Low-Temperature Oxidation of Dimethyl Ether to Polyoxymethylene Dimethyl Ethers over CNT-Supported Rhenium Catalyst
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effects of PW12 Content on the Performance of 5%Re-PW12/CNTs
2.2. Effects of Reaction Temperature on the Performance of 5%Re-30%PW12/CNTs
2.3. Effects of Gas Hourly Space Velocity on the Performance of 5%Re-30%PW12/CNTs
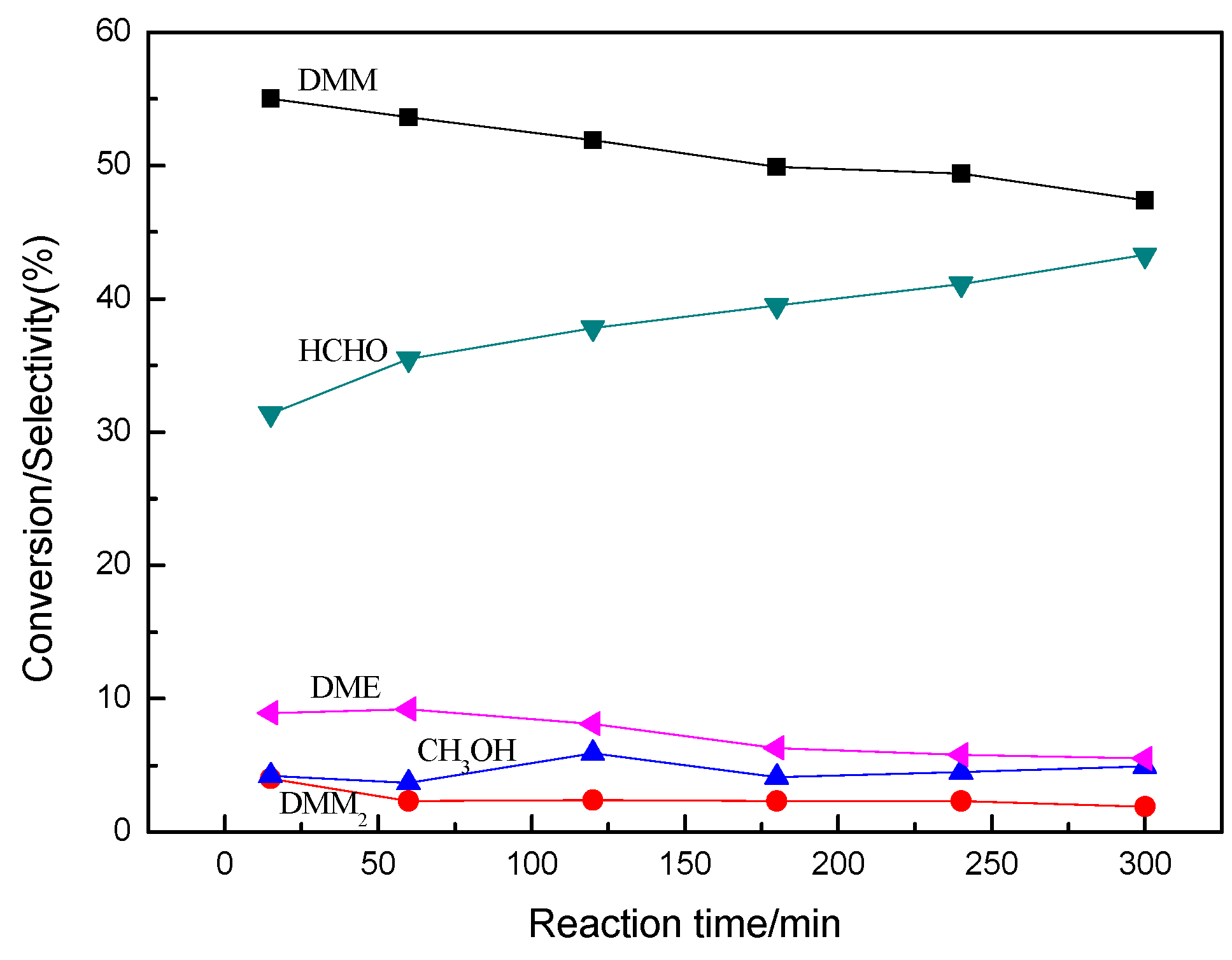
2.4. Effects of Reaction Time on the Performance of 5%Re-30%PW12/CNTs
2.5. Catalyst Characterization
2.5.1. XRD
2.5.2. BET Surface Area
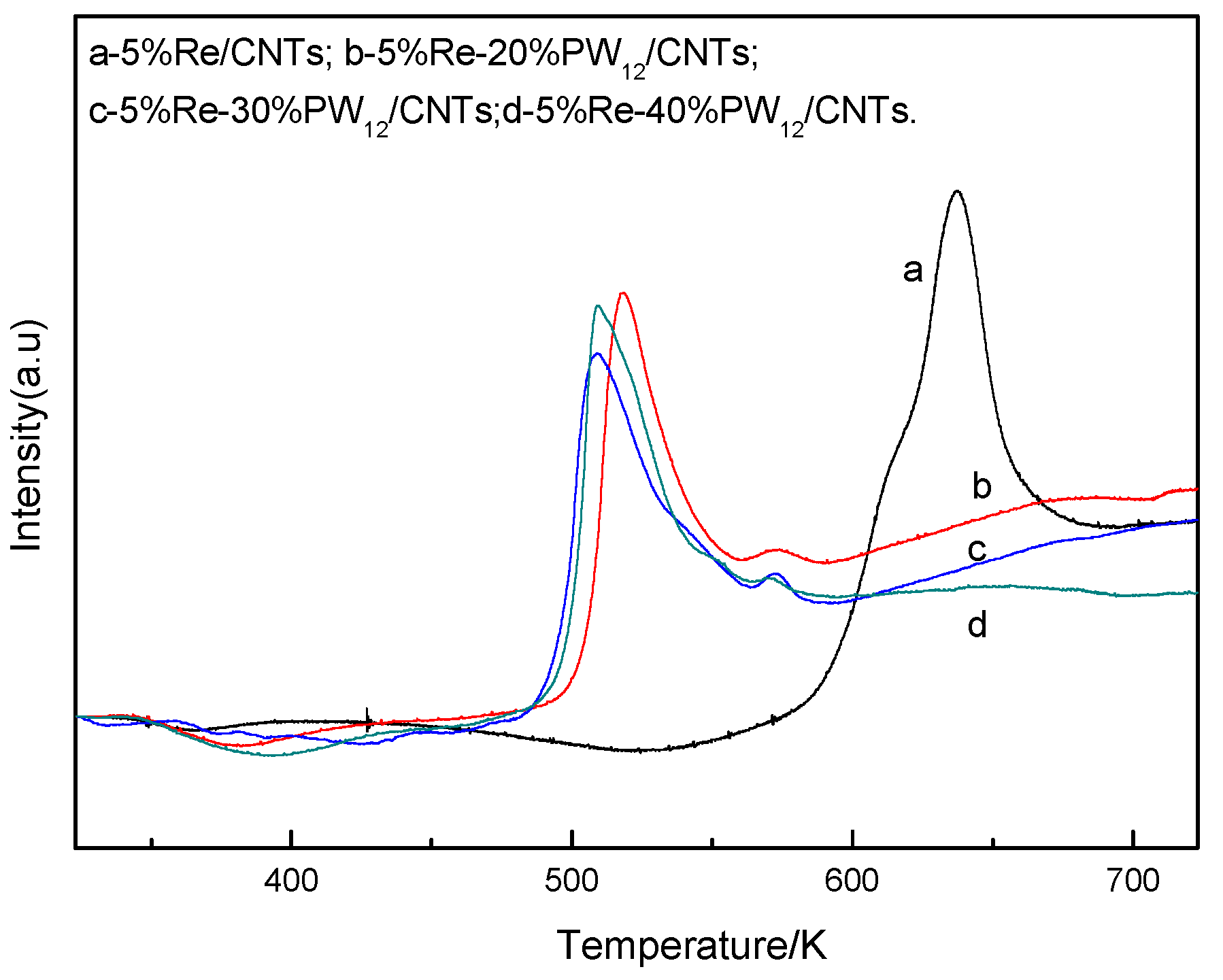
2.5.3. NH3-TPD
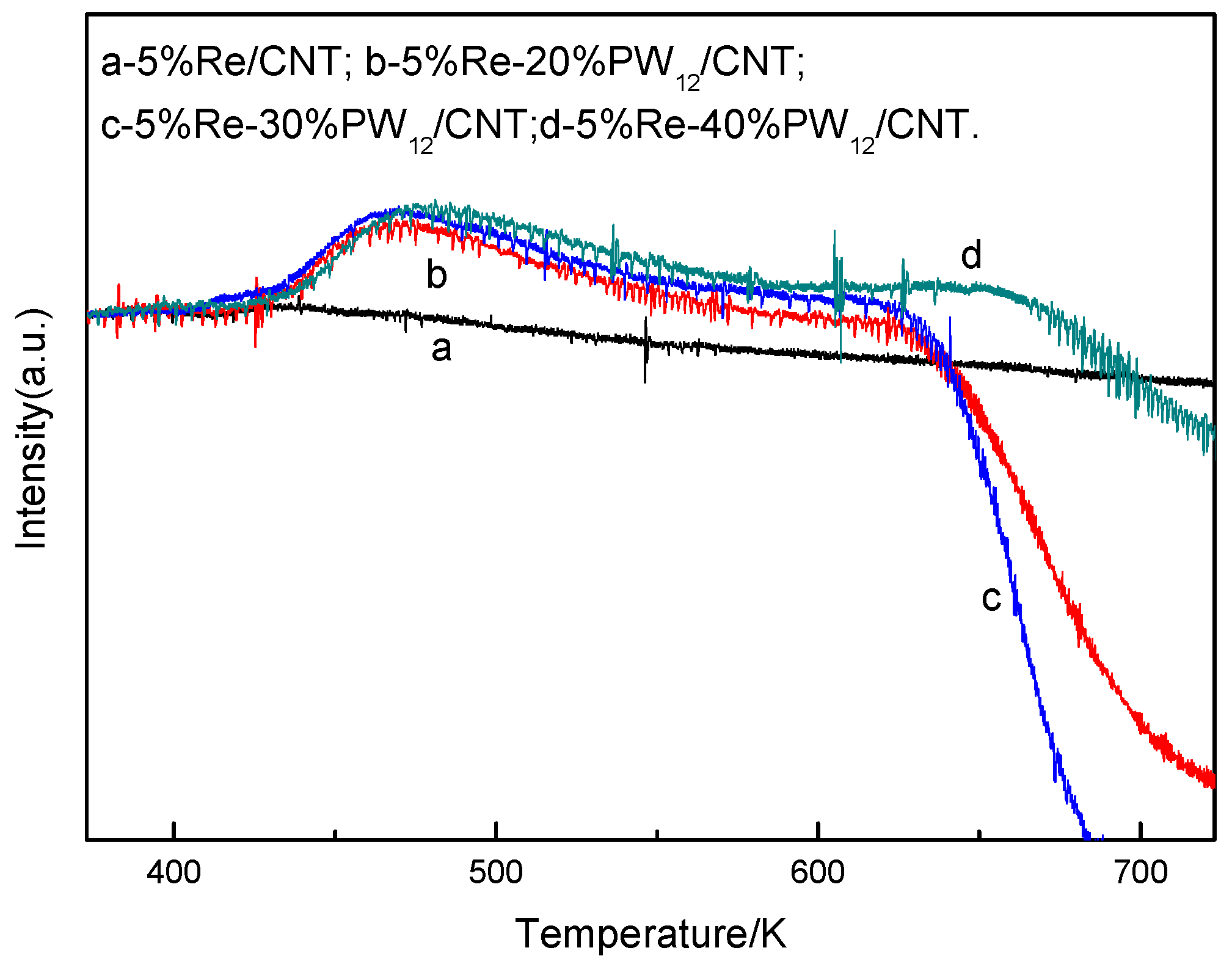
2.5.4. H2-TPR
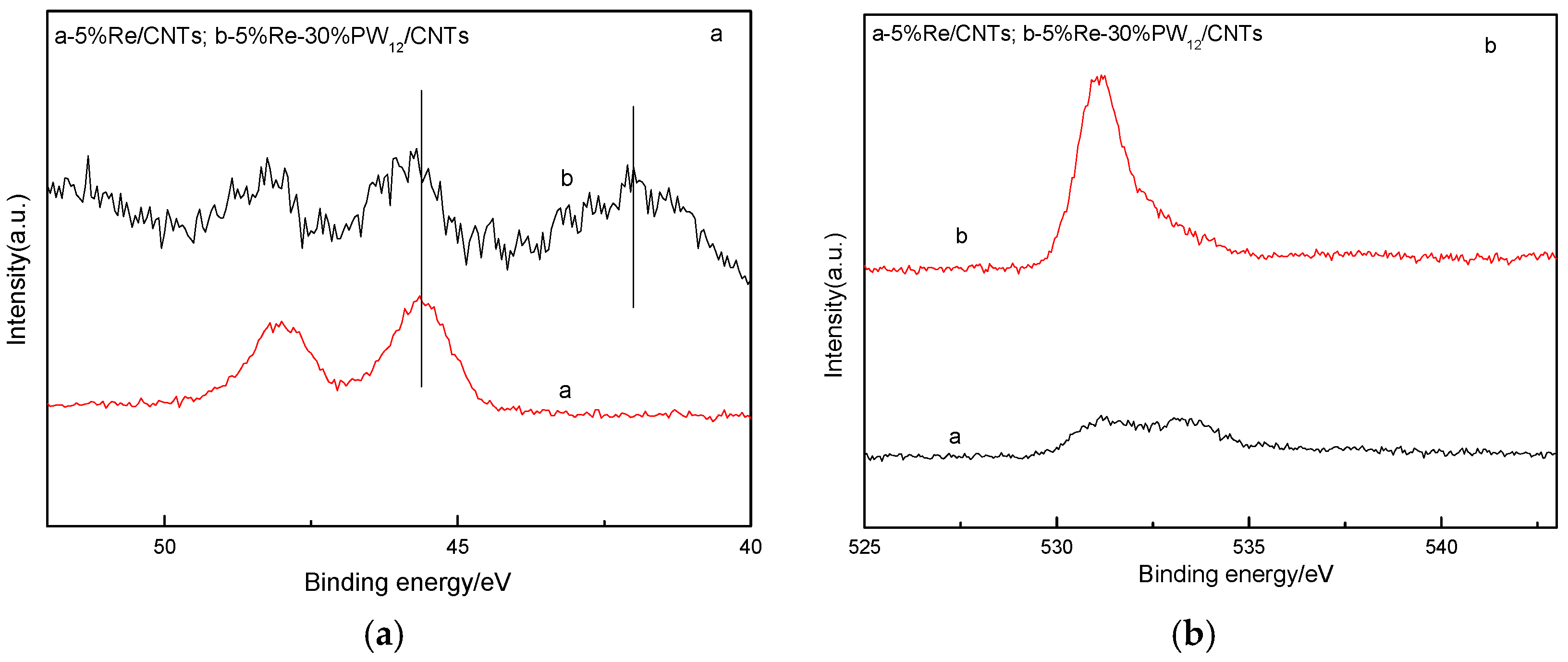
2.5.5. XPS
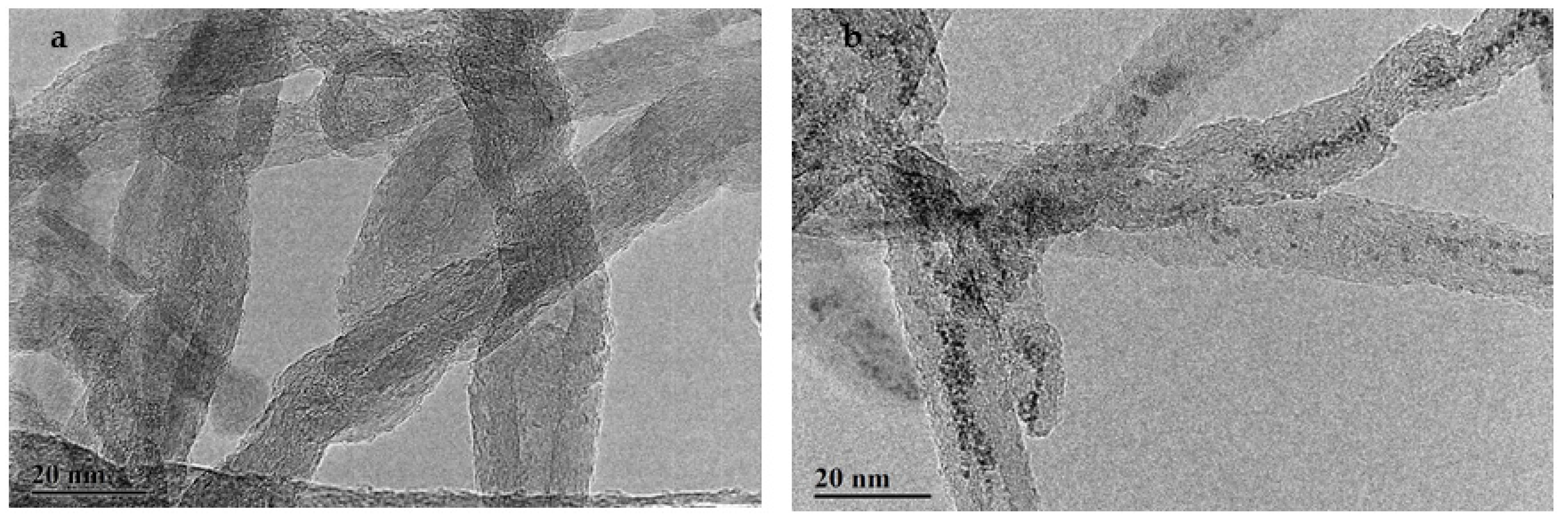
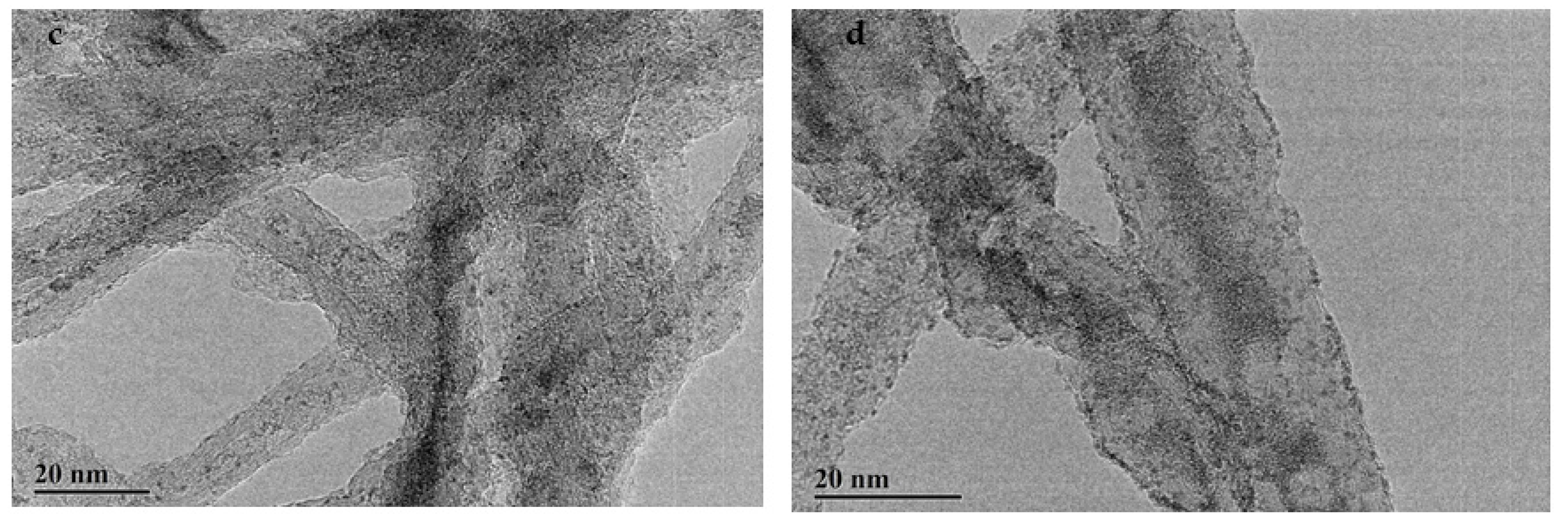
2.5.6. TEM
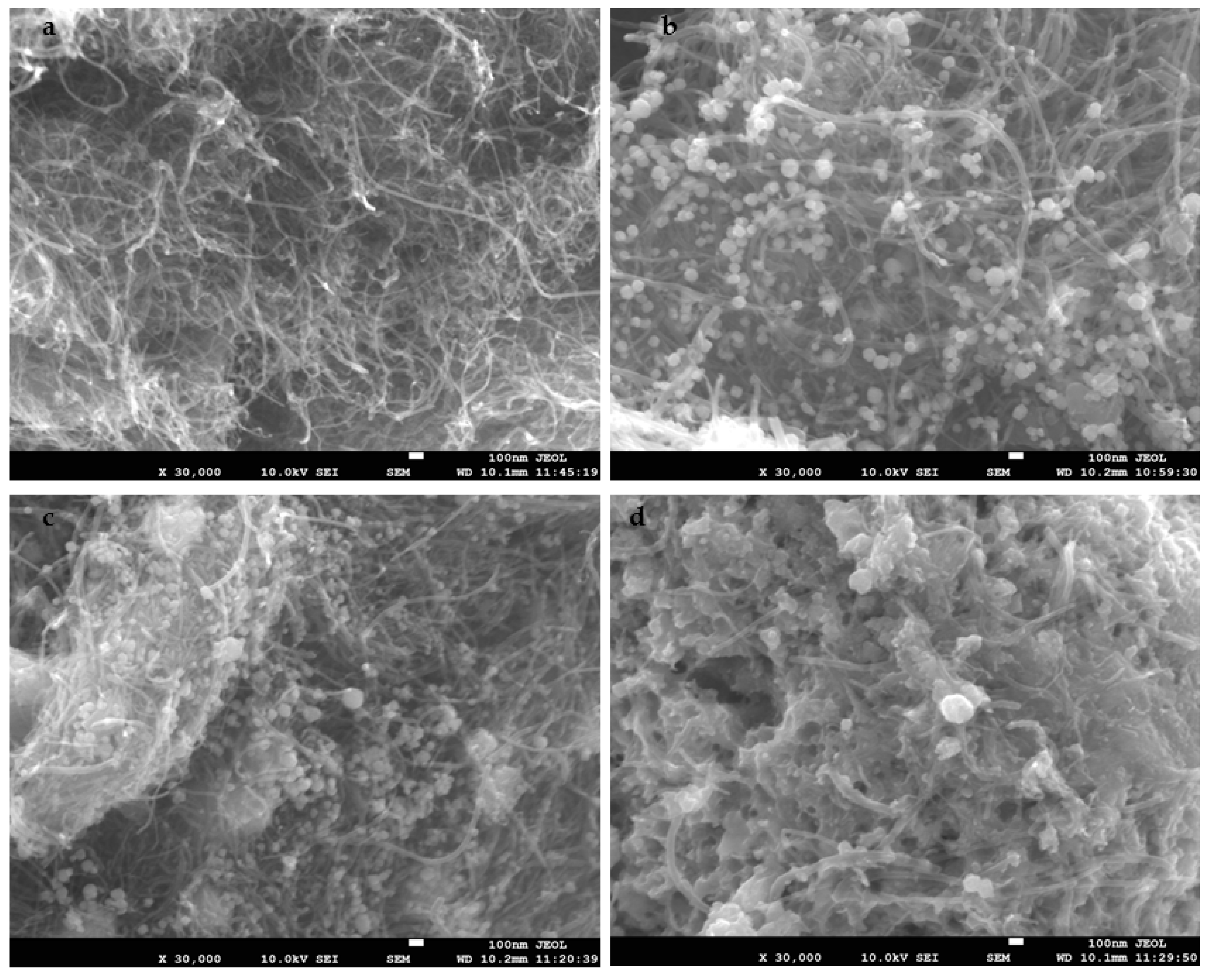
2.5.7. SEM
3. Experimental Section
3.1. Catalyst Preparation
3.2. Catalytic Oxidation of DME
3.3. Structure and Properties Characterization
3.3.1. BET Surface Area
3.3.2. X-ray Diffraction (XRD)
3.3.3. Temperature Programmed Desorption (TPD)
3.3.4. Temperature Programmed Reduction (TPR)
3.3.5. X-ray Photoelectron Spectra (XPS)
3.3.6. Transmission Electron Microscope (TEM)
3.3.7. Scanning Electron Microscope (SEM)
