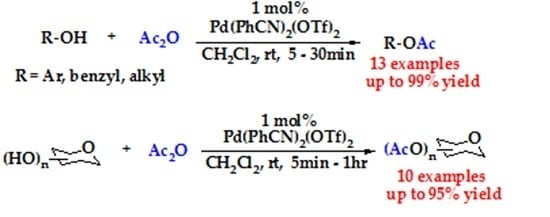
Highly Efficient Cationic Palladium Catalyzed Acetylation of Alcohols and Carbohydrate-Derived Polyols
Abstract
:1. Introduction
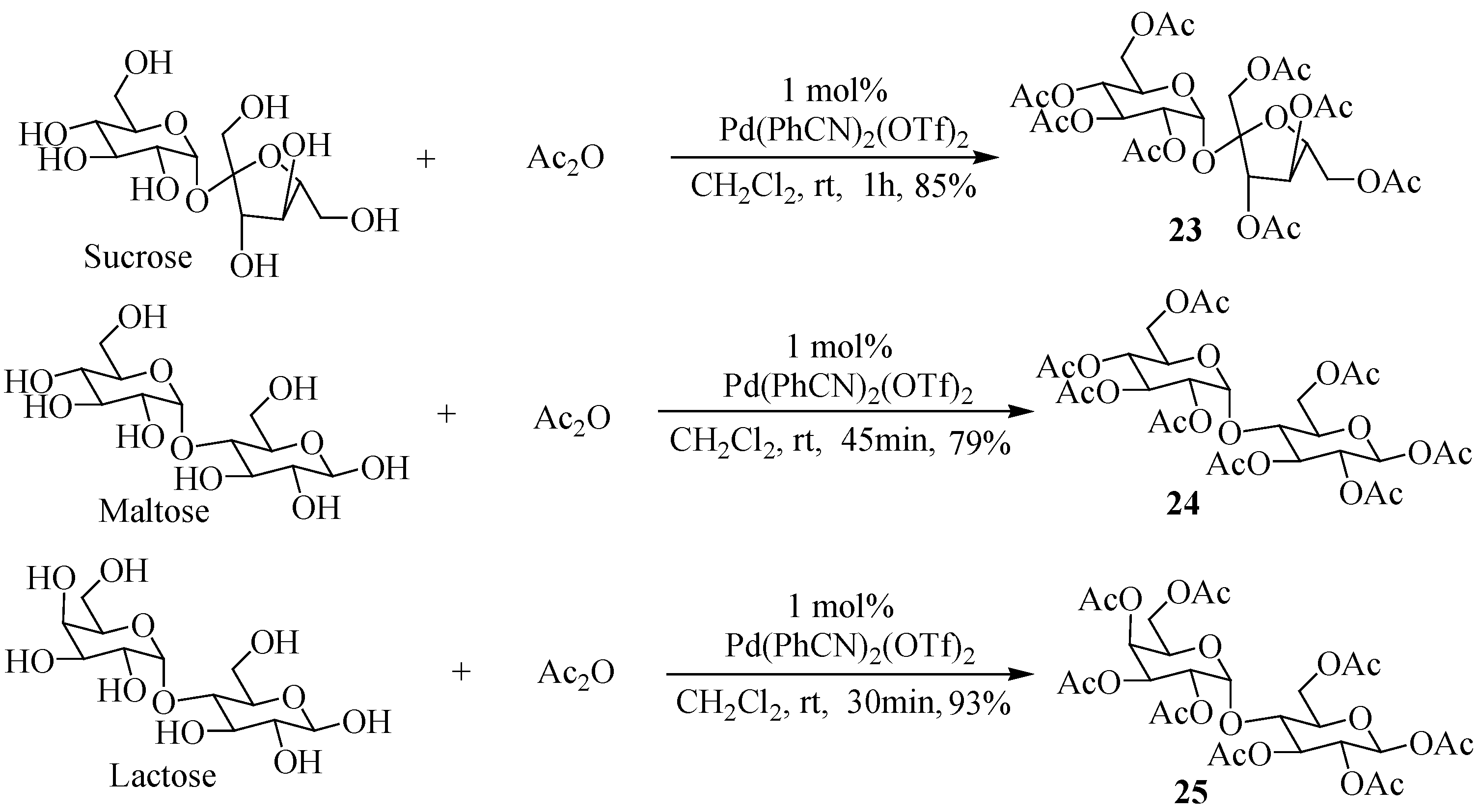
2. Results and Discussion
| Entry | Catalyst | Loading (mol %) | Temperature | Time (min) | Yield (%) b |
|---|---|---|---|---|---|
| 1 | Pd(CH3CN)4(BF4)2 | 10 | 25 °C | 30 | 75 |
| 2 | Pd(CH3CN)4(BF4)2 | 5 | 25 °C | 30 | 72 |
| 3 | Pd(PhCN)2(OTf)2 a | 10 | 25 °C | 5 | 95 |
| 4 | Pd(PhCN)2(OTf)2 a | 7 | 25 °C | 5 | 93 |
| 5 | Pd(PhCN)2(OTf)2 a | 5 | 25 °C | 5 | 96 |
| 6 | Pd(PhCN)2(OTf)2 a | 2 | 25 °C | 5 | 95 |
| 7 | Pd(PhCN)2(OTf)2 a | 1 | 25 °C | 5 | 95 |
| Entry | Catalyst | Catalyst Loading (mol %) | Time (min) | Yield (%) | References |
|---|---|---|---|---|---|
| 1 | RuCl3 | 5 | 10 | 95 | [18] |
| 2 | Cu(OTf)2 | 2 | 30 | 97 | [16] |
| 3 | Bi(TFA)3 | 5 | 60 | 96 | [37] |
| 4 | Cp2ZrCl2 | 1 | 600 | 93 | [38] |
| 5 | Pd(PhCN)2(OTf)2 | 1 | 5 | 95 | This work |
| Entry | Substrate | Product | Time (min) | Yield (%) b |
|---|---|---|---|---|
| 1 |  |  | 5 | 95 |
| 2 |  |  | 10 | 98 |
| 3 |  |  | 10 | 94 |
| 4 |  |  | 30 | 92 |
| 5 |  |  | 25 | 90 |
| 6 |  |  | 30 | 86 |
| 7 |  |  | 10 | 95 |
| 8 |  |  | 6 | 93 |
| 9 |  |  | 5 | 92 |
| 10 |  |  | 10 | 82 |
| 11 |  |  | 15 | 99 |
| 12 |  |  | 10 | 78 |
| 13 |  |  | 9 | 92 |
| Entry | Substrate | Product | Yield (%) c |
|---|---|---|---|
| 1 |  |  | 94 |
| 2 |  |  | 95 |
| 3 |  |  | 82 |
| 4 |  |  | 90 |
| 5 |  |  | 86 |
| 6 |  |  | 82 |
| 7 |  |  | 85 |
| Entry | Catalyst | Loading | Additive | Time | Yield (%) c |
|---|---|---|---|---|---|
| 1 | No catalyst | - | - | 5 h | < 1 |
| 2 | Pd(PhCN)2Cl2 | 1 mol% | - | 5 h | < 1 |
| 3 | AgOTf | 2 mol% | - | 5 h | 5 |
| 4 | Pd(PhCN)2(OTf)2 a | 1 mol% | - | 5 min | 95 |
| 5 | Pd(PhCN)2(OTf)2 a | 1 mol% | DTBP b | 2 h | 90 |
| 6 | TfOH | 2 mol% | - | 8 min | 86 |
| 7 | Pd(PhCN)2(OTf)2 a | 1 mol% | Hg(0) | 5 min | 93 |
