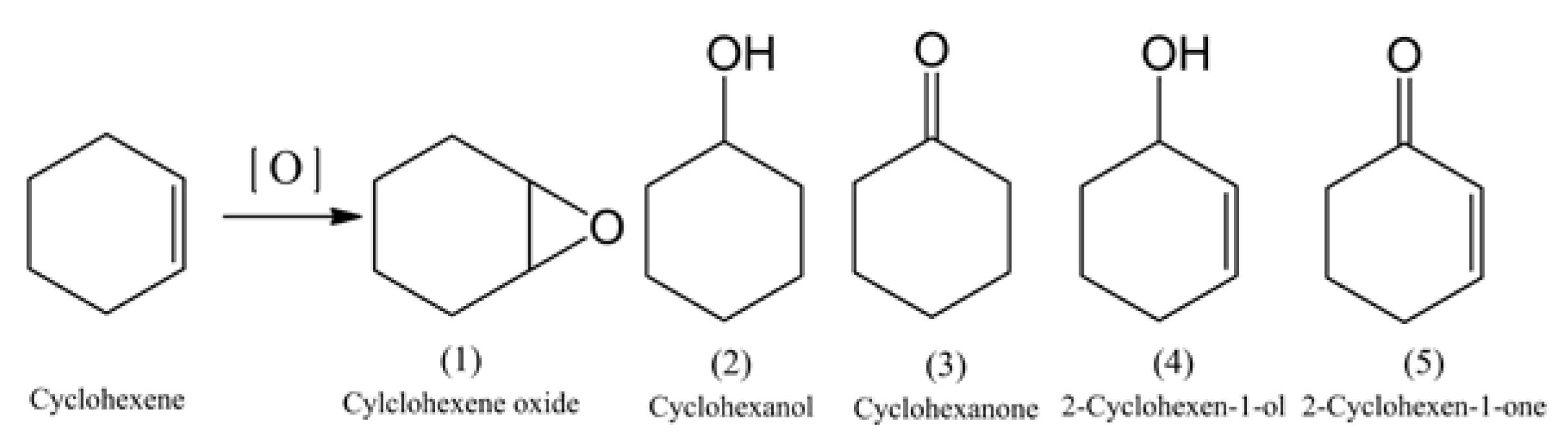
Aerobic Catalytic Oxidation of Cyclohexene over TiZrCo Catalysts
Abstract
:1. Introduction
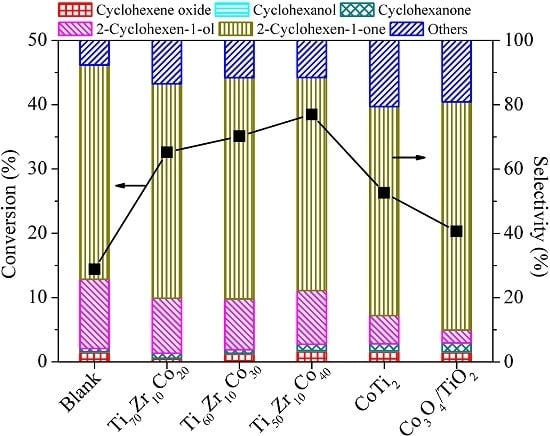
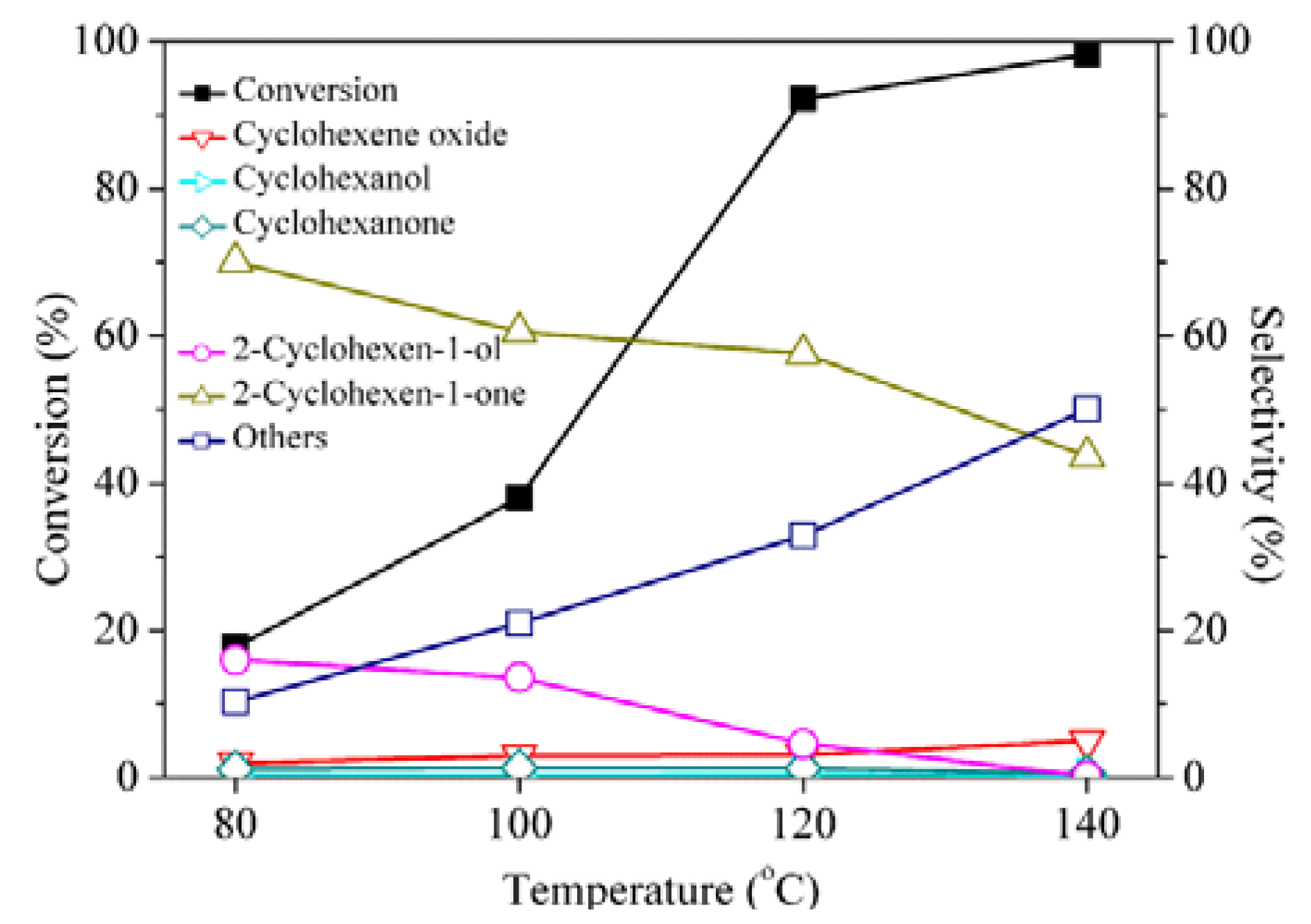
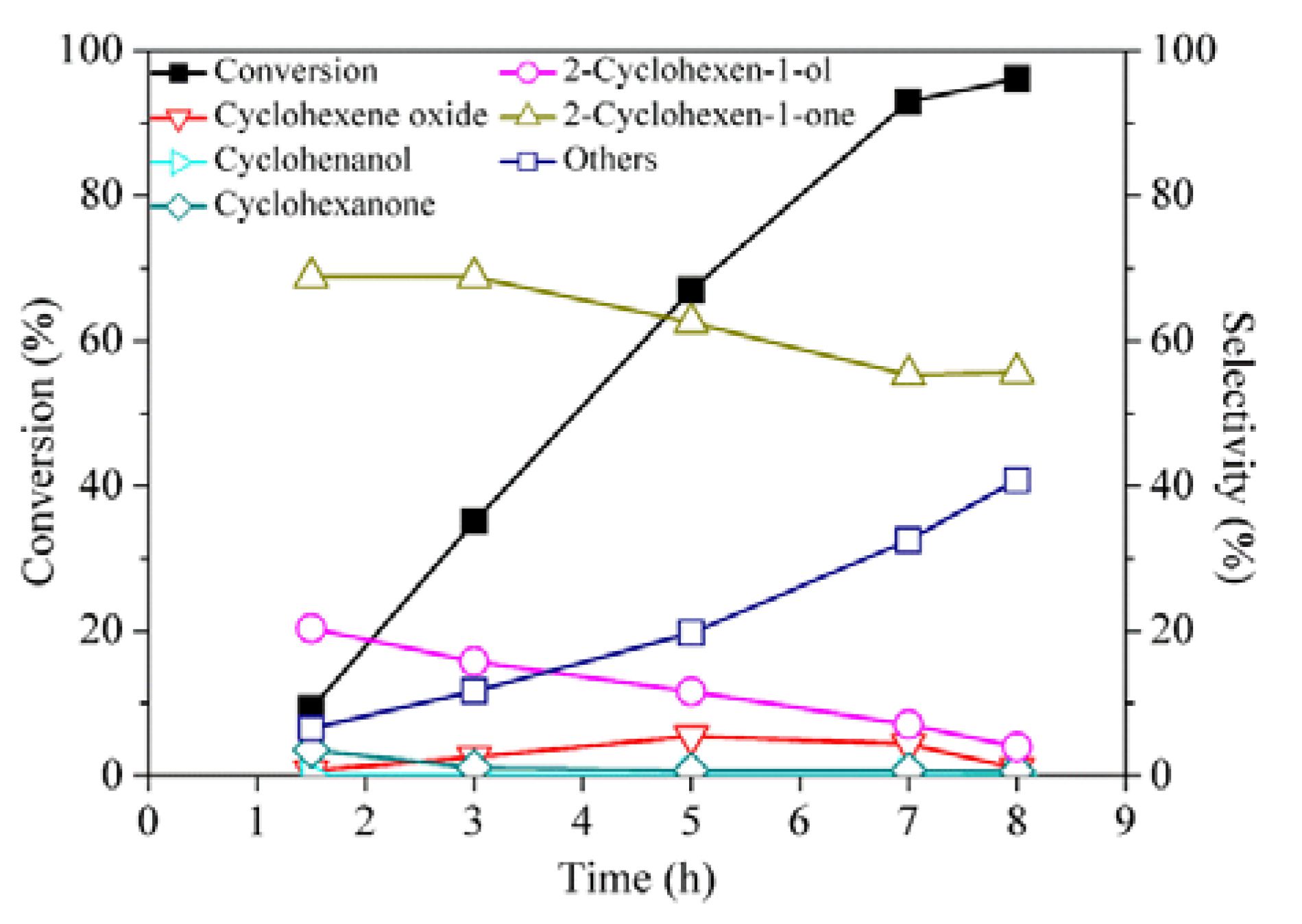
2. Results and Discussion
| Solvent | Conversion (%) | Selectivity (%) a | |||||
|---|---|---|---|---|---|---|---|
| (1) | (2) | (3) | (4) | (5) | Others b | ||
| Acetone | 43.9 | 7.3 | 0 | 1.5 | 13.3 | 71.8 | 6.1 |
| Acetonitrile | 38.0 | 3.0 | 0.4 | 1.4 | 13.6 | 60.6 | 21.0 |
| Ethanol | 22.0 | 1.4 | 0 | 0.3 | 4.7 | 25.0 | 68.6 c |
| Cyclohexane | 6.2 | - | 0.2 | 7.4 | 9.9 | 37.8 | 44.7 d |
| Entry | Cyclohexene (%) a | Time (h) | Conversion (%) | Selectivity (%) | |||||
|---|---|---|---|---|---|---|---|---|---|
| (1) | (2) | (3) | (4) | (5) | Others b | ||||