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wu, J.B.; Zhu, H.Q.; Wu, Z.W.; Qin, Z.F.; Yan, L.; Du, B.L.; Fan, W.B.; Wang, J.G. High Si/Al ratio HZSM-5 zeolite: An efficient catalyst for the synthesis of polyoxymethylene dimethyl ethers from dimethoxymethane and trioxymethylene. Green Chem. 2015, 17, 2353–2357. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, H.; Qin, Z.F.; Wu, Z.W.; Wu, J.B.; Fan, W.B.; Wang, J.G. Synthesis of polyoxymethylene dimethyl ethers from methanol and trioxymethylene with molecular sieves as catalysts. J. Fuel Chem. Tech. 2011, 39, 918–923. [Google Scholar] [CrossRef]
- Liu, H.C.; Cheung, P.; Iglesia, E. Structure and support effects on the selective oxidation of dimethyl ether to formaldehyde catalyzed by MoOx domains. J. Catal. 2003, 217, 222–232. [Google Scholar] [CrossRef]
- Guo, H.J.; Sun, W.T.; Haas, F.M.; Farouk, T.; Dryer, F.L.; Ju, Y.G. Measurements of H2O2 in low temperature dimethyl ether oxidation. P. Combust. Inst. 2013, 34, 573–581. [Google Scholar] [CrossRef]
- Liu, H.C.; Iglesia, E. Selective oxidation of dimethyl ether to formaldehyde on small molybdenum oxide domains. J. Catal. 2002, 208, 1–5. [Google Scholar] [CrossRef]
- Yu, L.; Xu, J.Y.; Sun, M.; Wang, X.T. Catalytic oxidation of dimethyl ether to hydrocarbons over SnO2/MgO and SnO2/CaO catalysts. J. Nat. Gas. Chem. 2007, 16, 200–203. [Google Scholar] [CrossRef]
