The Performance of Electron-Mediator Modified Activated Carbon as Anode for Direct Glucose Alkaline Fuel Cell
Abstract
:1. Introduction
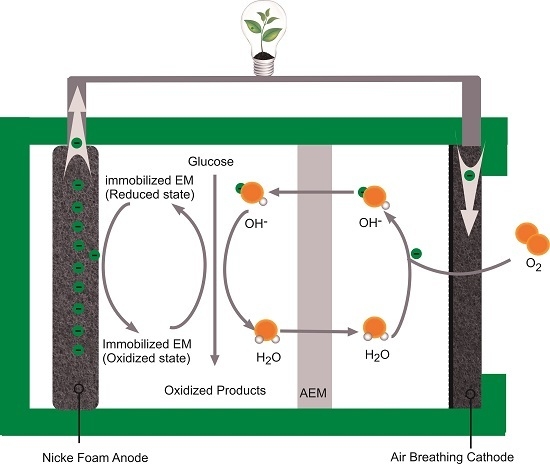
2. Results and Discussions
2.1. Effects of Doping with Different Electron Mediators on DGAFC Performance
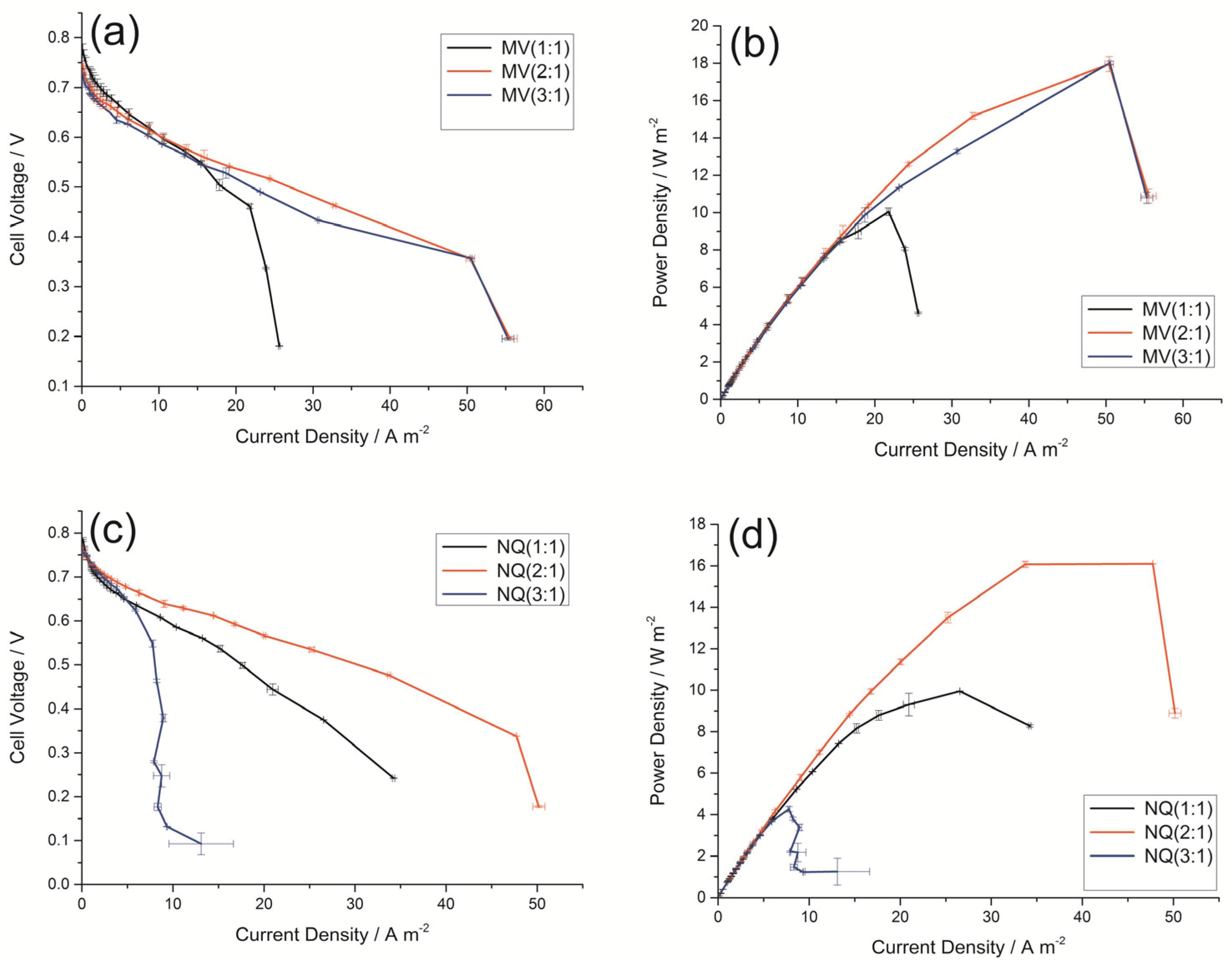
2.2. Effect of Different Mass Ratio of Electron Mediator/AC on DGAFC Performance
2.3. Electrochemical Characterization
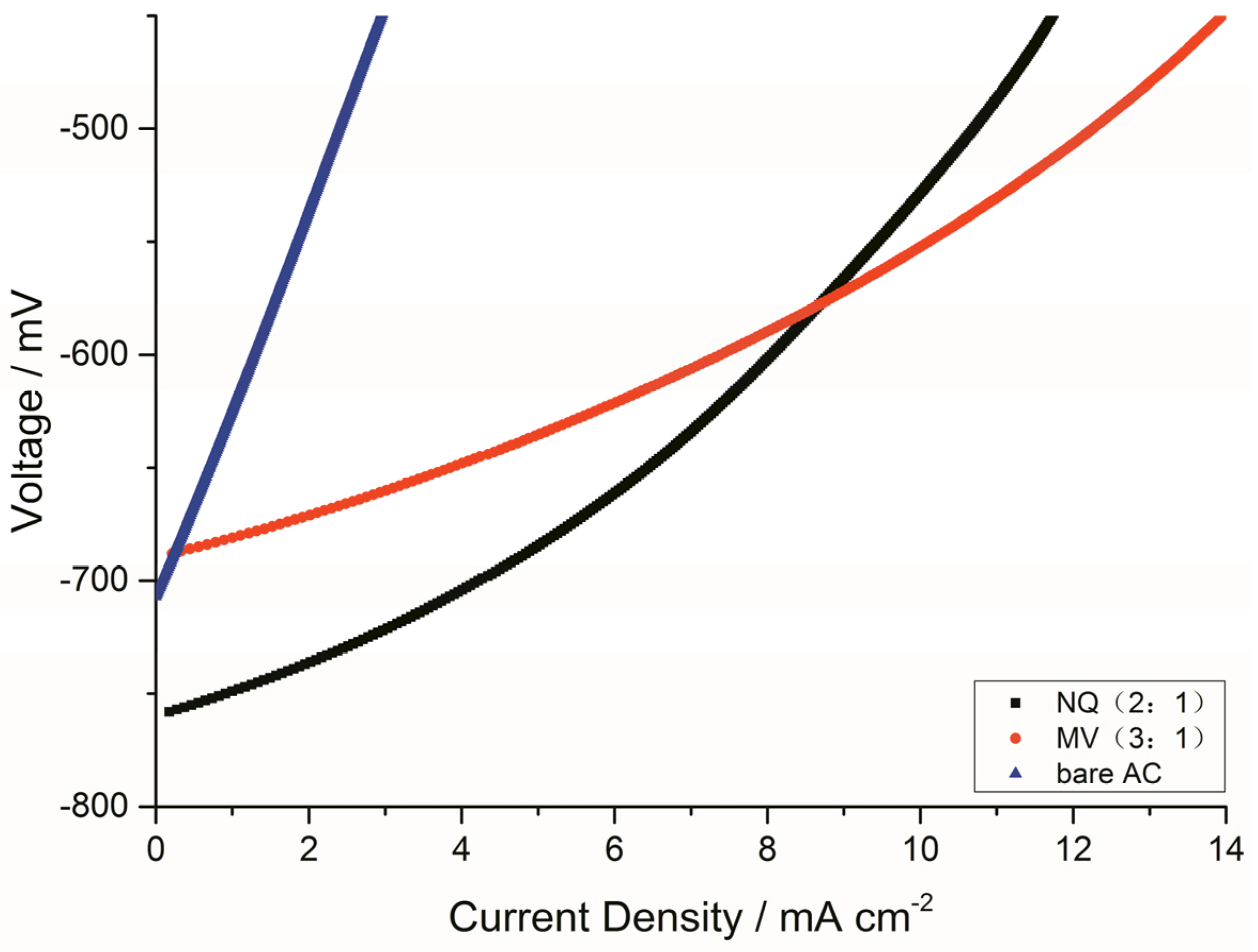
2.3.1. LSV Polarization Curves of the Anodes
2.3.2. EIS of the Anodes
2.3.3. Tafel Curves of the Anodes
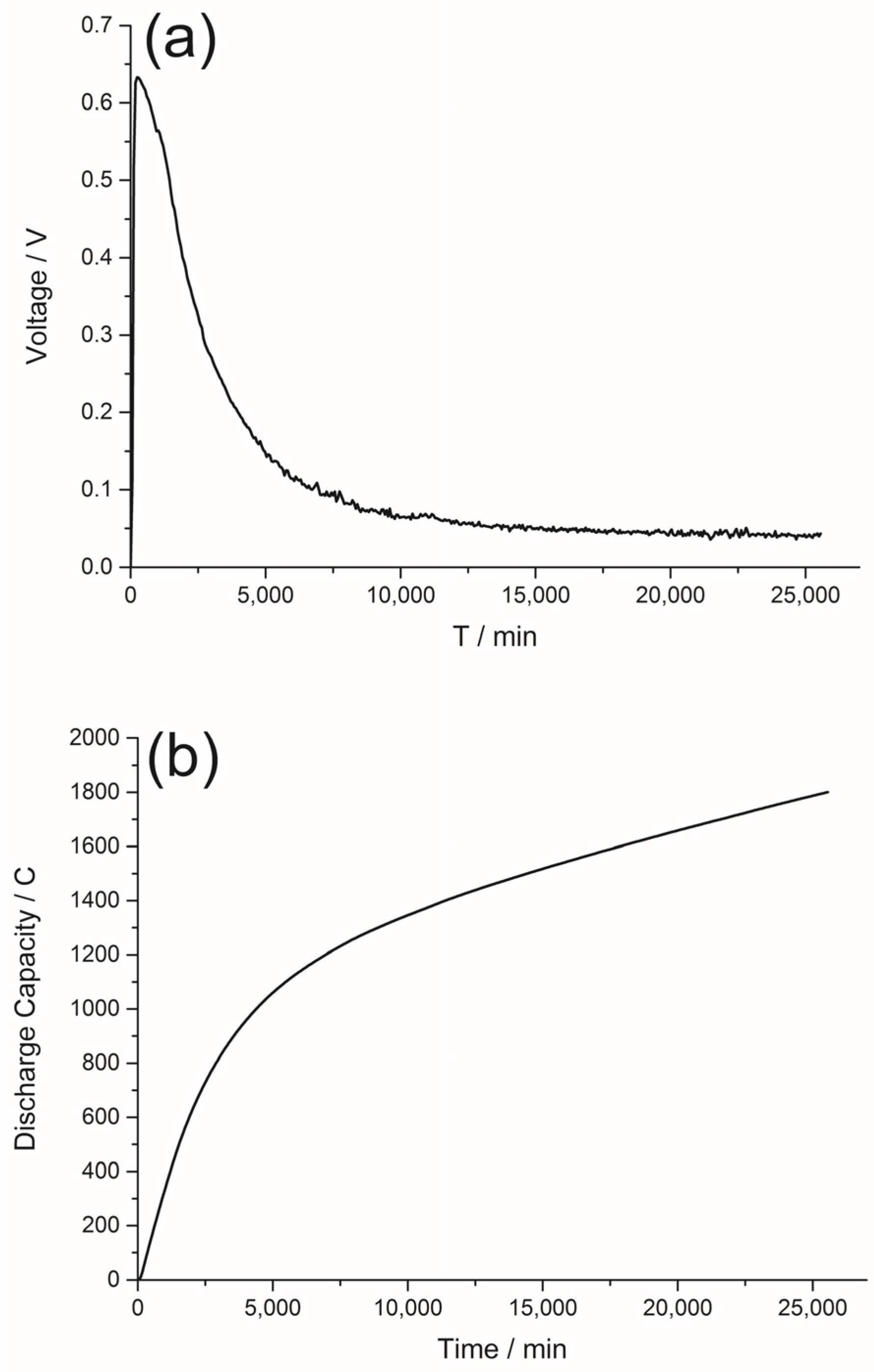
2.4. The Discharge Curve of the DGAFC Equipped with NQ-AC Anode
3. Experimental Section
3.1. Materials
3.2. Preparation of the Electrodes
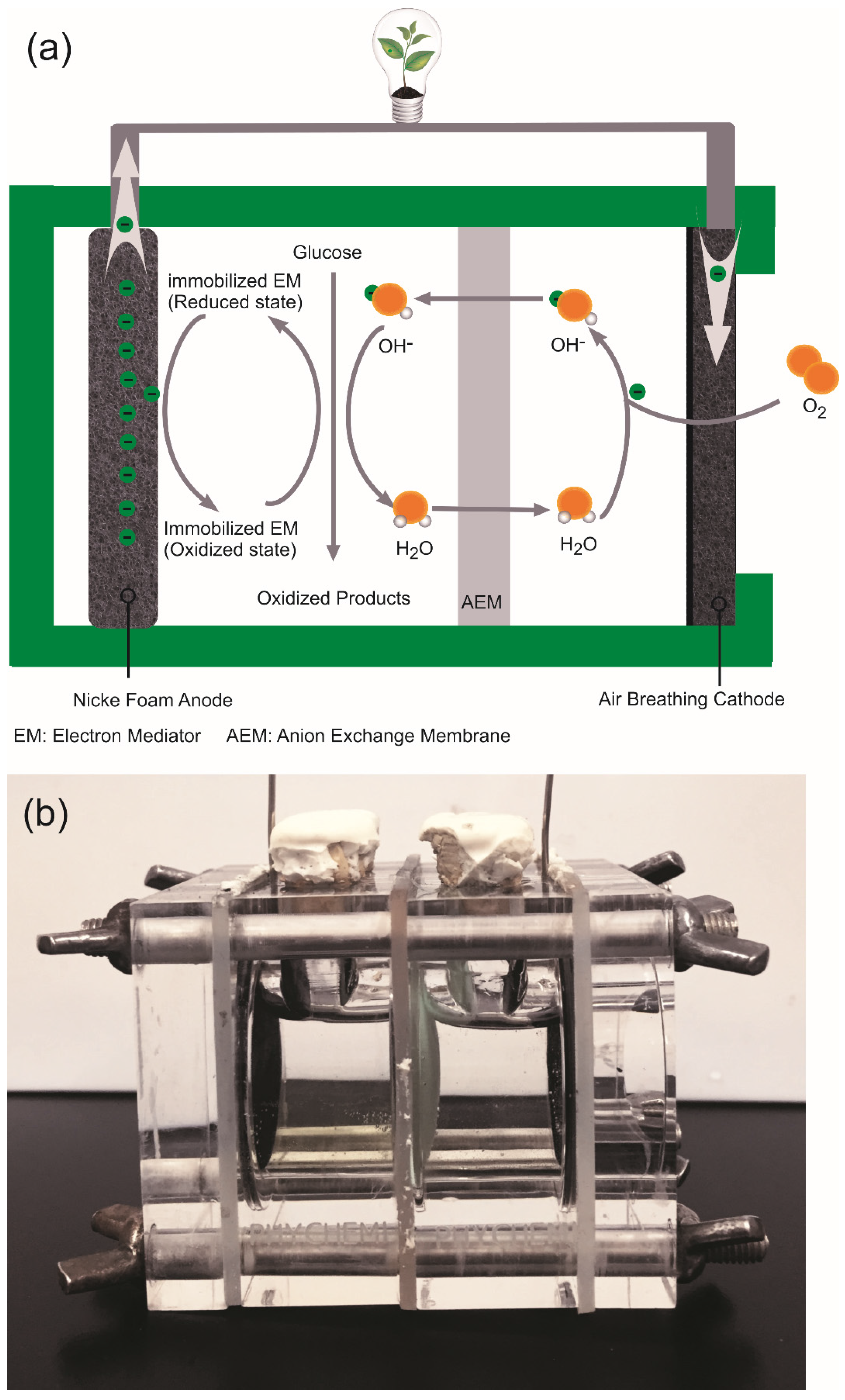
3.3. Fuel Cell Apparatus and Assembly
3.4. Electrochemical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dranga, B.-A.; Lazar, L.; Koeser, H. Oxidation catalysts for elemental mercury in flue gases—A review. Catalysts 2012, 2, 139–170. [Google Scholar] [CrossRef]