| 8 | Pd(CH3CN)4(OTf)2 | 1 mol% | - | 5 min | 92 |
3. Experimental Section
3.1. Materials and Methods
3.2. Typical Experimental Procedure for Acetylation of Alcohols
4. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Mojtahedi, M.M.; Samadian, S. Efficient and Rapid Solvent-Free Acetylation of Alcohols, Phenols, and Thiols Using Catalytic Amounts of Sodium Acetate Trihydrate. J. Chem. 2013, 2013, 1–7. [Google Scholar] [CrossRef]
- Petursson, S. Diarylmethyl ethers for the protection of polyols. J. Chem. 2013, 2013. Article ID 183049:1–183049:10. [Google Scholar] [CrossRef]
- Shirini, F.; Zolfigol, M.A.; Safari, A. A mild and efficient method for the acetylation of alcohols. Indian J. Chem. 2005, 44B, 201–203. [Google Scholar] [CrossRef]
- Heravi, M.M.; Behbahani, F.K.; Bamoharram, F.F. Acetylation of alcohols, phenols and salicylic acid by heteropoly acids in acetic anhydride: A green and eco-friendly protocol for synthesis of acetyl salicylic acid (Aspirin). ARKIVOC 2007, xvi, 123–131. [Google Scholar]
- Zhdanov, R.I.; Zhenodarova, S.M. A Mild and Efficient Method for the Acetylation of Alcohols. Synthesis 1975, 44B, 222. [Google Scholar]
- Vedejs, E.; Bennett, N.S.; Conn, L.M.; Diver, S.T.; Gingras, M.; Lin, S.; Oliver, P.A.; Peterson, M.J. Tributylphosphine-catalyzed acylations of alcohols: Scope and related reactions. J. Org. Chem. 1993, 58, 7286–7288. [Google Scholar] [CrossRef]
- Vedejs, E.; Diver, S.T. Tributylphosphine: A remarkable Acylation Catalyst. J. Am. Chem. Soc. 1993, 115, 3358–3359. [Google Scholar] [CrossRef]
- Khan, A.T.; Choudhury, L.H.; Ghosh, S. Acetonyltriphenylphosphonium Bromide (ATPB): A Versatile Reagent for the Acylation of Alcohols, Phenols, Thiols and Amines and for 1,1-Diacylation of Aldehydes under Solvent-Free Conditions. Eur. J. Org. Chem. 2005, 2782–2787. [Google Scholar] [CrossRef]
- For p-Toluenesulfonic acid refer to Cope, A.C.; Herrich, E.C. Org. Synth. Coll. 1963, 4, 304.