- Zhang, Q.D.; Tan, Y.S.; Yang, C.H.; Han, Y.Z. MnCl2 modified H4SiW12O40/SiO2 catalysts for catalytic oxidation of dimethyl ether to dimethoxymethane. J. Mole. Catal. A 2007, 263, 149–155. [Google Scholar] [CrossRef]
- Liu, G.B.; Zhang, Q.D.; Han, Y.Z.; Tsubaki, N.; Tan, Y.S. Selective oxidation of dimethyl ether to methyl formate over trifunctional MoO3-SnO2 catalyst under mild conditions. Green Chem. 2013, 15, 1501–1504. [Google Scholar] [CrossRef]
- Yagita, H.; Asami, K.; Muramatsu, A. Oxidative dimerization of dimethyl ether with solid catalysts. Appl. Catal. 1989, 53, L5–L9. [Google Scholar] [CrossRef]
- Huang, X.M.; Liu, J.L.; Chen, J.L.; Xu, Y.D.; Shen, W.J. Mechanistic study of selective oxidation of dimethyl ether to formaldehyde over alumina-supported molybdenum oxide catalyst. Catal. Lett. 2006, 108, 79–86. [Google Scholar] [CrossRef]
- Liu, H.C.; Iglesia, E. Selective One-Step Synthesis of dimethoxymethane via methanol or dimethyl ether oxidation on H3+nVnMo12−nPO40 Keggin Structures. J. Phys. Chem. B 2003, 107, 10840–10847. [Google Scholar] [CrossRef]
- Liu, G.B.; Zhang, Q.D.; Han, Y.Z.; Tsubaki, N.; Tan, Y.S. Effects of MoO3 structure of Mo-Sn catalysts on dimethyl ether oxidation to methyl formate under mild conditions. Green Chem. 2015, 17, 1057–1064. [Google Scholar] [CrossRef]