- Wang, Y.; Zou, S.; Cai, W.-B. Recent advances on electro-oxidation of ethanol on Pt- and Pd-based catalysts: From reaction mechanisms to catalytic materials. Catalysts 2015, 5, 1507–1534. [Google Scholar] [CrossRef]
- Lo, K.; Wang, M.Y. Energy conservation in China’s twelfth five-year plan period: Continuation or paradigm shift? Renew. Sustain. Energy Rev. 2013, 18, 499–507. [Google Scholar] [CrossRef]
- Balat, M.; Balat, M. Political, economic and environmental impacts of biomass-based hydrogen. Int. J. Hydrog. Energy 2009, 34, 3589–3603. [Google Scholar] [CrossRef]
- Weng, Y.; Qiu, S.; Ma, L.; Liu, Q.; Ding, M.; Zhang, Q.; Zhang, Q.; Wang, T. Jet-fuel range hydrocarbons from biomass-derived sorbitol over Ni-HZSM-5/SBA-15 catalyst. Catalysts 2015, 5, 2147–2160. [Google Scholar] [CrossRef]
- Urbaniec, K.; Bakker, R.R. Biomass residues as raw material for dark hydrogen fermentation—A review. Int. J. Hydrog. Energy 2015, 40, 3648–3658. [Google Scholar] [CrossRef]
- Sopian, K.; Ali, B.; Asim, N. Strategies for renewable energy applications in the organization of Islamic conference (OIC) countries. Renew. Sustain. Energy Rev. 2011, 15, 4706–4725. [Google Scholar] [CrossRef]