- Breton, G.W.; Kurtz, M.J.; Kurtz, S.L. Acetylation of unsymmetrical diols in the presence of Al2O3. Tetrahedron Lett. 1997, 38, 3825–3828. [Google Scholar] [CrossRef]
- Ishihara, K.; Kubota, M.; Kurihara, H.; Yamamoto, H. Scandium Trifluoromethanesulfonate as an extremely active lewis acid catalyst in acylation of alcohols with acid anhydrides and mixed anhydrides. J. Org. Chem. 1996, 61, 4560–4567. [Google Scholar] [CrossRef] [PubMed]
- Bizier, N.P.; Atkins, S.R.; Helland, L.C.; Colvin, S.F.; Twitchell, J.R.; Cloninger, M.J. Indium triflate catalyzed peracetylation of carbohydrates. Carbohydr. Res. 2008, 343, 1814–1818. [Google Scholar]
- Chauhan, K.K.; Frost, C.G.; Love, I.; Waite, D. Indium triflate: An efficient catalyst for acylation reactions. Synlett 1999, 1743–1744. [Google Scholar] [CrossRef]
- Orita, A.; Tanahashi, C.; Kakuda, A.; Otera, J. Highly Efficient and versatile acylation of alcohols with Bi(OTf)3 as catalyst. Angew. Chem. Int. Ed. 2000, 39, 2877–2879. [Google Scholar]
- Procopiou, P.A.; Baugh, S.P.D.; Flack, S.S.; Inglis, G.G.A. An extremely fast and efficient acylation reaction of alcohols with acid anhydrides in the presence of trimethylsilyl trifluoromethanesulfonate as catalyst. Chem. Commun. 1996, 23, 2625–2626. [Google Scholar] [CrossRef]
- Saravanan, P.; Singh, V.K. An efficient method for acylation reactions. Tetrahedron Lett. 1999, 40, 2611–2614. [Google Scholar] [CrossRef]
- Dalpozzo, R.; De Nino, A.; Maiuolo, L.; Procopio, A.; Nardi, M.; Bartoli, M.; Romeo, R. Highly efficient and versatile acetylation of alcohols catalyzed by cerium(III) triflate. Tetrahedron Lett. 2003, 44, 5621–5624. [Google Scholar] [CrossRef]
- De, S.K. Ruthenium(III) chloride catalyzed acylation of alcohols, phenols, thiols, and amines. Tetrahedron Lett. 2004, 45, 2919–2922. [Google Scholar] [CrossRef]
- Jin, T.S.; Ma, Y.R.; Zhang, Z.H.; Li, T.S. Sulfamic acid catalysed acetylation of alcohols and phenols with acetic anhydride. Synth. Commun. 1998, 28, 3173–3177. [Google Scholar] [CrossRef]
- Bhaskar, P.M.; Loganathan, D. Per-O-acetylation of sugars catalysed by montmorillonite K-10. Tetrahedron Lett. 1998, 39, 2215–2218. [Google Scholar] [CrossRef]
- Adinolfi, M.; Barone, G.; Iadonisi, A.; Schiattarella, M. An easy approach for the acetylation of saccharidic alcohols. Applicability for regioselective protections. Tetrahedron Lett. 2003, 44, 4661–4663. [Google Scholar] [CrossRef]
- Dasgupta, F.; Singh, P.P.; Srivastava, H.C. Acetylation of carbohydrates using ferric chloride in acetic anhydride. Carbohydr. Res. 1980, 80, 346–349. [Google Scholar] [CrossRef]
- Pansare, S.V.; Malusara, M.G.; Rai, A.N. Magnesium bromide catalysed acylation of alcohols. Synth. Commun. 2000, 30, 2587–2592. [Google Scholar] [CrossRef]
- Chandrasekhar, S.; Ramachander, T.; Takhi, M. Acylation of alcohols with acetic anhydride catalyzed by TaCl5: Some implications in kinetic resolution. Tetrahedron Lett. 1998, 39, 3263–3266. [Google Scholar] [CrossRef]
- Choudary, B.M.; Kantam, M.L.; Neeraja, V.; Bandyopadhyay, T.; Reddy, P.N. Vanadyl(IV) acetate, a new reusable catalyst for acetylation of alcohols. J. Mol. Catal. A 1999, 140, 25–29. [Google Scholar] [CrossRef]