- Zhang, Z.Z.; Zhang, Q.D.; Jia, L.Y.; Wang, W.F.; Zhang, T.; Han, Y.Z.; Tsubaki, N.; Tan, Y.S. Effects of tetrahedral molybdenum oxide species and MoOx domains on the selective oxidation of dimethyl ether under mild conditions. Catal. Sci. Technol. 2016. [Google Scholar] [CrossRef]
- Zhang, Q.D.; Tan, Y.S.; Liu, G.B.; Zhang, J.F.; Han, Y.Z. Rhenium oxide modified H3PW12O40/TiO2 catalysts for selective oxidation of dimethyl ether to dimethoxy dimethyl ether. Green Chem. 2014, 16, 4708–4715. [Google Scholar] [CrossRef]
- Zhang, Q.D.; Tan, Y.S.; Yang, C.H.; Han, Y.Z. Research on catalytic oxidation of dimethyl ether to dimethoxymethane over MnCl2 modified heteropolyacid catalysts. Catal. Commun. 2008, 9, 1916–1919. [Google Scholar] [CrossRef]
- Zhang, Q.D.; Tan, Y.S.; Liu, G.B.; Yang, C.H.; Han, Y.Z. Promotional effects of Sm2O3 on Mn-H4SiW12O40/SiO2 catalyst for dimethyl ether direct-oxidation to dimethoxymethane. J. Ind. Eng. Chem. 2014, 20, 1869–1874. [Google Scholar] [CrossRef]
- Liu, H.C.; Gaigneaux, E.M.; Imoto, H.; Shido, T.; Iwasawa, Y. Novel Re-Sb-O catalysts for the selective oxidation of isobutene and isobutylene. Appl. Catal. A 2000, 202, 251–264. [Google Scholar] [CrossRef]
- Yuan, Y.Z.; Liu, H.C.; Imoto, H.; Shido, T.; Iwasawa, Y. Performance and characterization of a new crystalline SbRe2O6 catalyst for selective oxidation of methanol to methylal. J. Catal. 2000, 195, 51–61. [Google Scholar] [CrossRef]
- Salameh, A.; Joubert, J.; Baudouin, A.; Lukens, W.; Delbecq, F.; Sautet, P.; Basset, J.M.; Coperet, C. CH3ReO3 on γ-Al2O3: Understanding its structure, initiation, and reactivity in olefin metathesis. Angew. Chew. Int. Edit. 2007, 46, 3870–3873. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.Z.; Iwasawa, Y. Performance and characterization of supported rhenium oxide catalysts for selective oxidation of methanol to methylal. J. Phys. Chem. B 2002, 106, 4441–4449. [Google Scholar] [CrossRef]
- Kusakari, T.; Sasaki, T.; Iwasawa, Y. Selective oxidation of benzene to phenol with molecular oxygen on rhenium/zeolite catalysts. Chem. Commun. 2004, 8, 992–993. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.Z.; Shido, T.; Iwasawa, Y. The new catalytic property of supported rhenium oxides for selective oxidation of methanol to methylal. Chem. Commun. 2000, 15, 1421–1422. [Google Scholar] [CrossRef]
- Nikonova, O.A.; Capron, M.; Fang, G.; Faye, J.; Mamede, A.S.; Jalowiecki-Duhamel, L.; Dumeignil, F.; Seisenbaeva, G.A. Novel approach to rhenium oxide catalysts for selective oxidation of methanol to DMM. J. Catal. 2011, 279, 310–318. [Google Scholar] [CrossRef]
- Zhang, F.; Ren, P.J.; Pan, X.L.; Liu, J.Y.; Li, M.R.; Bao, X.H. Self-Assembly of atomically thin and unusual face-centered cubic Re nanowires within carbon nanotubes. Chem. Mater. 2015, 27, 1569–1573. [Google Scholar] [CrossRef]