- Bindig, R.; Butt, S.; Hartmann, I.; Matthes, M.; Thiel, C. Application of heterogeneous catalysis in small-scale biomass combustion systems. Catalysts 2012, 2, 223–243. [Google Scholar] [CrossRef]
- Ojala, S.; Koivikko, N.; Laitinen, T.; Mouammine, A.; Seelam, P.; Laassiri, S.; Ainassaari, K.; Brahmi, R.; Keiski, R. Utilization of volatile organic compounds as an alternative for destructive abatement. Catalysts 2015, 5, 1092–1151. [Google Scholar] [CrossRef]
- Shabani, S.; Aghajani Delavar, M.; Azmi, M. Investigation of biomass gasification hydrogen and electricity co-production with carbon dioxide capture and storage. Int. J. Hydrog. Energy 2013, 38, 3630–3639. [Google Scholar] [CrossRef]
- Schechner, P.; Kroll, E.; Bubis, E.; Chervinsky, S.; Zussman, E. Silver-plated electrospun fibrous anode for glucose alkaline fuel cells. J. Electrochem. Soc. 2007, 154, B942–B948. [Google Scholar] [CrossRef]
- An, L.; Zhao, T.S.; Shen, S.Y.; Wu, Q.X.; Chen, R. Alkaline direct oxidation fuel cell with non-platinum catalysts capable of converting glucose to electricity at high power output. J. Power Sour. 2011, 196, 186–190. [Google Scholar] [CrossRef]
- Liang, B.; Guo, X.; Fang, L.; Hu, Y.; Yang, G.; Zhu, Q.; Wei, J.; Ye, X. Study of direct electron transfer and enzyme activity of glucose oxidase on graphene surface. Electrochem. Commun. 2015, 50, 1–5. [Google Scholar] [CrossRef]
- Kumar, R.; Leech, D. A glucose anode for enzymatic fuel cells optimized for current production under physiological conditions using a design of experiment approach. Bioelectrochemistry 2015, 106, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-Y.; Kim, T.G.; Cho, K.-S. Effects of proton exchange membrane on the performance and microbial community composition of air-cathode microbial fuel cells. J. Biotechnol. 2015, 211, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Kirubaharan, C.J.; Santhakumar, K.; Gnana kumar, G.; Senthilkumar, N.; Jang, J.-H. Nitrogen doped graphene sheets as metal free anode catalysts for the high performance microbial fuel cells. Int. J. Hydrog. Energy 2015, 40, 13061–13070. [Google Scholar] [CrossRef]
- Hernández-Fernández, F.J.; Pérez de los Ríos, A.; Salar-García, M.J.; Ortiz-Martínez, V.M.; Lozano-Blanco, L.J.; Godínez, C.; Tomás-Alonso, F.; Quesada-Medina, J. Recent progress and perspectives in microbial fuel cells for bioenergy generation and wastewater treatment. Fuel Process. Technol. 2015, 138, 284–297. [Google Scholar] [CrossRef]
