Preparation of PdCu Alloy Nanocatalysts for Nitrate Hydrogenation and Carbon Monoxide Oxidation
Abstract
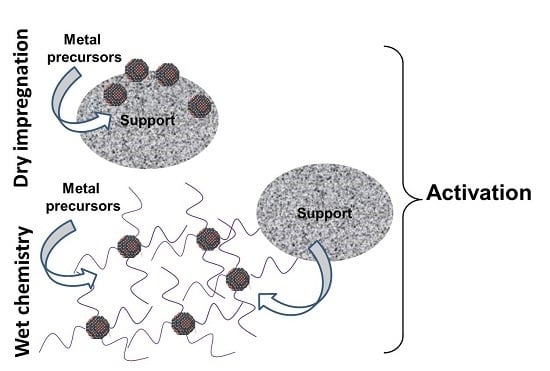
:1. Introduction
2. Results and Discussion
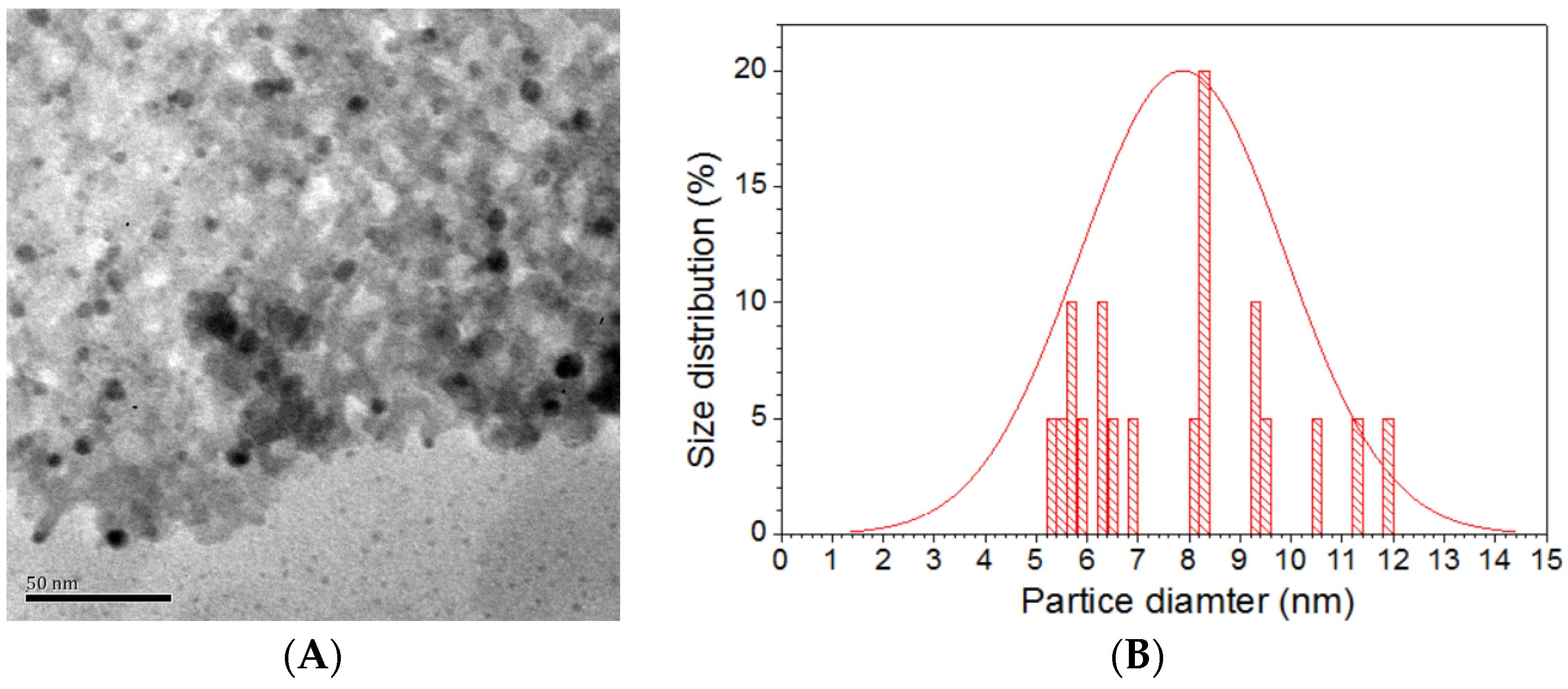
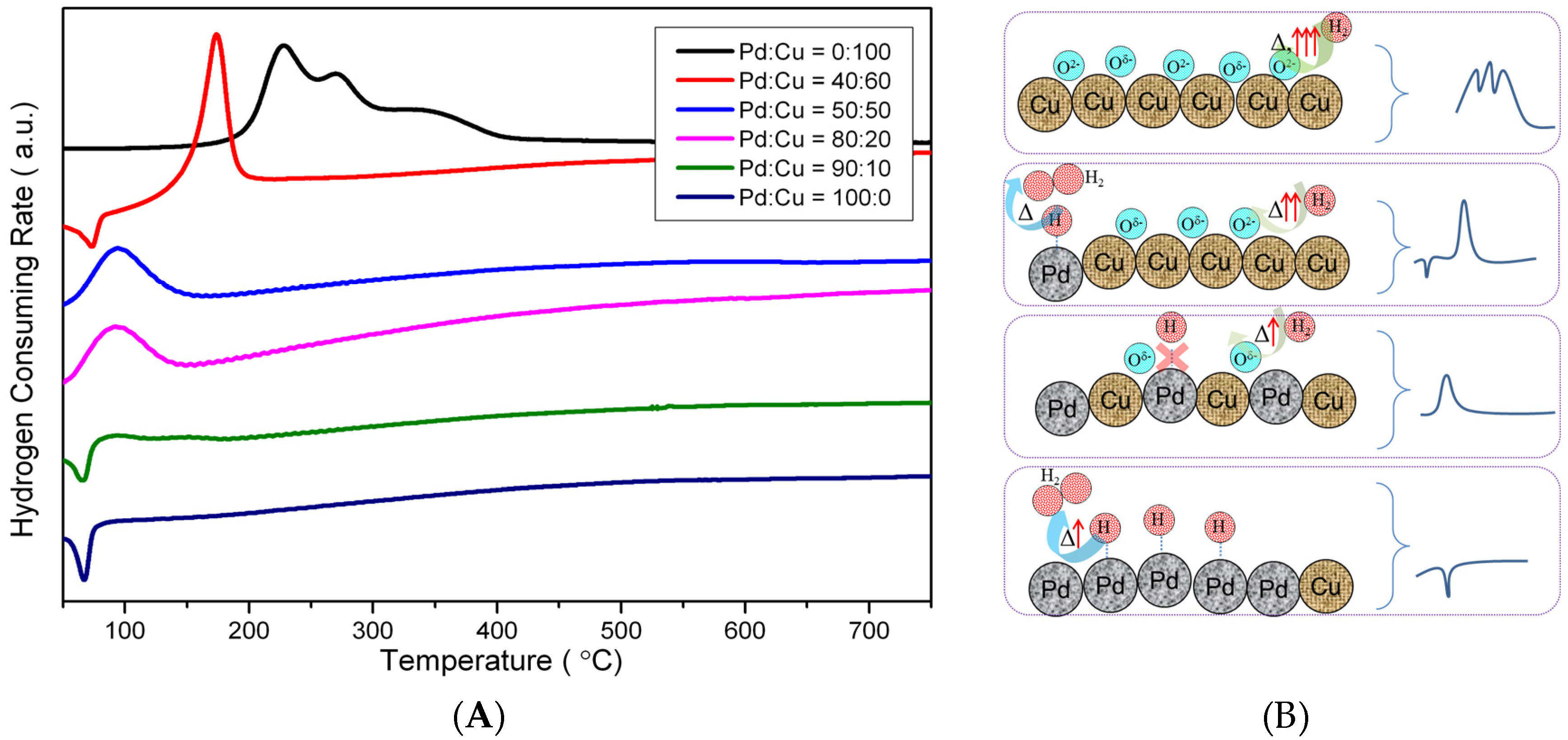
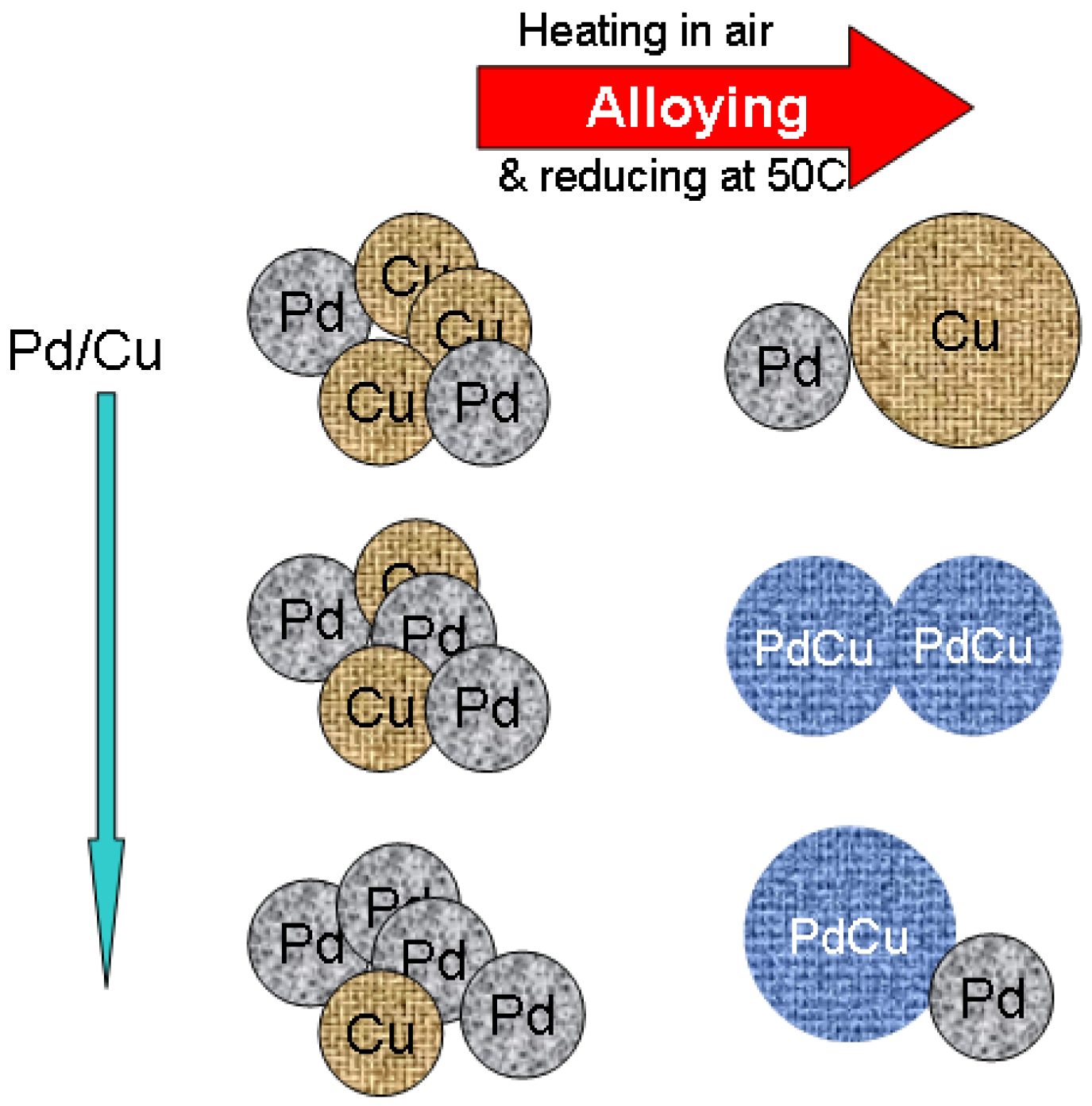
2.1. Catalyst Characterizations
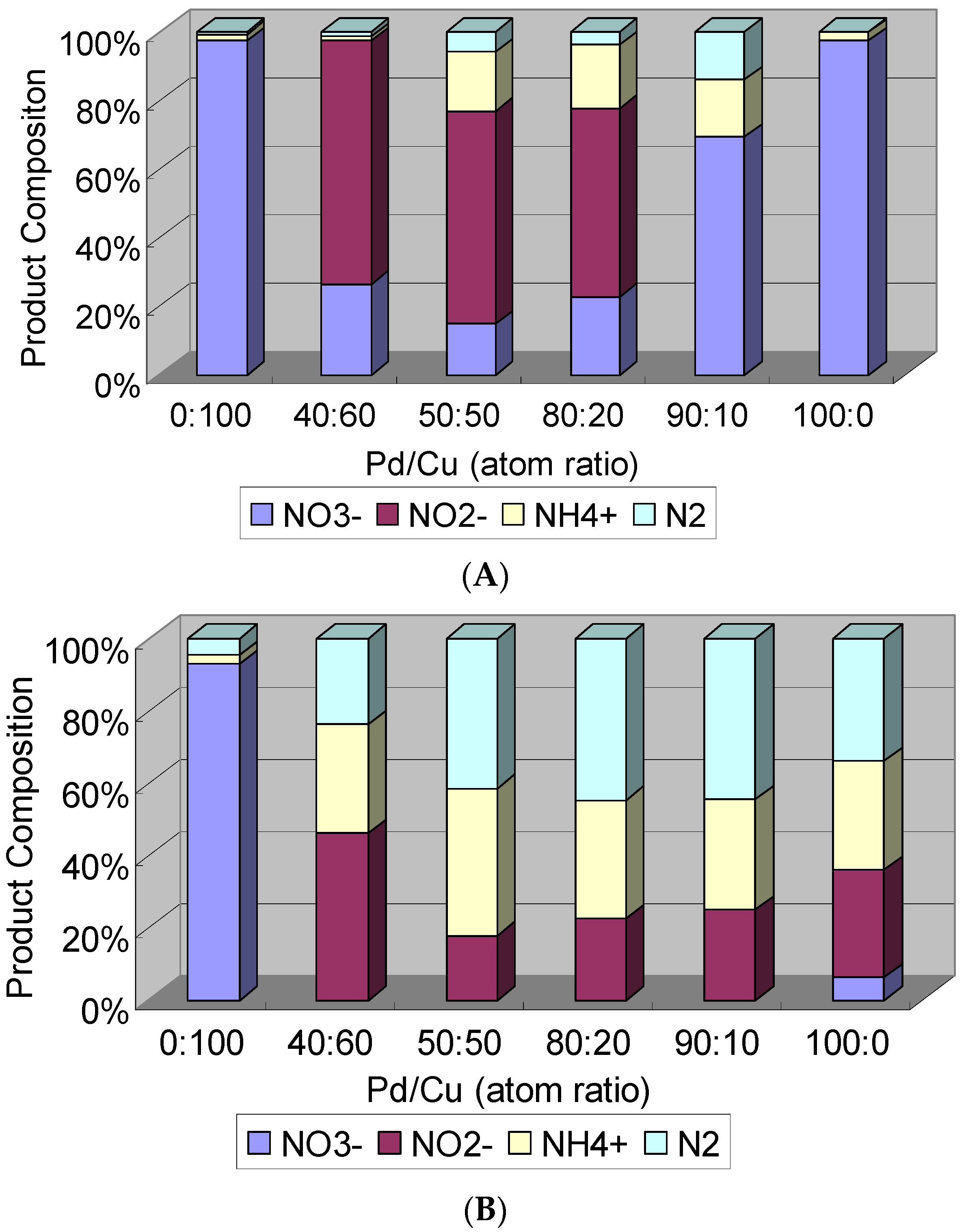
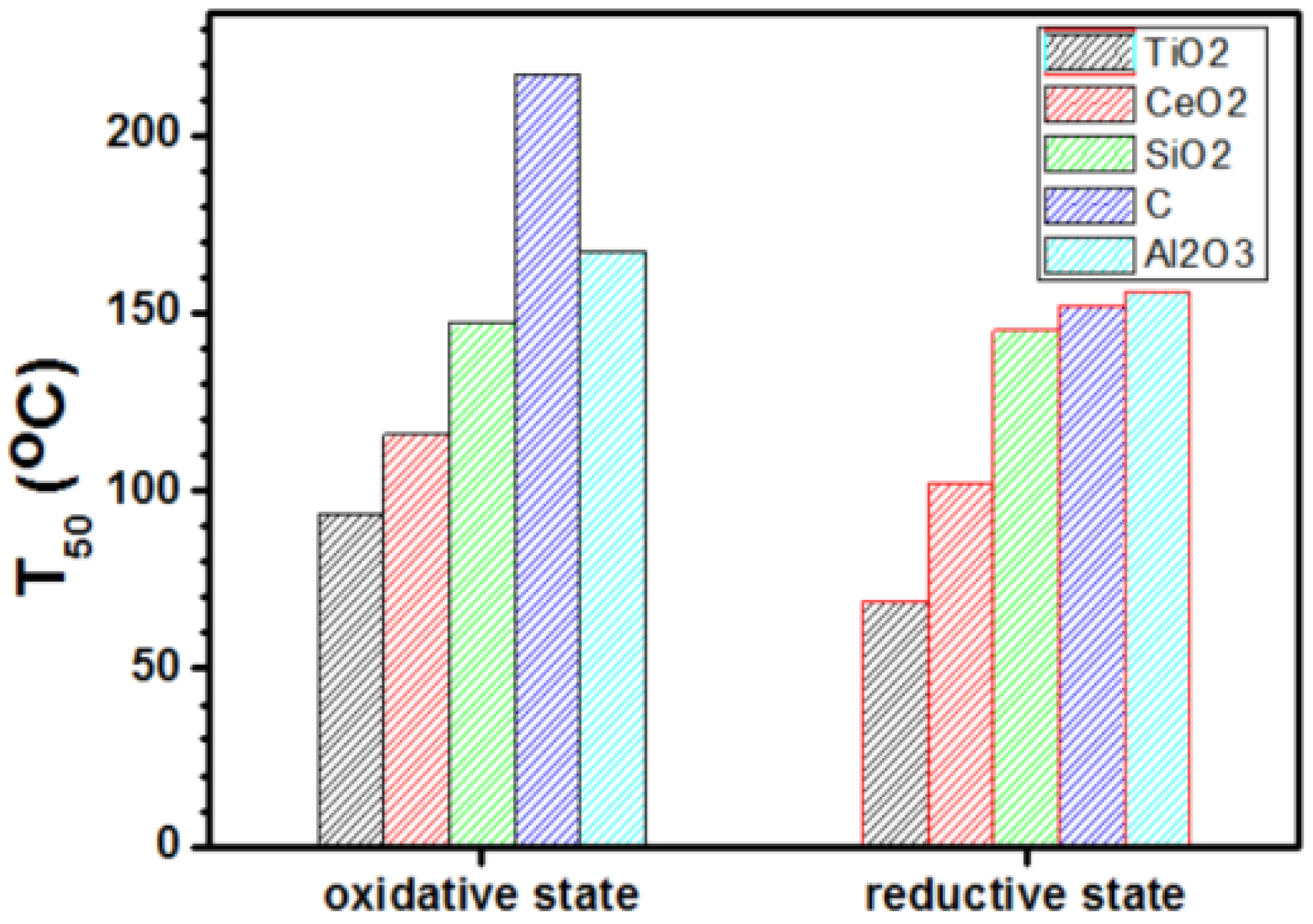
2.2. Catalytic Activity Evaluation
2.2.1. Catalytic Denitrification
2.2.2. Catalytic CO Oxidation
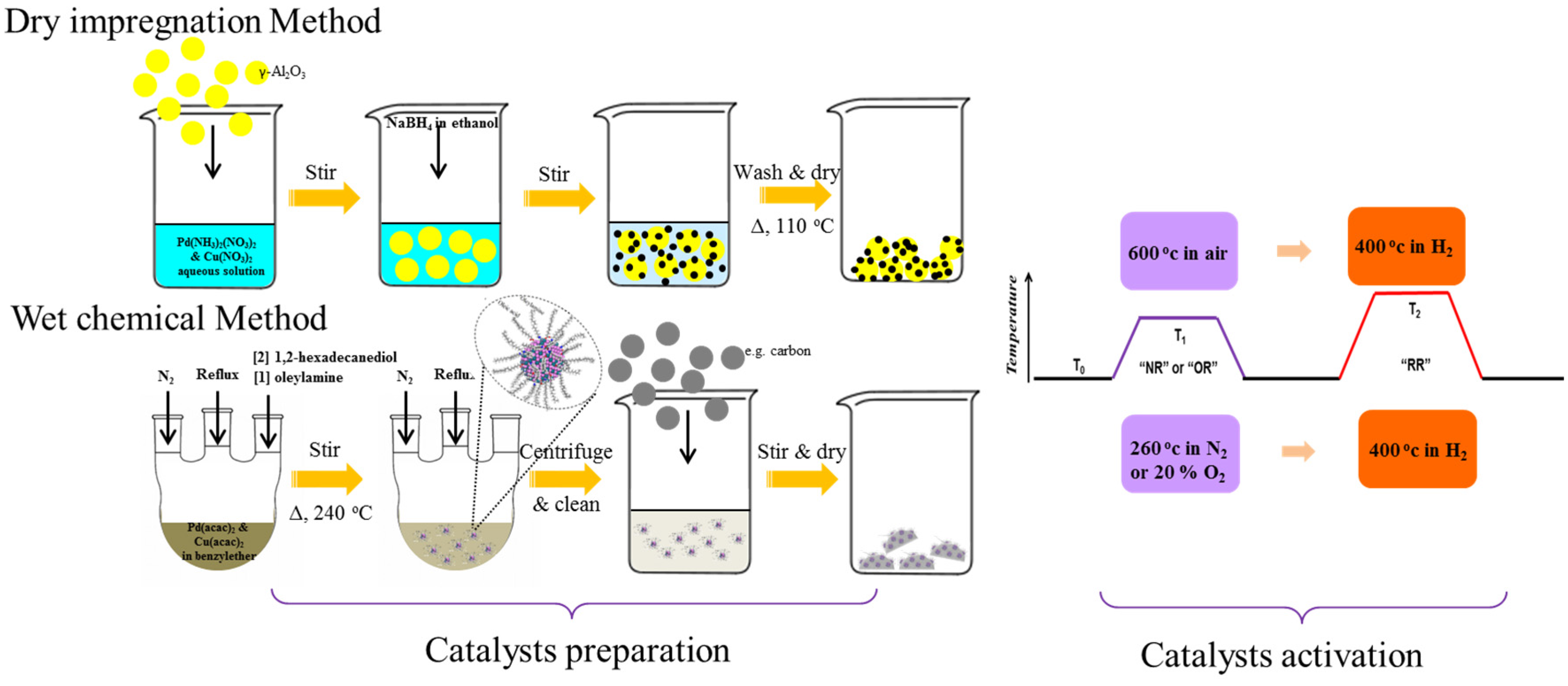
3. Experimental Section
3.1. Chemicals
3.2. Catalyst Preparations
3.2.1. Dry Impregnation Method
3.2.2. Catalyst Preparation by Wet Chemical Synthesis
3.3. Catalytic Activity Measurements
3.3.1. Denitrification Activity Measurement
3.3.2. CO Oxidation Activity Measurement
3.4. Instrumentation Used for Characterizations of Catalysts
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhong, C.J.; Regalbuto, J.R. Metal Nanoparticle Synthesis. Surface Inorganic Chemistry and Heterogeneous Catalysis. In Comprehensive Inorganic Chemistry II; Schloegl, R., Niemantsverdriet, J.W., Reedijk, J., Poeppelmeier, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; Chapter 4; Volume 7. [Google Scholar]
- Zhang, H.; Jin, M.S.; Xia, Y.N. Enhancing the catalytic and electrocatalytic properties of Pt-based catalysts by forming bimetallic nanocrystals with Pd. Chem. Soc. Rev. 2012, 41, 8035–8049. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.J.; Watanabe, T.; Okumura, M.; Haruta, M.; Toshima, N. Catalytically highly active top gold atom on palladium nanocluster. Nat. Mater. 2012, 11, 49–52. [Google Scholar]
