A Novel Delafossite Structured Visible-Light Sensitive AgFeO2 Photocatalyst: Preparation, Photocatalytic Properties, and Reaction Mechanism
Abstract
:1. Introduction
2. Result and Discussion
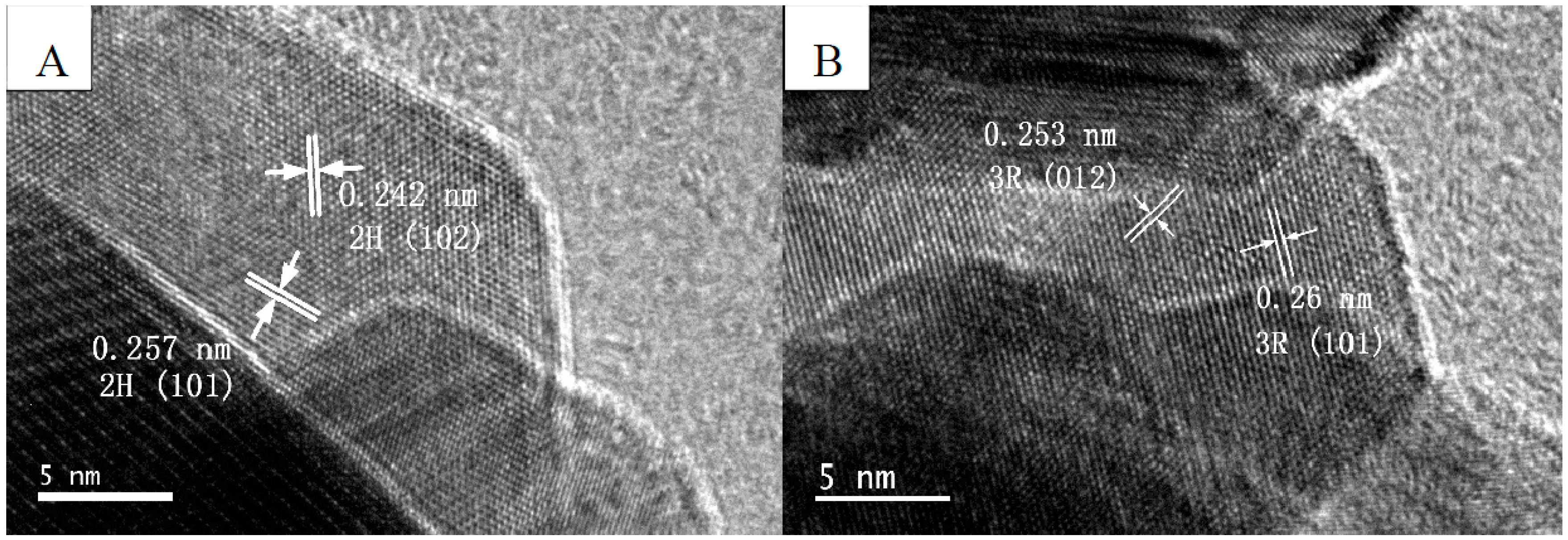
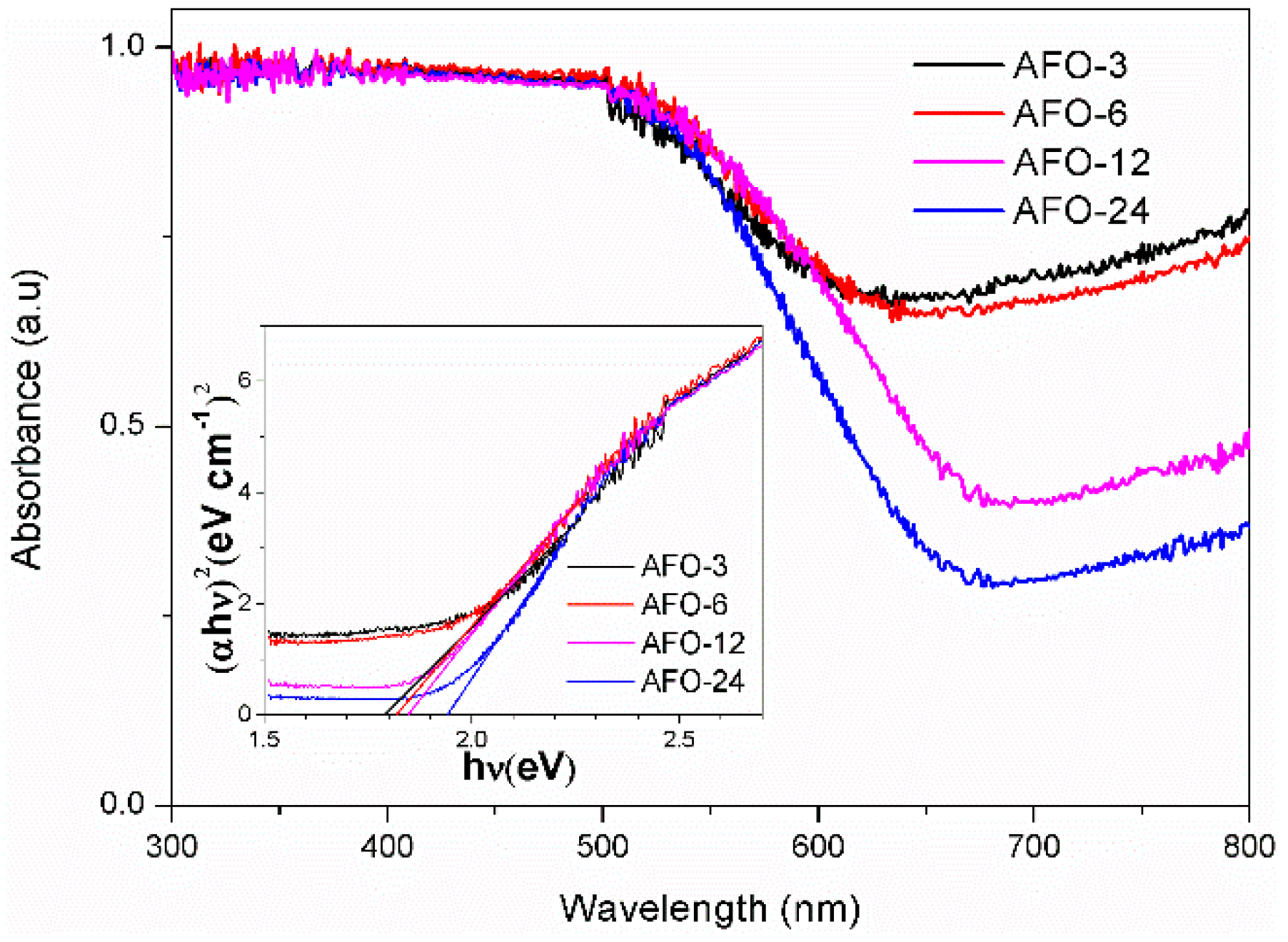
2.1. Structure, Morphology, and Optical Properties
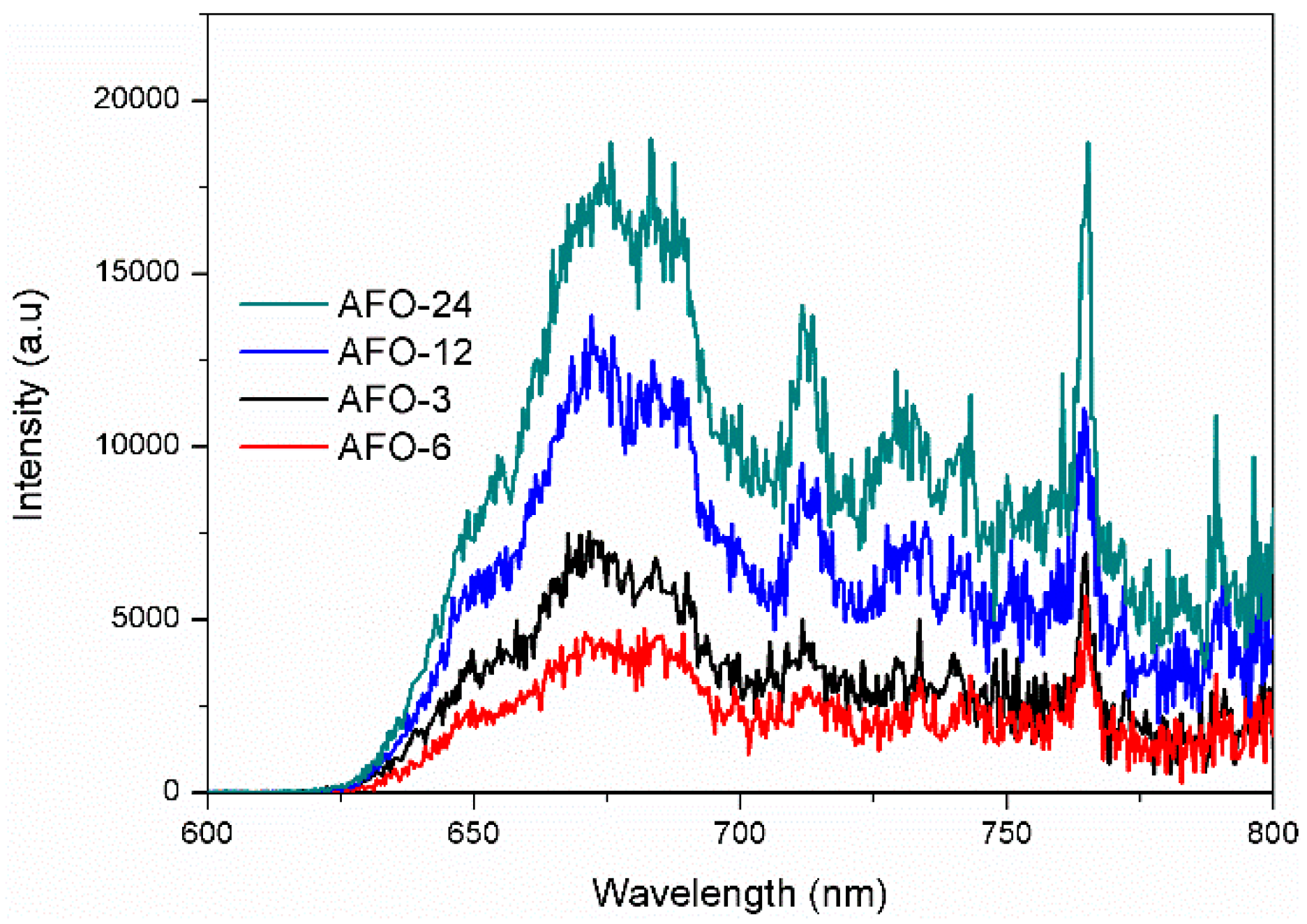
2.2. Photoluminescence and Photocurrent Analysis
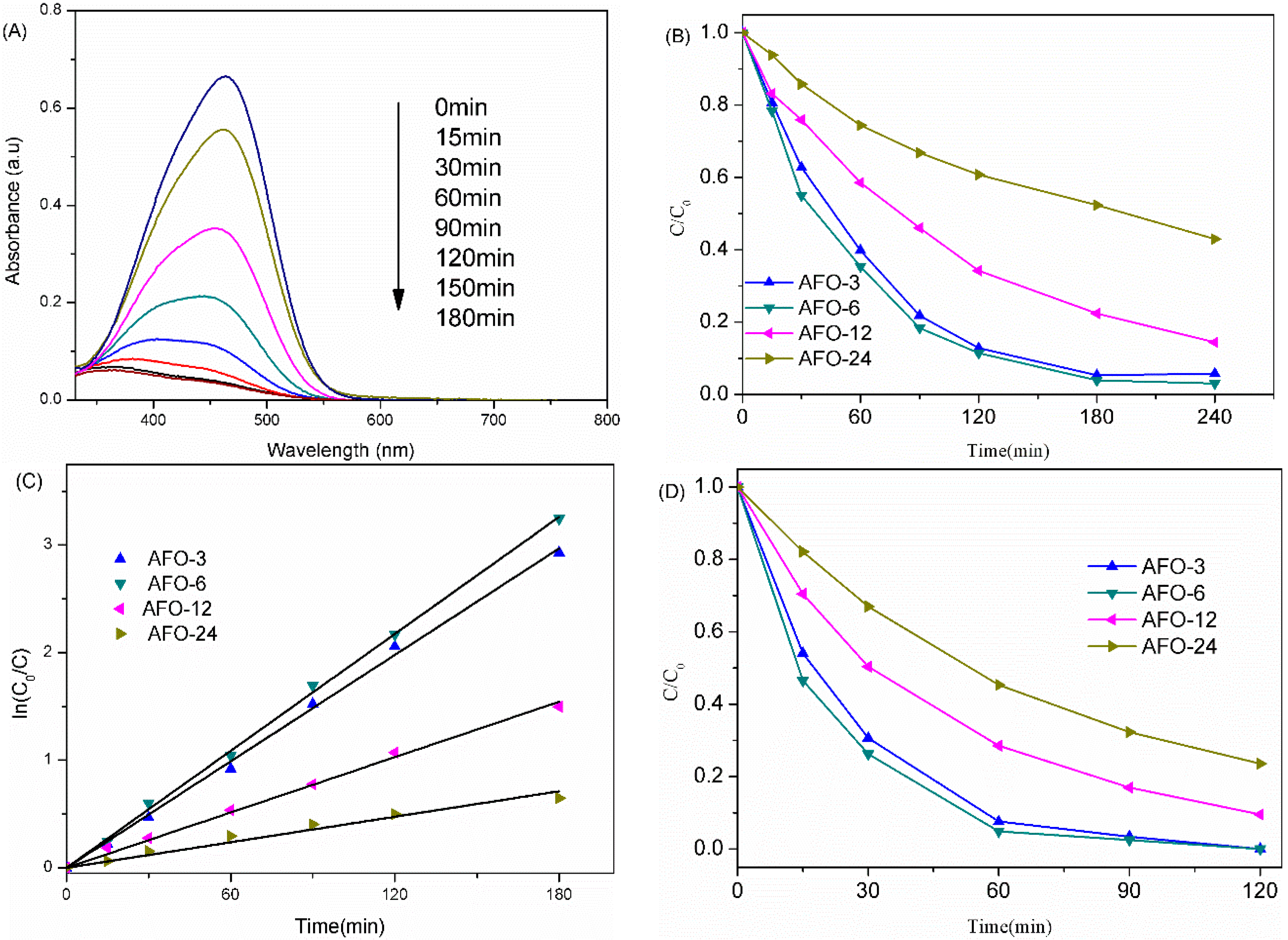
2.3. Photocatalytic Activity of AgFeO2
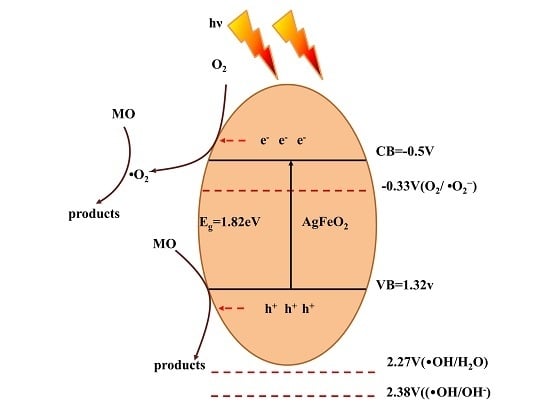
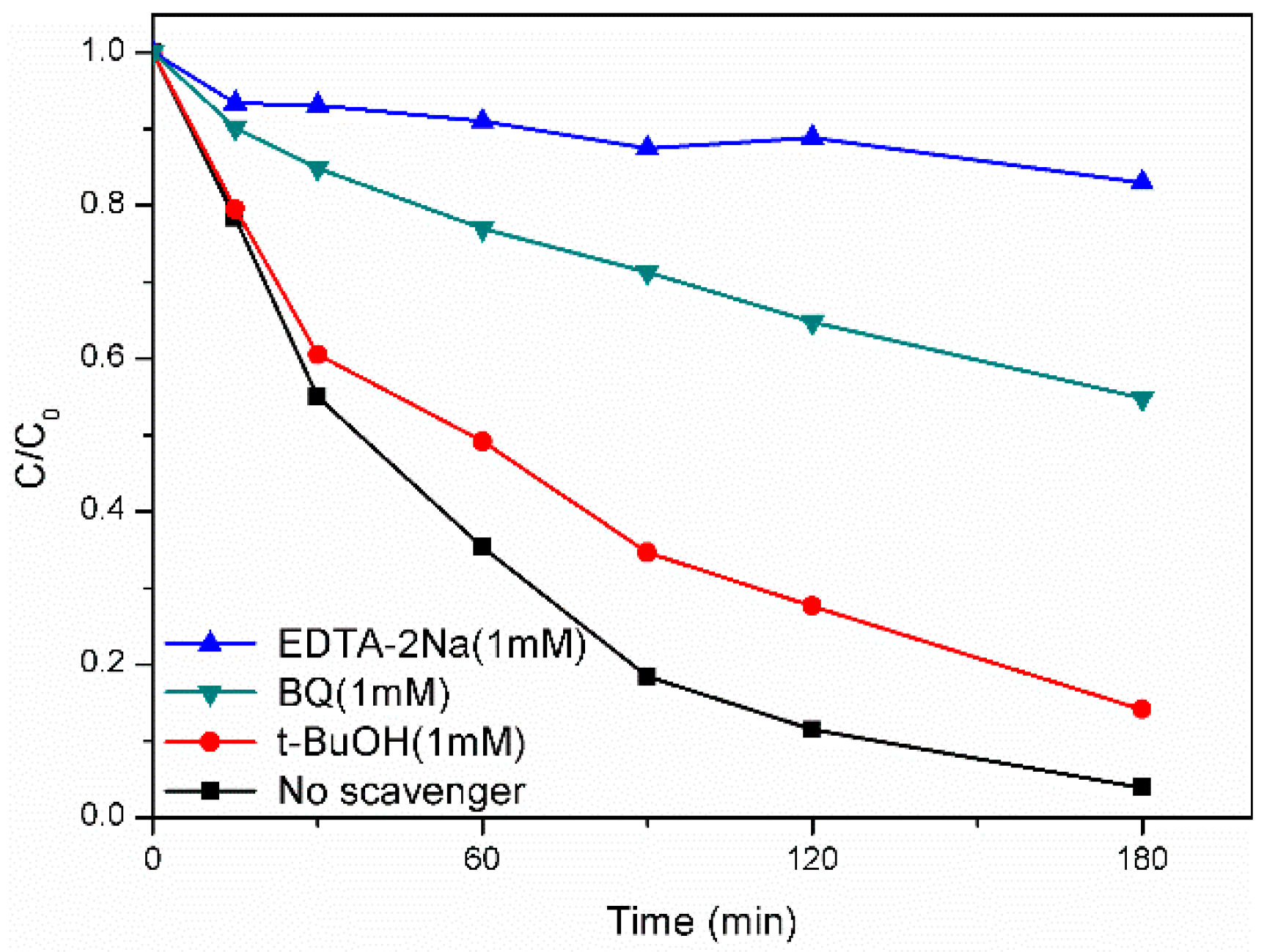
2.4. Photocatalytic Mechanism of AgFeO2
3. Materials and Methods
3.1. Synthesis
3.2. Characterization
3.3. Photocatalytic Evaluation
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ge, S.; Zhang, L. Efficient visible light driven photocatalytic removal of RhB and NO with low temperature synthesized In(OH)xSy hollow nanocubes: A comparative study. Environ. Sci. Technol. 2011, 45, 3027–3033. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, P.; Jin, L.; Shao, T.; Li, Z.; Cao, J. Efficient photocatalytic decomposition of perfluorooctanoic acid by indium oxide and its mechanism. Environ. Sci. Technol. 2012, 46, 5528–5534. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Yang, X. Photocatalytic oxidation for indoor air purification: A literature review. Build. Environ. 2003, 38, 645–654. [Google Scholar] [CrossRef]
- Herrmann, J.M. Heterogeneous photocatalysis: Fundamentals and applications to the removal of various types of aqueous pollutants. Catal. Today 1999, 53, 115–129. [Google Scholar] [CrossRef]
- Xiao, X.; Hu, R.; Liu, C.; Xing, C.; Zuo, X.; Nan, J.; Wang, L. Facile microwave synthesis of novel hierarchical Bi24O31Br10 nanoflakes with excellent visible light photocatalytic performance for the degradation of tetracycline hydrochloride. Chem. Eng. J. 2013, 225, 790–797. [Google Scholar] [CrossRef]
- Andersen, J.; Pelaez, M.; Guay, L.; Zhang, Z.; O’Shea, K.; Dionysiou, D.D. NF-TiO2 photocatalysis of amitrole and atrazine with addition of oxidants under simulated solar light: Emerging synergies, degradation intermediates, and reusable attributes. J. Hazard. Mater. 2013, 260, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Guo, Y.; Faiz, Y.; Yuan, W.T.; Sun, C.; Wang, S.M.; Deng, Y.H.; Zhuang, Y.; Li, Y.; Wang, X.; et al. Facile in-suit synthesis of Ag/AgVO3 one-dimensional hybrid nanoribbons with enhanced performance of plasmonic visible-light photocatalysis. Appl. Catal. B 2015, 163, 288–297. [Google Scholar] [CrossRef]