| 1 | 4.8 | 5 | 33.8 | 3.1 | - | 1.7 | 19.1 | 71.3 | 4.9 |
| 2 | 9.1 | 5 | 67.0 | 5.5 | - | 0.7 | 11.6 | 62.5 | 19.8 |
| 3 | 13.0 | 5 | 97.5 | - | - | 0.6 | 3.7 | 49.5 | 46.1 |
| 4 c | 16.7 | 3.3 | 92.8 | 1.9 | 0.9 | 1.4 | 6.2 | 30.2 | 59.4 |
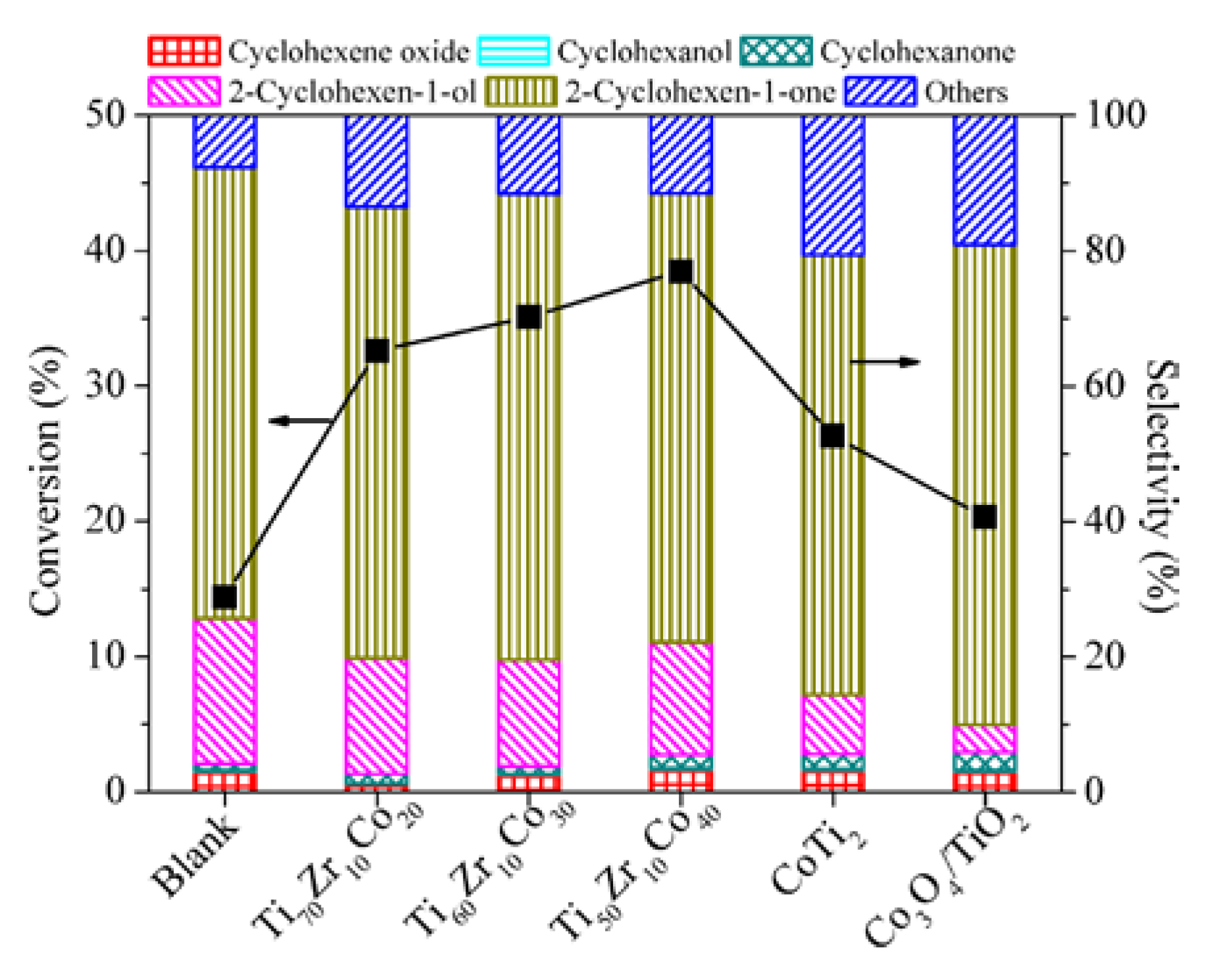


| Entry | Catalyst | Bulk Phase a | Surface Content of Co (%) b | SBET (m2/g) c | ||
|---|---|---|---|---|---|---|
| Co | CoO | Co3O4 | ||||
| 1 | CoTi2 | CoTi2, I-phase | 31.5 | 37.8 | 30.7 | - |
| 2 | Ti70Zr10Co20 | CoTi2, I-phase | 26.9 | 31.2 | 40.9 | 189 |
| 3 | Ti60Zr10Co30 | CoTi2 | 20.1 | 32.8 | 47.1 | 70 |
| 4 | Ti50Zr10Co40 | CoTi2, CoTi | 11.5 | 70.3 | 18.2 | 76 |
3. Experimental Section
3.1. Ti-Zr-Co Alloy Preparation
3.2. Catalyst Characterization
3.3. Catalytic Tests