- Hutchings, G.J. Nanocrystalline gold and gold-palladium alloy oxidation catalysts: A personal reflection on the nature of the active sites. Dalton Trans. 2008, 5523–5536. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.S.; Kumar, D.; Yi, C.W.; Goodman, D.W. The promotional effect of gold in catalysis by palladium-gold. Science 2005, 310, 291–293. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Goodman, D.W. Pd-Au bimetallic catalysts: Understanding alloy effects from planar models and (supported) nanoparticles. Chem. Soc. Rev. 2012, 41, 8009–8020. [Google Scholar] [CrossRef] [PubMed]
- Hutchings, G.J.; Kiely, C.J. Strategies for the Synthesis of Supported Gold Palladium Nanoparticles with Controlled Morphology and Composition. Acc. Chem. Res. 2013, 46, 1759–1772. [Google Scholar] [CrossRef] [PubMed]
- Shao, M.H.; Sasaki, K.; Adzic, R.R. Pd-Fe nanoparticles as electrocatalysts for oxygen reduction. J. Am. Chem. Soc. 2006, 128, 3526–3527. [Google Scholar] [CrossRef] [PubMed]
- Shao, M.H.; Shoemaker, K.; Peles, A.; Kaneko, K.; Protsailo, L. Pt Mono layer on Porous Pd-Cu Alloys as Oxygen Reduction Electrocatalysts. J. Am. Chem. Soc. 2010, 132, 9253–9255. [Google Scholar] [CrossRef] [PubMed]