- Tran, P.D.; Wong, L.H.; Barber, J.; Loo, J.S.C. Recent advances in hybrid photocatalysts for solar fuel production. Energ. Environ. Sci. 2012, 5, 5902–5918. [Google Scholar] [CrossRef]
- Pan, B.; Xie, Q.; Wang, H.; Zhu, J.; Zhang, Y.; Su, W.; Wang, X. Synthesis and photocatalytic hydrogen production of a novel photocatalyst LaCO3OH. J. Mater. Chem. A 2013, 1, 6629–6634. [Google Scholar] [CrossRef]
- Konta, R.; Kato, H.; Kobayashi, H.; Kudo, A. Photophysical properties and photocatalytic activities under visible light irradiation of silver vanadates. Phys. Chem. Chem. Phys. 2003, 5, 3061–3065. [Google Scholar] [CrossRef]
- Feng, M.; Song, J.M.; Li, X.G.; Yu, S.H. Ultralong Silver Trimolybdate Nanowires: Synthesis, Phase Transformation, Stability, and Their Photocatalytic, Optical, and Electrical Properties. ACS Nano 2011, 5, 6726–6735. [Google Scholar] [CrossRef] [PubMed]
- Yi, Z.; Ye, J.; Kikugawa, N.; Kako, T.; Ouyang, S.; Stuart-Williams, H.; Yang, H.; Cao, J.; Luo, W.; Li, Z. An orthophosphate semiconductor with photooxidation properties under visible-light irradiation. Nat. Mater. 2010, 9, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Liu, Y.; Huang, B.; Li, H.; Qin, X.; Zhang, X.; Dai, Y. Preparation, characterization, and photocatalytic properties of silver carbonate. Appl. Surf. Sci. 2011, 257, 8732–8736. [Google Scholar] [CrossRef]
- Yoshihiko Maruyama, H.I.; Hashimoto, K. Visible Light Sensitive Photocatalyst, Delafossite Structured r-AgGaO2. J. Phys. Chem. B 2006, 110, 23274–23278. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Cao, S.; Zhang, J.; Cheng, B.; Yu, J. Effects of the preparation method on the structure and the visible-light photocatalytic activity of Ag2CrO4. Beilstein J. Nanotechnol. 2014, 5, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, H.N.; Talib, A.; Wu, H.F. Facile synthesis of water soluble silver ferrite (AgFeO2) nanoparticles and their biological application as antibacterial agents. RSC Adv. 2015, 5, 34594–34602. [Google Scholar] [CrossRef]
- Shi, Z.; Wang, T.; Lin, H.; Wang, X.; Ding, J.; Shao, M. Excellent surface-enhanced Raman scattering (SERS) based on AgFeO2 semiconductor nanoparticles. Nanoscale 2013, 5, 10029–10033. [Google Scholar] [CrossRef] [PubMed]
- Jessica, K.K.; Durham, L.; Takeuchi, E.S.; Marschilokac, A.C.; Takeuchi, K.J. Synthetic control of composition and crystallite size of silver ferrite composites profound electrochemistry impacts. Chem. Comm. 2015, 51, 5120–5123. [Google Scholar]
- Ong, K.P.; Bai, K.; Blaha, P.; Wu, P. Electronic Structure and Optical Properties of AFeO2 (A = Ag Cu) within GGA Calculations. Chem. Mater. 2007, 19, 634–640. [Google Scholar] [CrossRef]
- Dong, H.; Li, Z.; Xu, X.; Ding, Z.; Wu, L.; Wang, X.; Fu, X. Visible light-induced photocatalytic activity of delafossite AgMO2 (M = Al, Ga, In) prepared via a hydrothermal method. Appl. Catal. B 2009, 89, 551–556. [Google Scholar] [CrossRef]
- Krehula, S.; Musić, S. Formation of AgFeO2, α-FeOOH, and Ag2O from mixed Fe(NO3)3–AgNO3 solutions at high pH. J. Mol. Struct. 2013, 1044, 221–230. [Google Scholar] [CrossRef]
- Farley, K.E.; Marschilok, A.C.; Takeuchi, E.S.; Takeuchi, K.J. Synthesis and Electrochemistry of Silver Ferrite. Electrochem. Solid State Lett. 2012, 15, A23–A27. [Google Scholar] [CrossRef]
- Murthy, Y.L.N.; Kondala Rao, T.; Kasi viswanath, I.V.; Singh, R. Synthesis and characterization of nano silver ferrite composite. J. Magn. Magn. Mater. 2010, 322, 2071–2074. [Google Scholar] [CrossRef]
- William, E.M.; Sheets, C.; Barnabe, A.; Marks, T.J.; Poeppelmeier, K.R. Hydrothermal Synthesis of Delafossite-Type Oxides. Chem. Mater. 2006, 18, 7–20. [Google Scholar]