- Fujiwara, N.; Yamazaki, S.-I.; Siroma, Z.; Ioroi, T.; Senoh, H.; Yasuda, K. Nonenzymatic glucose fuel cells with an anion exchange membrane as an electrolyte. Electrochem. Commun. 2009, 11, 390–392. [Google Scholar] [CrossRef]
- Gu, Y.; Liu, Y.; Yang, H.; Li, B.; An, Y. Electrocatalytic glucose oxidation via hybrid nanomaterial catalyst of multi-wall TiO2 nanotubes supported Ni(OH)2 nanoparticles: Optimization of the loading level. Electrochim. Acta 2015, 160, 263–270. [Google Scholar] [CrossRef]
- Ramsurn, H.; Gupta, R.B. Nanotechnology in solar and biofuels. ACS Sustain. Chem. Eng. 2013, 1, 779–797. [Google Scholar] [CrossRef]
- Liu, C.; Burghaus, U.; Besenbacher, F.; Wang, Z.L. Preparation and characterization of nanomaterials for sustainable energy production. ACS Nano 2010, 4, 5517–5526. [Google Scholar] [CrossRef] [PubMed]
- Apblett, C.A.; Ingersoll, D.; Sarangapani, S.; Kelly, M.; Atanassov, P. Direct glucose fuel cell: Noble metal catalyst anode polymer Electrolyte Membrane fuel cell with Glucose Fuel. J. Electrochem. Soc. 2010, 157, B86–B89. [Google Scholar] [CrossRef]