- Enache, D.I.; Edwards, J.K.; Landon, P.; Solsona-Espriu, B.; Carley, A.F.; Herzing, A.A.; Watanabe, M.; Kiely, C.J.; Knight, D.W.; Hutchings, G.J. Solvent-free oxidation of primary alcohols to aldehydes using Au-Pd/TiO2 catalysts. Science 2006, 311, 362–365. [Google Scholar] [CrossRef] [PubMed]
- Kesavan, L.; Tiruvalam, R.; Ab Rahim, M.H.; Saiman, M.I.; Enache, D.I.; Jenkins, R.L.; Dimitratos, N.; Lopez-Sanchez, J.A.; Taylor, S.H.; Knight, D.W.; et al. Solvent-Free Oxidation of Primary Carbon-Hydrogen Bonds in Toluene Using Au-Pd Alloy Nanoparticles. Science 2011, 331, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Antolini, E. Catalysts for direct ethanol fuel cells. J. Power Sources 2007, 170, 1–12. [Google Scholar] [CrossRef]
- Cargnello, M.; Delgado Jaen, J.J.; Hernandez Garrido, J.C.; Bakhmutsky, K.; Montini, T.; Calvino Gamez, J.J.; Gorte, R.J.; Fornasiero, P. Exceptional Activity for Methane Combustion over Modular Pd@CeO2 Subunits on Functionalized Al2O3. Science 2012, 337, 713–717. [Google Scholar] [CrossRef] [PubMed]
- Paalanen, P.; Weckhuysen, B.M.; Sankar, M. Progress in controlling the size, composition and nanostructure of supported gold-palladium nanoparticles for catalytic applications. Catal. Sci. Technol. 2013, 3, 2869–2880. [Google Scholar] [CrossRef]
- Ward, T.; Delannoy, L.; Hahn, R.; Kendell, S.; Pursell, C.J.; Louis, C.; Chandler, B.D. Effects of Pd on Catalysis by Au: CO Adsorption, CO Oxidation, and Cyclohexene Hydrogenation by Supported Au and Pd-Au Catalysts. ACS Catal. 2013, 3, 2644–2653. [Google Scholar] [CrossRef]