- Moniz, S.J.; Shevlin, S.A.; Martin, D.J.; Guo, Z.X.; Tang, J. Visible-light driven heterojunction photocatalysts for water splitting—A critical review. Energ. Environ. Sci. 2015, 8, 731–759. [Google Scholar] [CrossRef]
- Li, H.; Hou, W.; Tao, X.; Du, N. Conjugated polyene-modified Bi2MO6 (MMo or W) for enhancing visible light photocatalytic activity. Appl. Catal. B 2015, 172–173, 27–36. [Google Scholar] [CrossRef]
- Omeiri, Y.G.S.; Bouguelia, A.; Trari, M. Photoelectrochemical characterization of the delafossite CuFeO2: Application to removal of divalent metals ions. J. Electroanal. Chem. 2008, 614, 31–40. [Google Scholar] [CrossRef]
- Jeevanandam, P.; Mulukutla, R.; Phillips, M.; Chaudhuri, S.; Erickson, L.; Klabunde, K. Near infrared reflectance properties of metal oxide nanoparticles. J. Phys. Chem. C 2007, 111, 1912–1918. [Google Scholar] [CrossRef]
- Yamashita, H.; Ichihashi, Y.; Zhang, S.G.; Matsumura, Y.; Souma, Y.; Tatsumi, T.; Anpo, M. Photocatalytic decomposition of NO at 275 K on titanium oxide catalysts anchored within zeolite cavities and framework. Appl. Surf. Sci. 1997, 121, 305–309. [Google Scholar] [CrossRef]