- Ugarte, D.; Chatelain, A.; DeHeer, W.A. Nanocapillarity and chemistry in carbon nanotubes. Science 1996, 274, 1897–1899. [Google Scholar] [CrossRef]
- Castillejos, E.; Debouttiere, P.J.; Roiban, L.; Solhy, A.; Martinez, V.; Kihn, Y.; Ersen, O.; Philippot, K.; Chaudret, B.; Serp, P. An efficient strategy to drive nanoparticles into carbon nanotubes and the remarkable effect of confinement on their catalytic performance. Angew. Chem. Int. Edit. 2009, 48, 2529–2533. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Pan, X.L.; Hu, Y.F.; Yu, L.; Chen, X.Q.; Jiang, P.; Zhang, H.B.; Deng, S.B.; Bolin, J.T.B.; Zhang, S.; et al. Tuning the redox activity of encapsulated metal clusters via the metallic and semiconducting character of carbon nanotubes. P. Natl. Acad. Sci. USA 2013, 110, 14861–14866. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.; Su, D.S. Metal-free carbon catalysts for oxidative dehydrogenation reactions. ACS Catal. 2014, 4, 3212–3218. [Google Scholar] [CrossRef]
- Chen, S.; Wang, S.P.; Ma, X.B.; Gong, J.L. Selective oxidation of methanol to dimethoxymethane over bifunctional VOx/TS-1 catalysts. Chem. Commun. 2011, 47, 9345–9347. [Google Scholar] [CrossRef] [PubMed]

| Catalysts | DME Conversion (%) | Selectivity (C-mol%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| DMM | DMM2 | CH3OH | HCHO | MF | CO | CH4 | CO2 | ||
| Re/CNTs | 4.2 | 6.6 | 0 | 16.2 | 73.7 | 3.5 | 0 | 0 | 0 |
| Re-5%PW12/CNTs | 4.9 | 26.3 | 0.3 | 2.7 | 67.7 | 3.0 | 0 | 0 | 0 |
| Re-20%PW12/CNTs | 6.1 | 45.9 | 4.1 | 2.6 | 45.0 | 2.4 | 0 | 0 | 0 |
| Re-30%PW12/CNTs | 8.9 | 55.0 | 4.0 | 4.2 | 31.4 | 5.4 | 0 | 0 | 0 |
| Re-40%PW12/CNTs | 9.5 | 50.7 | 3.7 | 4.4 | 38.9 | 2.3 | 0 | 0 | 0 |
| Re-80%PW12/CNTs | 15.0 | 27.5 | 1.7 | 3.5 | 2.1 | 0.8 | 64.4 | 0 | 0 |
| 30%PW12/CNTs | 10.0 | 32.7 | 13.9 | 16.8 | 21.4 | 0.6 | 14.6 | 0 | 0 |
| Reaction Temperature (K) | DME Conversion (%) | Selectivity (C-mol%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| DMM | DMM2 | CH3OH | HCHO | MF | CO | CH4 | CO2 | ||
| 493 | 6.6 | 17.0 | 2.8 | 16.5 | 62.0 | 1.7 | 0 | 0 | 0 |
| 513 | 8.9 | 55.0 | 4.0 | 4.2 | 31.4 | 5.4 | 0 | 0 | 0 |
| 533 | 10.9 | 45.7 | 4.1 | 4.1 | 31.1 | 1.8 | 13.2 | 0 | 0 |
| 553 | 12.3 | 43.8 | 3.7 | 3.9 | 26.0 | 0.7 | 21.9 | 0 | 0 |
| GHSV (h−1) | DME Conversion (%) | Selectivity (C-mol%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| DMM | DMM2 | CH3OH | HCHO | MF | CO | CH4 | CO2 | ||
| 1200 | 9.8 | 28.3 | 2.0 | 28.2 | 34.2 | 7.3 | 0 | 0 | 0 |
| 1800 | 8.9 | 55.0 | 4.0 | 4.2 | 31.4 | 5.4 | 0 | 0 | 0 |
| 2400 | 5.0 | 35.7 | 5.1 | 8.8 | 50.4 | 0 | 0 | 0 | 0 |
| 3000 | 3.4 | 34.9 | 0.1 | 9.3 | 55.7 | 0 | 0 | 0 | 0 |