- Greeley, J.; Stephens, I.E.; Bondarenko, A.S.; Johansson, T.P.; Hansen, H.A.; Jaramillo, T.F.; Rossmeisl, J.; Chorkendorff, I.; Norskov, J.K. Alloys of platinum and early transition metals as oxygen reduction electrocatalysts. Nat. Chem. 2009, 1, 552–556. [Google Scholar]
- Jin, C.C.; Sun, X.J.; Chen, Z.D.; Dong, R.L. Electrocatalytic activity of PdNi/C catalysts for allyl alcohol oxidation in alkaline solution. Mater. Chem. Phys. 2012, 135, 433–437. [Google Scholar] [CrossRef]
- Xu, C.X.; Liu, A.H.; Qiu, H.J.; Liu, Y.Q. Nanoporous PdCu alloy with enhanced electrocatalytic performance. Electrochem. Commun. 2011, 13, 766–769. [Google Scholar] [CrossRef]
- Yin, Z.; Zhou, W.; Gao, Y.J.; Ma, D.; Kiely, C.J.; Bao, X.H. Supported Pd-Cu Bimetallic Nanoparticles That Have High Activity for the Electrochemical Oxidation of Methanol. Chem. Eur. J. 2012, 18, 4887–4893. [Google Scholar] [CrossRef] [PubMed]
- Gobal, F.; Arab, R. A preliminary study of the electro-catalytic reduction of oxygen on Cu-Pd alloys in alkaline solution. J. Electroanal. Chem. 2010, 647, 66–73. [Google Scholar] [CrossRef]
- Fouda-Onana, F.; Bah, S.; Savadogo, O. Palladium-copper alloys as catalysts for the oxygen reduction reaction in an acidic media I: Correlation between the ORR kinetic parameters and intrinsic physical properties of the alloys. J. Electroanal. Chem. 2009, 636, 1–2. [Google Scholar] [CrossRef]
- Yin, J.; Shan, S.; Ng, M.S.; Yang, L.F.; Mott, D.; Fang, W.; Kang, N.; Luo, J.; Zhong, C.J. Catalytic and Electrocatalytic Oxidation of Ethanol over Palladium-Based Nanoalloy Catalysts. Langmuir 2013, 29, 9249–9258. [Google Scholar] [CrossRef] [PubMed]