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Suresh, A.K.; Sharma, M.M.; Sridhar, T. Engineering aspects of industrial liquid-phase air oxidation of hydrocarbons. Ind. Eng. Chem. Res. 2000, 39, 3959–3997. [Google Scholar] [CrossRef]
- Thomas, J.M.; Raja, R.; Sankar, G.; Bell, R.G. Molecular sieve catalysts for the regioselective and shapeselective oxyfunctionalization of alkanes in air. Acc. Chem. Res. 2001, 34, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Patil, N.S.; Uphade, B.S.; Jana, P.; Bharagava, S.K.; Choudhary, V.R. Epoxidation of styrene by anhydrous t-butyl hydroperoxide over reusable gold supported on MgO and other alkaline earth oxides. J. Catal. 2004, 223, 236–239. [Google Scholar] [CrossRef]
- Kuznetsov, M.L.; Rocha, B.G.M.; Pombeiro, A.J.L.; Shul’pin, G.B. Oxidation of olefins with hydrogen peroxide catalyzed by bismuth salts: A mechanistic study. ACS Catal. 2015, 5, 3823–3835. [Google Scholar] [CrossRef]
- Wu, H.Y.; Zhang, X.L.; Yang, C.Y.; Chen, X.; Zheng, X.C. Alkali-hydrothermal synthesis and characterization of W-MCM-41 mesoporous materials with various Si/W molar ratios. Appl. Surf. Sci. 2013, 270, 590–595. [Google Scholar] [CrossRef]
- Ganji, S.; Bukya, P.; Vakati, V.; Rao, K.S.R.; Burri, D.R. Highly efficient and expeditious PdO/SBA-15 catalysts for allylic oxidation of cyclohexene to cyclohexenone. Catal. Sci. Technol. 2013, 3, 409–414. [Google Scholar] [CrossRef]
- Cai, Z.Y.; Zhu, M.Q.; Chen, J.; Shen, Y.Y.; Zhao, J.; Tang, Y.; Chen, X.Z. Solvent-free oxidation of cyclohexene over catalysts with molecular oxygen. Catal. Commun. 2010, 12, 197–201. [Google Scholar] [CrossRef]
- Weiner, H.; Trovarelli, A.; Finke, R.G. Expanded product, plus kinetic and mechanistic, studies of polyoxoanion-based cyclohexene oxidation catalysis: The detection of similar to 70 products at higher conversion leading to a simple, product-based test for the presence of olefin autoxidation. J. Mol. Catal. A 2003, 191, 217–252. [Google Scholar] [CrossRef]
- Chen, K.X.; Zhang, P.F.; Wang, Y.; Li, H.R. Metal-free allylic/benzylic oxidation strategies with molecular oxygen: Recent advances and future prospects. Green Chem. 2014, 16, 2344–2374. [Google Scholar] [CrossRef]
- Zhang, P.F.; Gong, Y.T.; Lv, Y.Q.; Guo, Y.; Wang, Y.; Wang, C.M.; Li, H.R. Ionic liquids with metal chelate anions. Chem. Commun. 2012, 48, 2334–2336. [Google Scholar] [CrossRef] [PubMed]
- