Mechanistic Investigation of the Reduction of NOx over Pt- and Rh-Based LNT Catalysts
Abstract
:1. Introduction
2. Results and Discussion
2.1. Reactivity of NOx Species Stored at 350 °C (Nitrates)
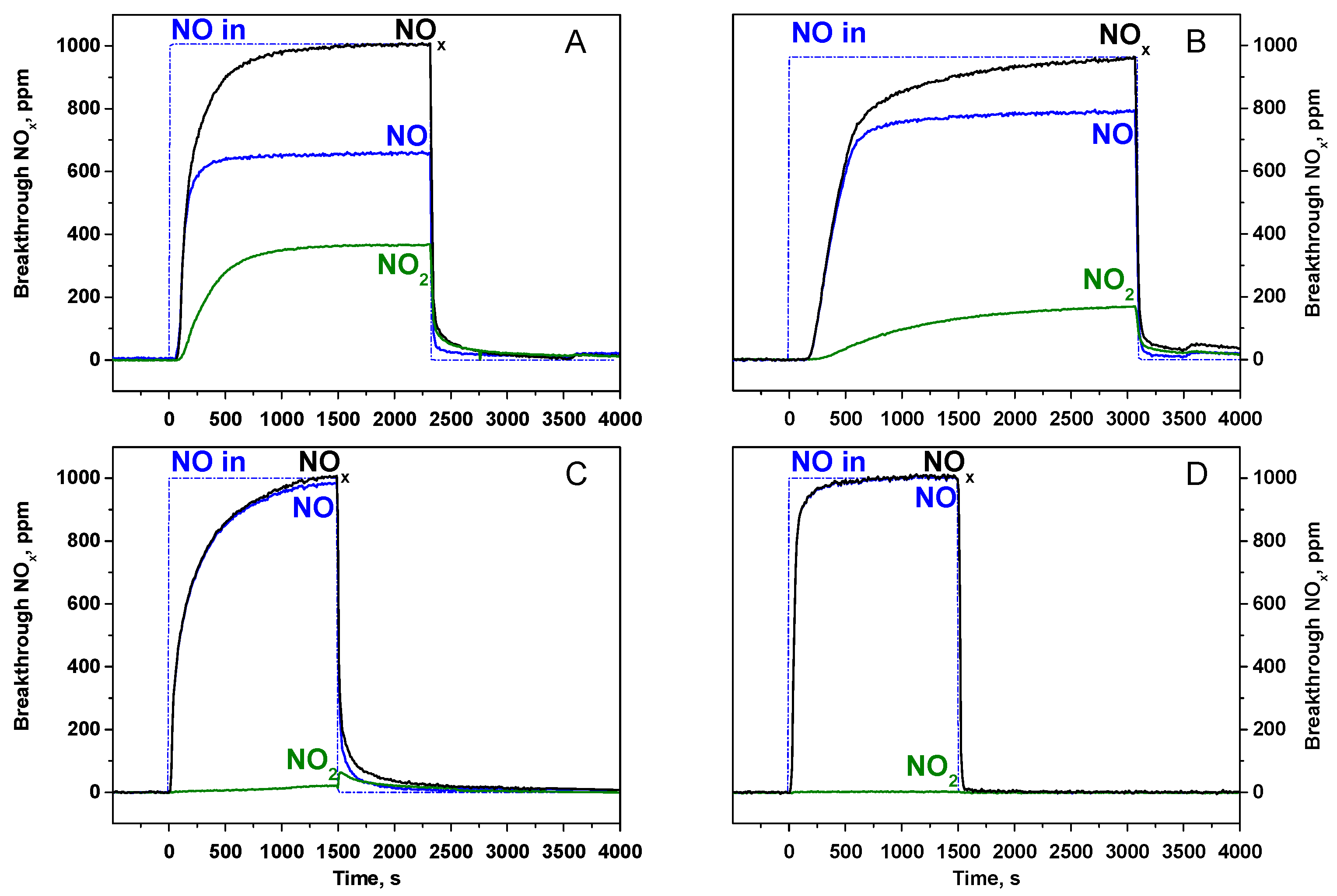
2.1.1. Adsorption of NOx at 350 °C
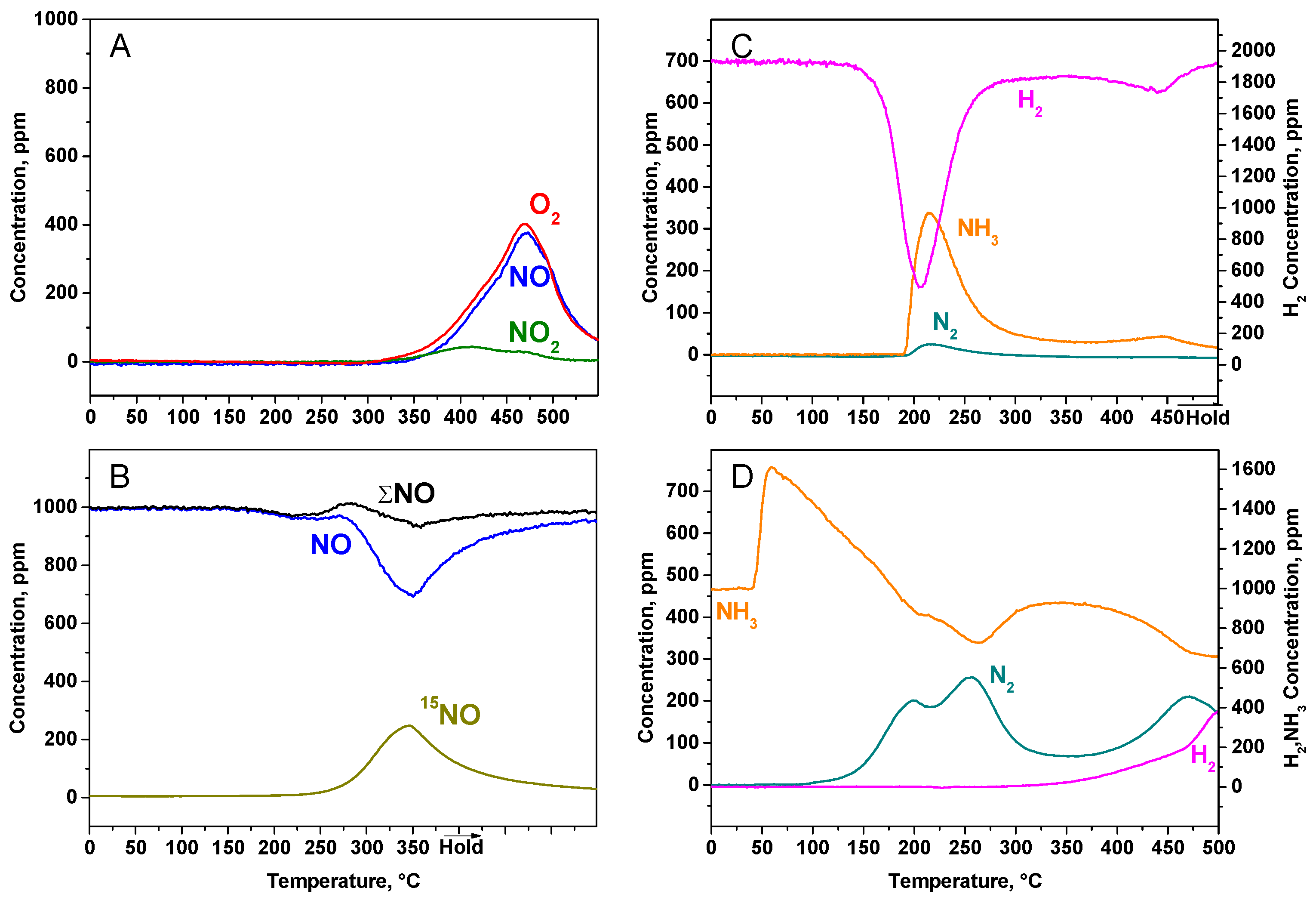
2.1.2. TPD Experiments
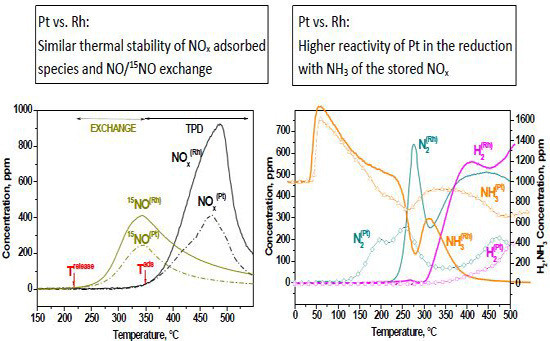
2.1.3. TPIE Experiments
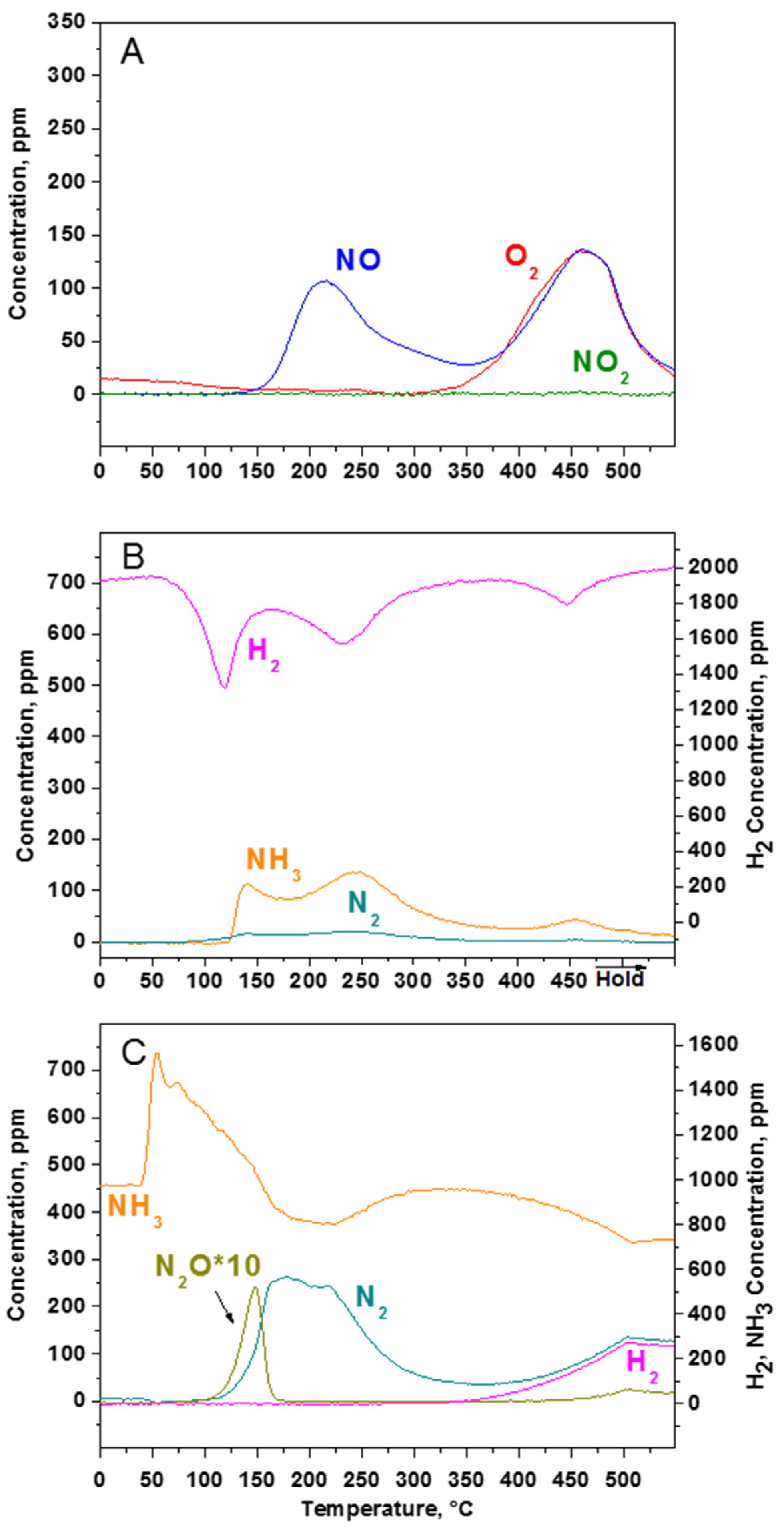
2.1.4. TPSR Experiments
2.2. Reactivity of NOx Species Stored at 150 °C (Nitrites)
2.2.1. Adsorption of NOx at 150 °C
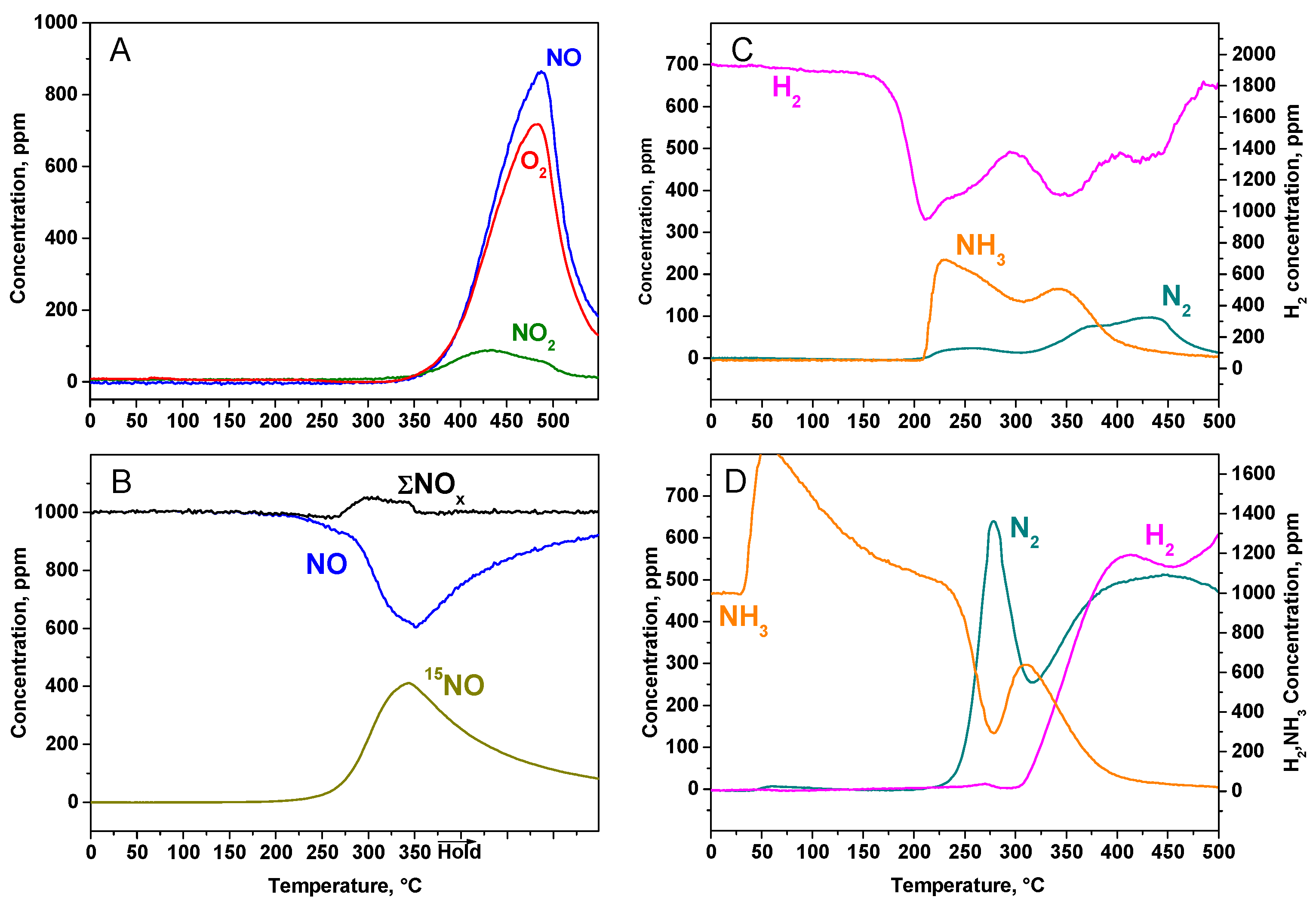
2.2.2. TPD Experiments
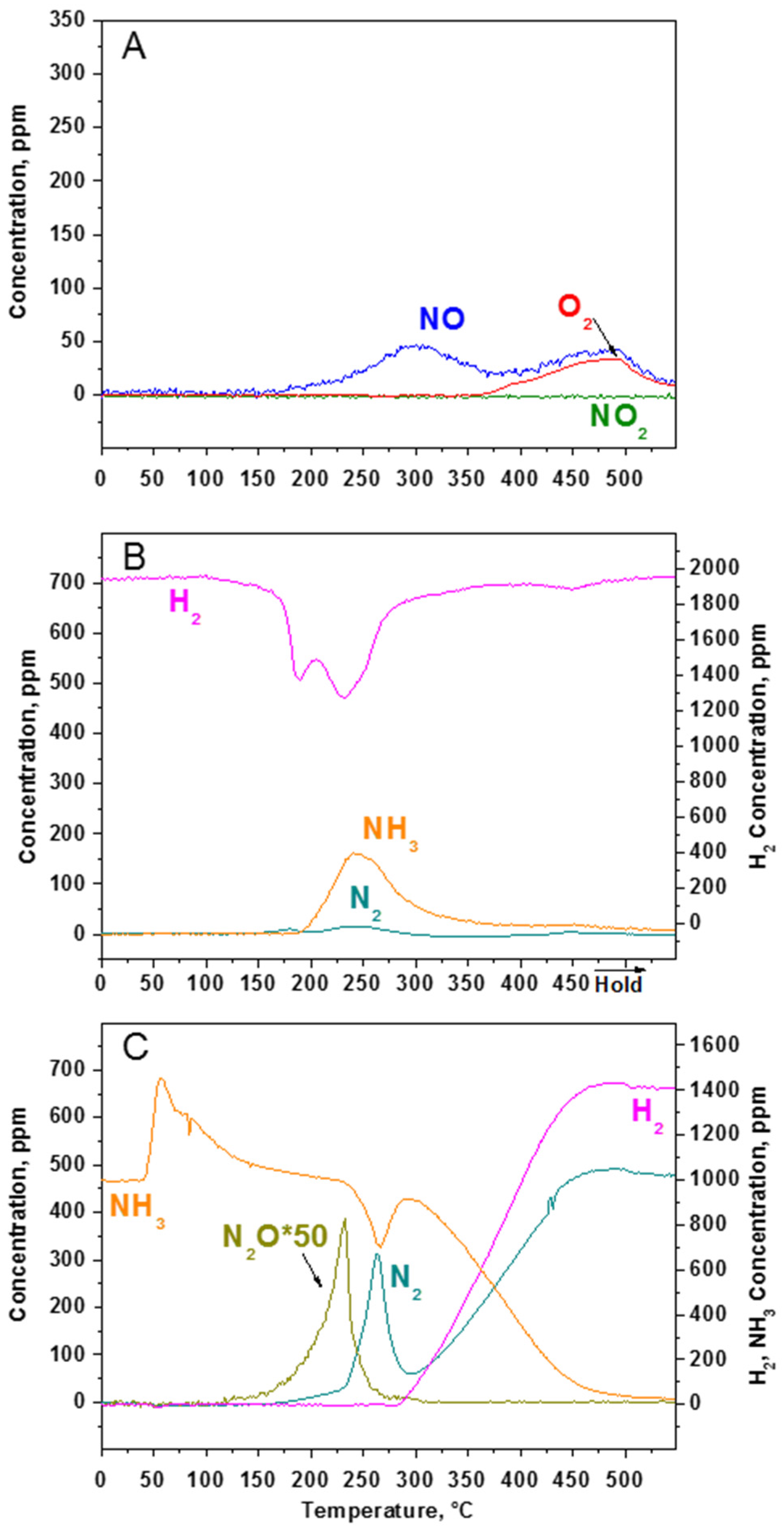
2.2.3. TPSR Experiments
3. Experimental Section
4. Concluding Remarks
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Miyoshi, N.; Matsumoto, S.; Katoh, K.; Tanaka, T.; Harada, J.; Takahashi, N.; Yokota, K.; Sugiura, M.; Kasahara, K. Development of new concept three-way catalyst for automotive lean-burn engines. SAE Tech. Pap. 1995. Available online: http://papers.sae.org/950809/ (accessed on 7 March 2016). [Google Scholar] [CrossRef]
- Takahashi, N.; Shinjoh, H.; Iijima, T.; Suzuki, T.; Yamazaki, K.; Yokota, K.; Suzuki, H.; Miyoshi, N.; Matsumoto, S.; Tanizawa, T.; et al. The new concept 3-way catalyst for automotive lean-burn engine: NOx storage and reduction catalyst. Catal. Today 1996, 27, 63–69. [Google Scholar] [CrossRef]
- Shoji, A.; Kamoshita, S.; Watanabe, T.; Tanaka, T.; Yabe, M. Development of a simultaneous reduction system of NOx and particulate matter for light-duty truck. SAE Tech. Pap. 2004. Available online: http://papers.sae.org/2004-01-0579/ (accessed on 7 March 2016). [Google Scholar] [CrossRef]
- Miller, W.; Klein, J.; Mueller, R.; Doelling, W.; Zuerbig, J. The Development of Urea-SCR Technology for US Heavy Duty Trucks. SAE Tech. Pap. 2000. Available online: http://papers.sae.org/2000-01-0190/ (accessed on 7 March 2016). [Google Scholar] [CrossRef]
- Matsumoto, S. Recent advances in automobile exhaust catalysts. Catal. Today 2004, 90, 183–190. [Google Scholar] [CrossRef]
- Epling, W.S.; Campbell, L.E.; Yezerets, A.; Currier, N.W.; Parks, J.E. Overview of the fundamental reactions and degradation mechanisms of NOx storage/reduction catalysts. Catal. Rev. Eng. 2004, 46, 163–245. [Google Scholar] [CrossRef]
- Nova, I.; Castoldi, L.; Lietti, L.; Tronconi, E.; Forzatti, P.; Prinetto, F.; Ghiotti, G. NOx adsorption study over Pt-Ba/alumina catalysts: FT-IR and pulse experiments. J. Catal. 2004, 222, 377–388. [Google Scholar] [CrossRef]
- Fridell, E.; Skoglundh, M.; Johansson, S.; Smedler, G. NOx Storage in Barium-Containing Catalysts. J. Catal. 1999, 183, 196–209. [Google Scholar] [CrossRef]
- Lietti, L.; Forzatti, P.; Nova, I.; Tronconi, E. NOx Storage Reduction over Pt–Ba/γ-Al2O3 Catalyst. J. Catal. 2001, 204, 175–191. [Google Scholar] [CrossRef]
- Prinetto, F.; Ghiotti, G.; Nova, I.; Lietti, L.; Tronconi, E.; Forzatti, P. FT-IR and TPD investigation of the NOx storage properties of BaO/Al2O3 and Pt–BaO/Al2O3 catalysts. J. Phys. Chem. B 2001, 105, 12732–12745. [Google Scholar] [CrossRef]
- Lesage, T.; Verrier, C.; Bazin, P.; Saussey, J.; Daturi, M. Studying the NOx-trap mechanism over a Pt-Rh/Ba/Al2O3 catalyst by operando FT-IR spectroscopy. Phys. Chem. Chem. Phys. 2003, 5, 4435–4440. [Google Scholar] [CrossRef]
- Prinetto, F.; Ghiotti, G.; Nova, I.; Castoldi, L.; Lietti, L.; Tronconi, E.; Forzatti, P. In situ FT-IR and reactivity study of NOx storage over Pt–Ba/Al2O3 catalysts. Phys. Chem. Chem. Phys. 2003, 5, 4428–4434. [Google Scholar] [CrossRef]
- Westerberg, B.; Fridell, E. A transient FTIR study of species formed during NOx storage in the Pt/BaO/Al2O3 system. J. Mol. Catal. A 2001, 165, 249–263. [Google Scholar] [CrossRef]
- Pihl, J.A.; Parks, J.E., II; Daw, C.S.; Root, T.W. Product selectivity during regeneration of lean NOx trap catalysts. SAE Tech. Pap. 2006. Available online: http://papers.sae.org/2006-01-3441/ (accessed on 7 March 2016). [Google Scholar] [CrossRef]
- Fridell, E.; Persson, H.; Olsson, L.; Skoglundh, M. The mechanism for NOx storage. Catal. Lett. 2000, 66, 71–74. [Google Scholar] [CrossRef]
- Amberntsson, A.; Fridell, E.; Skoglundh, M. Influence of platinum and rhodium composition on the NOx storage and sulphur tolerance of a barium based NOx storage catalyst. Appl. Catal. B 2003, 46, 429–439. [Google Scholar] [CrossRef]
- Salasc, S.; Skoglundh, M.; Fridell, E. A comparison between Pt and Pd in NOx storage catalysts. Appl. Catal. B 2002, 36, 145–160. [Google Scholar] [CrossRef]
- Büchel, R.; Pratsinis, S.E.; Baiker, A. Mono- and bimetallic Rh and Pt NSR-catalysts prepared by controlled deposition of noble metals on support or storage component. Appl. Catal. B 2012, 113–114, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Büchel, R.; Pratsinis, S.E.; Baiker, A. Influence of controlled spatial deposition of Pt and Pd in NOx storage-reduction catalysts on their efficiency. Appl. Catal. B 2011, 101, 682–689. [Google Scholar] [CrossRef]
- Andonova, S.; Marchionni, V.; Borelli, M.; Nedyalkova, R.; Lietti, L.; Olsson, L. Mechanistic investigations of the promoting role of Rh on the NSR performance of NOx storage BaO-based catalysts. Appl. Catal. B 2013, 132–133, 266–281. [Google Scholar] [CrossRef]
- Lietti, L.; Daturi, M.; Blasin-Aubé, V.; Ghiotti, G.; Prinetto, F.; Forzatti, P. Relevance of the nitrite route in the NOx adsorption mechanism over Pt–Ba/Al2O3 NOx storage reduction catalysts investigated by using operando FTIR spectroscopy. ChemCatChem 2012, 4, 55–58. [Google Scholar] [CrossRef]
- Clayton, R.D.; Harold, M.P.; Balakotaiah, V.; Wan, C.Z. Pt dispersion effects during NOx storage and reduction on Pt/BaO/Al2O3 catalysts. Appl. Catal. B 2009, 90, 662–676. [Google Scholar] [CrossRef]
- Nova, I.; Castoldi, L.; Lietti, L.; Tronconi, E. A low temperature pathway operating the reduction of stored nitrates in Pt–Ba/Al2O3 lean NOx trap systems. SAE Tech. Pap. 2006. Available online: http://papers.sae.org/2006-01-1368/ (accessed on 7 March 2016). [Google Scholar] [CrossRef]
- Nova, I.; Lietti, L.; Castoldi, L.; Tronconi, E.; Forzatti, P. New insights in the NOx reduction mechanism with H2 over Pt–Ba/Al2O3 lean NOx trap catalysts under near-isothermal conditions. J. Catal. 2006, 239, 244–254. [Google Scholar] [CrossRef]
- Castoldi, L.; Righini, L.; Matarrese, R.; Lietti, L.; Forzatti, P. Mechanistic aspects of the release and the reduction of NOx stored on Pt–Ba/Al2O3. J. Catal. 2015, 328, 270–279. [Google Scholar] [CrossRef]
- Castoldi, L.; Righini, L.; Kubiak, L.; Matarrese, R.; Morandi, S.; Lietti, L.; Forzatti, P. New insights on the release and reduction of NOx stored over PGM-based LNT catalysts. Top. Catal. 2016. submitted for publication. [Google Scholar]
- Lietti, L.; Nova, I.; Forzatti, P. Role of ammonia in the reduction by hydrogen of NOx stored over Pt–Ba/Al2O3 lean NOx trap catalysts. J. Catal. 2008, 257, 270–282. [Google Scholar] [CrossRef]