| Catalysts | BET Surface Area | Total Pore Volume | Average Pore Diameter |
|---|---|---|---|
| A (m2·g−1) | v (cm3·g−1) | d (nm) | |
| 5%Re/CNTs | 217.4 | 1.054 | 19.399 |
| 5%Re-30%PW12/CNTs | 90.9 | 0.467 | 20.539 |
| 5%Re-30%PW12/CNTs after reaction | 81.1 | 0.523 | 25.795 |
| 5%Re-80%PW12/CNTs | 15.2 | 0.062 | 16.402 |
| Catalysts | Weak Acid Sites Area(S1) | Strong Acid Sites Area(S2) | Ratio S1/S2 |
|---|---|---|---|
| 5%Re/CNTs | 100 | - | - |
| 5%Re-20%PW12/CNTs | 89.8 | 10.2 | 8.8 |
| 5%Re-30%PW12/CNTs | 90.0 | 10.0 | 9.0 |
| 5%Re-40%PW12/CNTs | 86.9 | 13.1 | 6.6 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Wang, W.; Zhang, Z.; Han, Y.; Tan, Y. Low-Temperature Oxidation of Dimethyl Ether to Polyoxymethylene Dimethyl Ethers over CNT-Supported Rhenium Catalyst. Catalysts 2016, 6, 43. https://doi.org/10.3390/catal6030043
Zhang Q, Wang W, Zhang Z, Han Y, Tan Y. Low-Temperature Oxidation of Dimethyl Ether to Polyoxymethylene Dimethyl Ethers over CNT-Supported Rhenium Catalyst. Catalysts. 2016; 6(3):43. https://doi.org/10.3390/catal6030043
Chicago/Turabian StyleZhang, Qingde, Wenfeng Wang, Zhenzhou Zhang, Yizhuo Han, and Yisheng Tan. 2016. "Low-Temperature Oxidation of Dimethyl Ether to Polyoxymethylene Dimethyl Ethers over CNT-Supported Rhenium Catalyst" Catalysts 6, no. 3: 43. https://doi.org/10.3390/catal6030043