- Zhao, C.X.; Wang, K.; Yan, H.; Xu, G. Output current increase in alkaline glucose fuel cells. J. Electrochem. Soc. 2011, 158, B1055–B1059. [Google Scholar] [CrossRef]
- Basu, D.; Basu, S. A Study on Direct Glucose and Fructose Alkaline Fuel Cell. Electrochim. Acta 2010, 55, 5775–5779. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, C.X.; Zhi, M.M.; Wang, K.; Deng, L.; Xu, G. Alkaline direct oxidation glucose fuel cell system using silver/nickel foams as electrodes. Electrochim. Acta 2012, 66, 133–138. [Google Scholar] [CrossRef]
- Zhang, D.; Popov, B.N.; White, R.E. Electrochemical investigation of CRO 2.65 doped LiMn2O4 as a cathode material for lithium-ion batteries. J. Power Sour. 1998, 99, 466–472. [Google Scholar]
- Yang, Y.-L.; Liu, X.-H.; Hao, M.-Q.; Zhang, P.-P. Performance of a low-cost direct glucose fuel cell with an anion-exchange membrane. Int. J. Hydrog. Energy 2015, 40, 10979–10984. [Google Scholar] [CrossRef]
- Chen, X.Y.; He, Y.Y.; Song, H.; Zhang, Z.J. Structure and electrochemical performance of highly nanoporous carbons from benzoate-metal complexes by a template carbonization method for supercapacitor application. Carbon 2014, 72, 410–420. [Google Scholar] [CrossRef]
- Liu, X.; Hao, M.; Feng, M.; Zhang, L.; Zhao, Y.; Du, X.; Wang, G. A one-compartment direct glucose alkaline fuel cell with methyl viologen as electron mediator. Appl. Energy 2013, 106, 176–183. [Google Scholar] [CrossRef]
- Eustis, R.; Tsang, T.M.; Yang, B.; Scott, D.; Liaw, B.Y. Seeking effective dyes for a mediated glucose-air alkaline battery/fuel cell. J. Power Sour. 2014, 248, 1133–1140. [Google Scholar] [CrossRef]
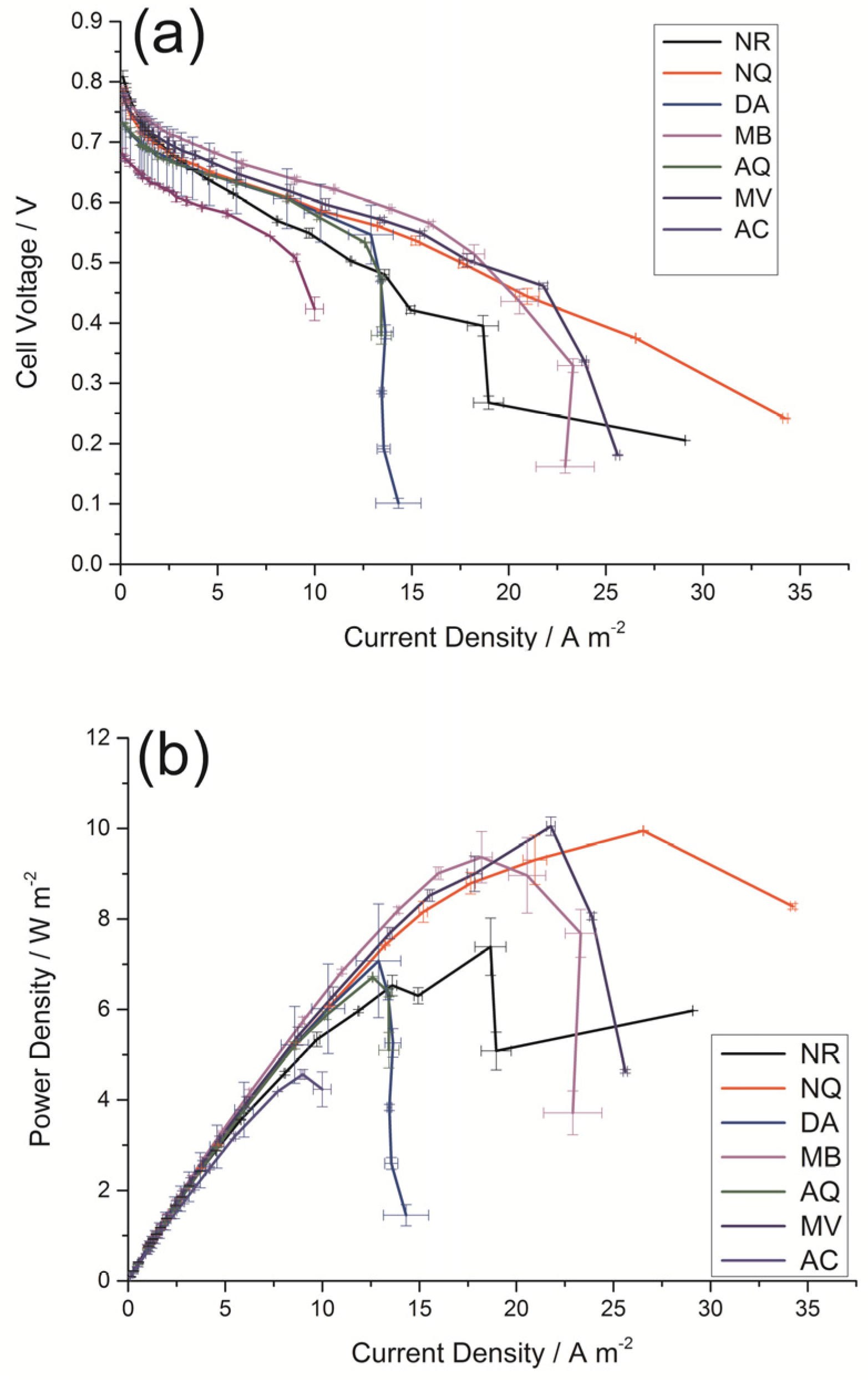



| Compound Name | Molar Mass (g·mol−1) | Molecular Structure | Molecular Formula | Soluble in Ethanol | Market Price (RMB/g) | OCV a (V) | PPD b (Wm−2) | PCD c (Am−2) |
|---|---|---|---|---|---|---|---|---|
| NR | 288.78 |  | C15H17ClN4 | Yes | 0.5 | 0.81 | 7.39 | 29.08 |
| NQ | 174.15 |  | C10H6O3 | Yes | 15 | 0.79 | 9.95 | 34.23 |
| DA | 277.10 |  | C14H6Cl2O2 | No | 12 | 0.73 | 7.07 | 14.31 |
| MB | 319.85 |  | C16H18ClN3S | Yes | 1 | 0.79 | 9.37 | 22.90 |
| AQ | 208.22 |  | C14H8O2 | No | 4 | 0.73 | 6.70 | 13.43 |