- Karimi, B.; Seradj, H. N-Bromosuccinimide (NBS), a novel and highly effective catalyst for acetylation of alcohols under mild reaction conditions. Synlett 2001, 519–520. [Google Scholar] [CrossRef]
- Tale, R.H.; Adude, R.N. A novel 3-nitrobenzeneboronic acid as an extremely mild and environmentally benign catalyst for the acetylation of alcohols under solvent-free conditions. Tetrahedron Lett. 2006, 47, 7263–7265. [Google Scholar] [CrossRef]
- Lu, K.C.; Hsieh, S.Y.; Patkar, L.N.; Chen, C.T.; Lin, C.C. Simple and efficient per-O-acetylation of carbohydrates by lithium perchlorate catalyst. Tetrahedron 2004, 60, 8967–8973. [Google Scholar] [CrossRef]
- Breton, G.W. Selective monoacetylation of unsymmetrical diols catalyzed by silica gel-supported sodium hydrogen sulfate. J. Org. Chem. 1997, 62, 8952–8954. [Google Scholar] [CrossRef]
- Lugemwa, F.N.; Shaikh, K.; Hochstedt, E. Facile and efficient acetylation of primary alcohols and phenols with acetic anhydride aatalyzed by dried sodium bicarbonate. Catalysts 2013, 3, 954–965. [Google Scholar] [CrossRef]
- Borah, R.; Deka, N.; Sarma, J.C. Iodine as an Acetyl Transfer Catalyst. J. Chem. Res. 1997, 110–111. [Google Scholar] [CrossRef]
- Kartha, K.P.R.; Field, R.A. Iodine: A versatile reagent in carbohydrate chemistry IV. Per-O-acetylation, regioselective acylation and acetolysis. Tetrahedron 1997, 53, 11753–11766. [Google Scholar]
- Mensah, E.A.; Azzarelli, J.M.; Nguyen, H.M. Palladium-controlledβ-selective glycosylation in the absence of the C(2)-ester participatory group. J. Org. Chem. 2009, 74, 1650–1657. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.; Cooper-Vanosdell, C.; Mensah, E.A.; Nguyen, H.M. Cationic palladium(II)-catalyzed stereoselective glycosylation with glycosyl trichloroacetimidates. J. Org. Chem. 2008, 73, 794–800. [Google Scholar]
- Reed, C.A. Carboranes: A new class of anions for strong electrophiles, oxidants and superacids. Acc. Chem. Res. 1998, 31, 133–139. [Google Scholar] [CrossRef]
- Mecking, S. Cationic nickel and palladium complexes with bidentate ligands for the C–C linkage of olefins. Coord. Chem. Rev. 2000, 203, 325–351. [Google Scholar] [CrossRef]
- Mohammadpoor-Baltork, I.; Aliyan, H.; Khosropour, A.R. Bismuth(III) salts as convenient and efficient catalysts for the selective acetylation and benzoylation of alcohols and phenols. Tetrahedron 2001, 57, 5851–5854. [Google Scholar] [CrossRef]
- Kantam, M.L.; Aziz, K.; Likhar, P.R. Bis(cyclopentadienyl) zirconium dichloride catalyzed acetylation of phenols, alcohols and amines. Catal. Commun. 2006, 7, 484–487. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mensah, E.A.; Reyes, F.R.; Standiford, E.S. Highly Efficient Cationic Palladium Catalyzed Acetylation of Alcohols and Carbohydrate-Derived Polyols. Catalysts 2016, 6, 27. https://doi.org/10.3390/catal6020027
Mensah EA, Reyes FR, Standiford ES. Highly Efficient Cationic Palladium Catalyzed Acetylation of Alcohols and Carbohydrate-Derived Polyols. Catalysts. 2016; 6(2):27. https://doi.org/10.3390/catal6020027
Chicago/Turabian StyleMensah, Enoch A., Francisco R. Reyes, and Eric S. Standiford. 2016. "Highly Efficient Cationic Palladium Catalyzed Acetylation of Alcohols and Carbohydrate-Derived Polyols" Catalysts 6, no. 2: 27. https://doi.org/10.3390/catal6020027