- Ohtani, B.; Ogawa, Y.; Nishimoto, S.I. Photocatalytic activity of amorphous-anatase mixture of titanium (IV) oxide particles suspended in aqueous solutions. J. Phys. Chem. B 1997, 101, 3746–3752. [Google Scholar] [CrossRef]
- Carp, O.; Huisman, C.L.; Reller, A. Photoinduced reactivity of titanium dioxide. Prog. Solid State Chem. 2004, 32, 33–177. [Google Scholar] [CrossRef]
- Tanaka, K.; Capule, M.F.; Hisanaga, T. Effect of crystallinity of TiO2 on its photocatalytic action. Chem. Phys. Lett. 1991, 187, 73–76. [Google Scholar] [CrossRef]
- Zhu, Y.P.; Ma, T.Y.; Ren, T.Z.; Li, J.; Du, G.H.; Yuan, Z.Y. Highly dispersed photoactive zinc oxide nanoparticles on mesoporous phosphonated titania hybrid. Appl. Catal. B 2014, 156–157, 44–52. [Google Scholar] [CrossRef]
- Arendt, R. The molten salt synthesis of single magnetic domain BaFe12O19 and SrFe12O19 crystals. J. Solid State Chem. 1973, 8, 339–347. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, W.; Zhang, Z.; Fang, X. High-efficiency visible-light-driven Ag3PO4/AgI photocatalysts: Z-scheme photocatalytic mechanism for their enhanced photocatalytic activity. J. Phys. Chem. C 2013, 117, 19346–19352. [Google Scholar] [CrossRef]
- Li, F.; Wang, X.; Zhao, Y.; Liu, J.; Hao, Y.; Liu, R.; Zhao, D. Ionic-liquid-assisted synthesis of high-visible-light-activated N–B–F-tri-doped mesoporous TiO2 via a microwave route. Appl. Catal. B 2014, 144, 442–453. [Google Scholar] [CrossRef]
- Kim, J.; Lee, C.W.; Choi, W. Platinized WO3 as an environmental photocatalyst that generates OH radicals under visible light. Environ. Sci. Technol. 2010, 44, 6849–6854. [Google Scholar] [CrossRef] [PubMed]






© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, L.; Shi, Y.; Lu, L.; Fang, R.; Wan, X.; Shi, H. A Novel Delafossite Structured Visible-Light Sensitive AgFeO2 Photocatalyst: Preparation, Photocatalytic Properties, and Reaction Mechanism. Catalysts 2016, 6, 69. https://doi.org/10.3390/catal6050069
Yin L, Shi Y, Lu L, Fang R, Wan X, Shi H. A Novel Delafossite Structured Visible-Light Sensitive AgFeO2 Photocatalyst: Preparation, Photocatalytic Properties, and Reaction Mechanism. Catalysts. 2016; 6(5):69. https://doi.org/10.3390/catal6050069
Chicago/Turabian StyleYin, Lu, Yubin Shi, Lian Lu, Rongye Fang, Xiankai Wan, and Huixiang Shi. 2016. "A Novel Delafossite Structured Visible-Light Sensitive AgFeO2 Photocatalyst: Preparation, Photocatalytic Properties, and Reaction Mechanism" Catalysts 6, no. 5: 69. https://doi.org/10.3390/catal6050069