| MV | 257.16 |  | C12H14Cl2N2 | Yes | 200 | 0.78 | 10.05 | 25.61 |
| Resistance | Bare AC | NQ (2:1) | MV (3:1) |
|---|---|---|---|
| Ro(Ω) | 0.9891 | 0.9044 | 0.9182 |
| Rct(Ω) | 8.9720 | 0.4522 | 0.3101 |
| Rd(Ω) | 0.0779 | 0.0748 | 0.0786 |
| The Anode | Linear Fitting Equation (R2) | 10−3 i0 (Acm−2) |
|---|---|---|
| bare AC | y = −2.833 + 0.001x (0.998) | 1.4689 |
| NQ (2:1) | y = −2.028 + 0.00006x (0.995) | 9.3756 |
| MV (3:1) | y = −1.715 + 0.00009 x (0.991) | 19.2752 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Liu, X.; Liu, P.; Zhang, P. The Performance of Electron-Mediator Modified Activated Carbon as Anode for Direct Glucose Alkaline Fuel Cell. Catalysts 2016, 6, 95. https://doi.org/10.3390/catal6070095
Li Z, Liu X, Liu P, Zhang P. The Performance of Electron-Mediator Modified Activated Carbon as Anode for Direct Glucose Alkaline Fuel Cell. Catalysts. 2016; 6(7):95. https://doi.org/10.3390/catal6070095
Chicago/Turabian StyleLi, Zi, Xianhua Liu, Peng Liu, and Pingping Zhang. 2016. "The Performance of Electron-Mediator Modified Activated Carbon as Anode for Direct Glucose Alkaline Fuel Cell" Catalysts 6, no. 7: 95. https://doi.org/10.3390/catal6070095