- Infantes-Molina, A.; Righini, L.; Castoldi, L.; Loricera, C.V.; Fierro, J.L.G.; Sin, A.; Lietti, L. Characterization and reactivity of Ce-promoted PtBa lean NOx trap catalysts. Catal. Today 2012, 197, 178–189. [Google Scholar] [CrossRef]
- Lietti, L.; Artioli, N.; Righini, L.; Castoldi, L.; Forzatti, P. Pathways for N2 and N2O formation during the reduction of NOx over Pt–Ba/Al2O3 LNT catalysts investigated by labelling isotopic experiments. Ind. Eng. Chem. Res. 2012, 51, 7597–7605. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kubiak, L.; Castoldi, L.; Lietti, L.; Andonova, S.; Olsson, L. Mechanistic Investigation of the Reduction of NOx over Pt- and Rh-Based LNT Catalysts. Catalysts 2016, 6, 46. https://doi.org/10.3390/catal6030046
Kubiak L, Castoldi L, Lietti L, Andonova S, Olsson L. Mechanistic Investigation of the Reduction of NOx over Pt- and Rh-Based LNT Catalysts. Catalysts. 2016; 6(3):46. https://doi.org/10.3390/catal6030046
Chicago/Turabian StyleKubiak, Lukasz, Lidia Castoldi, Luca Lietti, Stanislava Andonova, and Louise Olsson. 2016. "Mechanistic Investigation of the Reduction of NOx over Pt- and Rh-Based LNT Catalysts" Catalysts 6, no. 3: 46. https://doi.org/10.3390/catal6030046