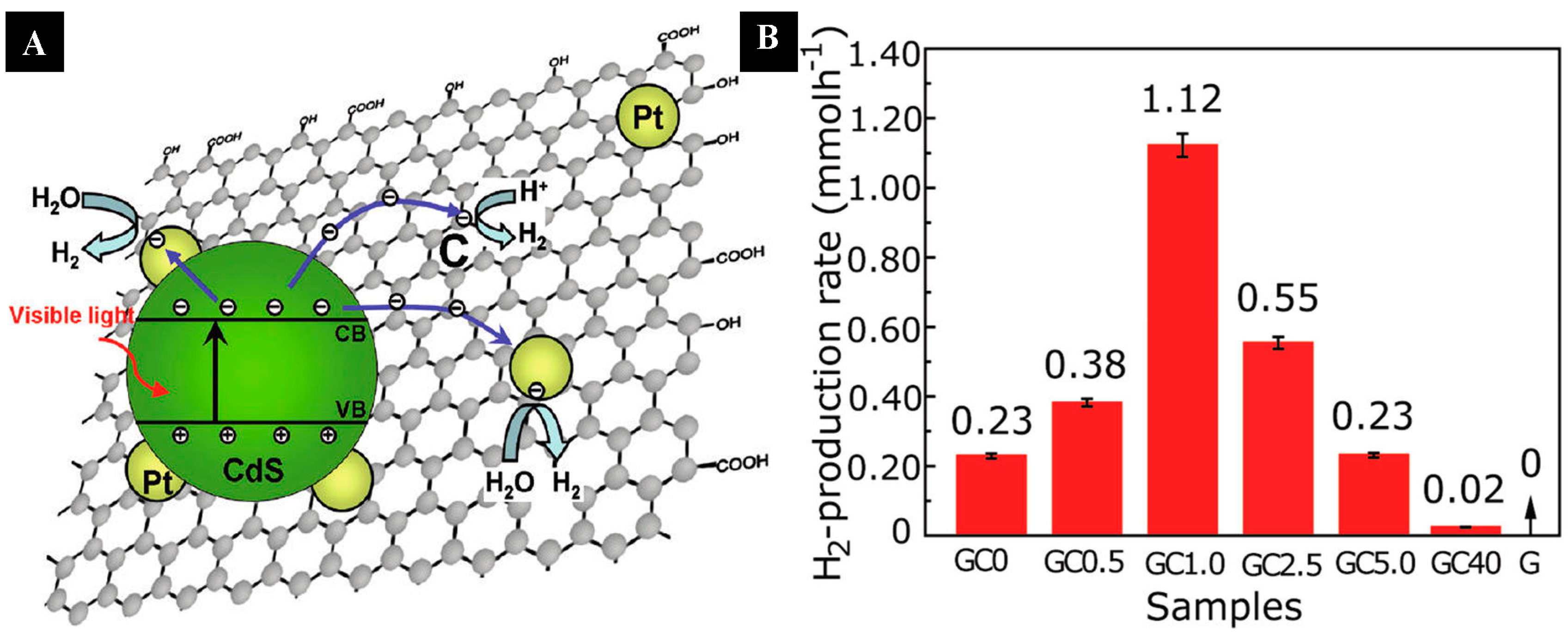
| Graphene | CdS cluster | 1% | H2 evolution rate of 1.12 mmol·h−1. (Xe lamp, λ ≥ 420 nm, 10 vol % lactic acid as sacrificial reagent and 0.5 wt% Pt as a cocatalyst) | [78] | |
| N-doped G | CdS | 2% | H2 evolution rate of 210 μmol·h−1. (Xe lamp, λ ≥ 420 nm, 0.1 Na2S and 0.1 M Na2SO3 as sacrificial reagent) | [79] | |
| Graphene | TiO2 | - | Photocurrent response. | [76] | |
| Graphene | TiO2 | 5% | H2 evolution rate of 8.6 μmol·h−1. (Xe lamp, UV-vis light, 0.1 Na2S and 0.04 M Na2SO3 as sacrificial reagent) | [74] | |
| Graphene | TiO2 | 5% | Selective transformation of alcohols to aldehydes | [73] | |
| N-doped G | TiO2 | 5% | Degrade of MO and Phenol aqueous | [83] | |
| Graphene | MoS2+TiO2 | 5% | H2 evolution rate of 165.3 μmol·h−1. (Xe lamp, 25 vol % ethanol as sacrificial reagent) | [88] | |
| Graphene | MCM41+TiO2 | 0.15% | Degrade of 2-propanol | [87] | |
| Graphene | CdS+TiO2 | 5% | Selective oxidation of benzylic alcohols and allylic alcohols | [91] | |
| 2D/1D | RGO | CdS nanowires | 5% | Selective reduction of nitro organics | [97] |
| RGO | CdS nanorods | 0.5% | Reduction of CO2 | [98] | |
| RGO | Zn0.5Cd0.5S ultrathin nanorods | 2% | H2 evolution rate of 30.8 μmol·h−1. (Xe lamp, λ ≥ 420 nm, 0.1 Na2S and 0.02 M Na2SO3 as sacrificial reagent) | [101] | |
| GO | TiO2 nanorods | - | Degrade of MB | [102] | |
| RGO | V-doped TiO2 nanorods | 10% | H2 evolution rate (Xe lamp, AM 1.5 Global, 20 vol % methanol as sacrificial reagent) | [94] | |
| N-dopedG | N-doped TiO2 nanowires | 7% | Degrade of MB | [103] | |
| 2D/2D | RGO | g-C3N4 | 0.1% | Degrade of phenol | [120] |
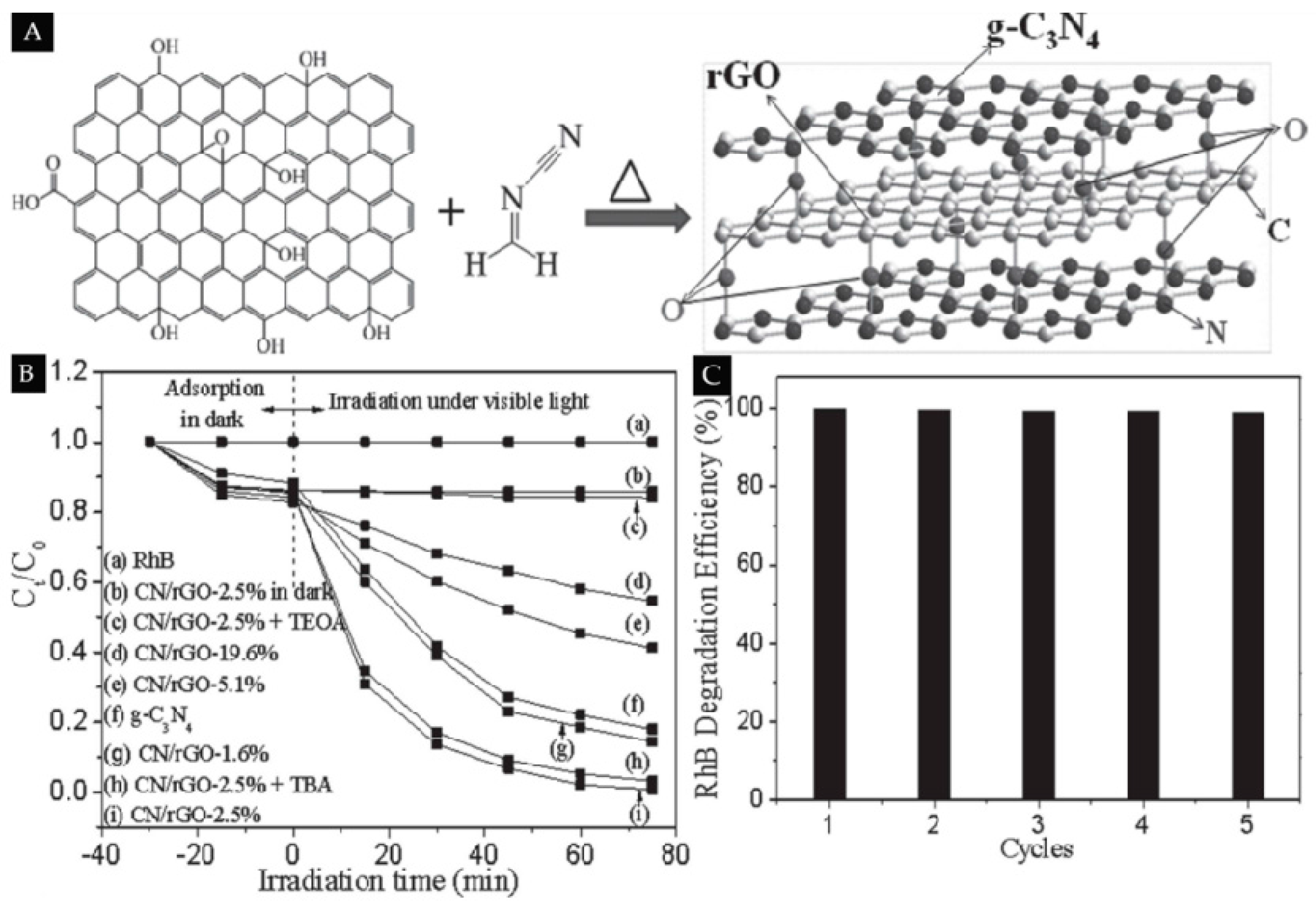
| RGO | g-C3N4 | 2.5% | Degrade of RhB and 4-nitrophenol | [122] | |
| GO | g-C3N4 | 5% | Degrade of MB | [124] | |
| GO | g-C3N4 | - | Degrade of MB | [117] | |
| graphene | TiO2 | 1% | H2 evolution rate of 36.8 μmol·h−1. (Xe lamp, 20 mW·cm-2, 25 vol % methanol as sacrificial reagent) | [125] | |
| N-doped graphene | MoS2+g-C3N4 | - | Degrade of MB and reduction of Cr(VI) | [128] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Z.; Shen, S.; Li, L.; Sun, M.; Yang, J. Nanocarbons with Different Dimensions as Noble-Metal-Free Co-Catalysts for Photocatalysts. Catalysts 2016, 6, 111. https://doi.org/10.3390/catal6080111
Wu Z, Shen S, Li L, Sun M, Yang J. Nanocarbons with Different Dimensions as Noble-Metal-Free Co-Catalysts for Photocatalysts. Catalysts. 2016; 6(8):111. https://doi.org/10.3390/catal6080111
Chicago/Turabian StyleWu, Zhujun, Shuling Shen, Long Li, Minquan Sun, and Junhe Yang. 2016. "Nanocarbons with Different Dimensions as Noble-Metal-Free Co-Catalysts for Photocatalysts" Catalysts 6, no. 8: 111. https://doi.org/10.3390/catal6080111