- Shan, S.; Petkov, V.; Prasai, B.; Wu, J.; Joseph, P.; Skeete, Z.; Kim, E.; Malis, D.M.O.; Luo, J.; Zhong, C.J. Catalytic Activity of Bimetallic Catalysts Highly Sensitive to Atomic Composition and Phase Structure at the Nanoscale. Nanoscale 2015, 7, 18936–18948. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Shan, S.; Luo, J.; Joseph, P.; Petkov, V.; Zhong, C.J. PdCu Nanoalloy Electrocatalysts in Oxygen Reduction Reaction: Role of Composition and Phase Properties in Catalytic Synergy. ACS Appl. Mater. Interfaces 2015, 7, 25906–25913. [Google Scholar] [CrossRef] [PubMed]
- Lopez, N.; Norskov, J.K. Synergetic effects in CO adsorption on Cu-Pd(111) alloys. Surf. Sci. 2001, 477, 59–75. [Google Scholar] [CrossRef]
- Jeroro, E.; Hyman, M.P.; Vohs, J.M. Ensemble vs. electronic effects on the reactivity of two-dimensional Pd alloys: A comparison of CO and CH3OH adsorption on Zn/Pd(111) and Cu/Pd(111). Phys. Chem. Chem. Phys. 2009, 11, 10457–10465. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.J.; Zhang, L.; Henkelman, G. Catalytic Activity of Pd/Cu Random Alloy Nanoparticles for Oxygen Reduction. J. Phys. Chem. Lett. 2011, 2, 1328–1331. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.Y.; Zhao, T.S.; Xu, J.B.; Li, Y.S. Synthesis of PdNi catalysts for the oxidation of ethanol in alkaline direct ethanol fuel cells. J. Power Sources 2010, 195, 1001–1006. [Google Scholar] [CrossRef]
- Persson, K.; Ersson, A.; Jansson, K.; Iverlund, N.; Jaras, S. Influence of co-metals on bimetallic palladium catalysts for methane combustion. J. Catal. 2005, 231, 139–150. [Google Scholar] [CrossRef]
- Kanra, B.C.; Bertolini, J.C.; Rousset, J.L. Effect of surface segregation on the catalytic activity of alloys: CO hydrogenation on Pd-Ni(111) surface. J. Mol. Catal. A Chem. 1998, 129, 233–240. [Google Scholar] [CrossRef]
- Yuan, X.; Sun, G.; Asakura, H.; Tanaka, T.; Chen, X.; Yuan, Y.; Laurenczy, G.; Kou, Y.; Dyson, P.J.; Yan, N. Development of palladium surface enriched heteronuclear Au Pd nanoparticle dehalogenation catalysts in an ionic liquid. Chem. Eur. J 2013, 19, 1227–1234. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Yuan, Y.; Philippot, K.; Yan, N. Ag-Pd and CuO-Pd nanoparticles in a hydroxyl-group functionalized ionic liquid: Synthesis, characterization and catalytic performance. Catal. Sci. Technol. 2015, 5, 1683–1692. [Google Scholar] [CrossRef]
- Niu, W.X.; Gao, Y.J.; Zhang, W.Q.; Yan, N.; Lu, X.M. Pd-Pb Alloy Nanocrystals with Tailored Composition for Semihydrogenation: Taking Advantage of Catalyst Poisoning. Angew. Chem. Int. Ed. 2015, 54, 8271–8274. [Google Scholar] [CrossRef] [PubMed]
- Soares, O.S.G.P.; Orfao, J.J.M.; Pereira, M.F.R. Bimetallic catalysts supported on activated carbon for the nitrate reduction in water: Optimization of catalysts composition. Appl. Catal. B Environ. 2009, 91, 441–448. [Google Scholar] [CrossRef]
- Wada, K.; Hirata, T.; Hosokawa, S.; Iwamoto, S.; Inoue, M. Effect of supports on Pd–Cu bimetallic catalysts for nitrate and nitrite reduction in water. Catal. Today 2012, 185, 81–87. [Google Scholar] [CrossRef]
- Francha, C.; Rodríguez-Castellón, E.; Reyes-Carmona, Á.; Palomares, A.E. Characterization of (Sn and Cu)/Pd catalysts for the nitrate reduction in natural water. Appl. Catal. A Gen. 2012, 425–426, 145–152. [Google Scholar] [CrossRef]
- Soares, O.S.G.P.; Orfao, J.J.M.; Pereira, M.F.R. Nitrate reduction with hydrogen in the presence of physical mixtures with mono and bimetallic catalysts and ions in solution. Appl. Catal. B Environ. 2011, 102, 424–432. [Google Scholar] [CrossRef]
- Fernandez-Garcia, M.; Martinez-Arias, A.; Belver, C.; Anderson, J.A.; Conesa, J.C.; Soria, J. Behavior of palladium–copper catalysts for CO and NO elimination. J. Catal. 2000, 190, 387–395. [Google Scholar] [CrossRef]
- Lobodacackovic, J.; Hammoudeh, A.; Mousa, M.S.; Block, J.H. Oxidation of CO on PdCu(110) single crystal alloy catalyst: Steady state, hysteresis and related surface phenomena. Vacuum 1995, 46, 411–415. [Google Scholar] [CrossRef]
- Wang, F.G.; Zhang, H.J.; He, D.N. Catalytic oxidation of low-concentration CO at ambient temperature over supported Pd-Cu catalysts. Environ. Technol. 2014, 35, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Matatov-Meytal, U.; Sheintuch, M. The relation between surface composition of Pd-Cu/ACC catalysts prepared by selective deposition and their denitrification behavior. Catal. Commun. 2009, 10, 1137–1141. [Google Scholar] [CrossRef]
- Witońska, I.; Karski, S.; Gołuchowska, J. Hydrogenation of nitrate in water over bimetallic Pd-Ag/Al2O3. React. Kinet. Catal. Lett. 2007, 90, 107–115. [Google Scholar] [CrossRef]
- Yang, L.; Shan, S.; Loukrakpam, R.; Petkov, V.; Ren, Y.; Wanjala, B.N.; Engelhard, M.H.; Luo, J.; Yin, J.; Chen, Y.; et al. Role of support-nanoalloy interactions in the atomic-scale structural and chemical ordering for tuning catalytic sites. J. Am. Chem. Soc. 2012, 134, 15048–15060. [Google Scholar] [CrossRef] [PubMed]
- Shan, S.Y.; Petkov, V.; Yang, L.F.; Mott, D.; Wanjala, B.N.; Cai, F.; Chen, B.H.; Luo, J.; Zhong, C.J. Oxophilicity and Structural Integrity in Maneuvering Surface Oxygenated Species on Nanoalloys in CO Oxidation. ACS Catal. 2013, 3, 3075–3085. [Google Scholar] [CrossRef]




© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, F.; Yang, L.; Shan, S.; Mott, D.; Chen, B.H.; Luo, J.; Zhong, C.-J. Preparation of PdCu Alloy Nanocatalysts for Nitrate Hydrogenation and Carbon Monoxide Oxidation. Catalysts 2016, 6, 96. https://doi.org/10.3390/catal6070096
Cai F, Yang L, Shan S, Mott D, Chen BH, Luo J, Zhong C-J. Preparation of PdCu Alloy Nanocatalysts for Nitrate Hydrogenation and Carbon Monoxide Oxidation. Catalysts. 2016; 6(7):96. https://doi.org/10.3390/catal6070096
Chicago/Turabian StyleCai, Fan, Lefu Yang, Shiyao Shan, Derrick Mott, Bing H. Chen, Jin Luo, and Chuan-Jian Zhong. 2016. "Preparation of PdCu Alloy Nanocatalysts for Nitrate Hydrogenation and Carbon Monoxide Oxidation" Catalysts 6, no. 7: 96. https://doi.org/10.3390/catal6070096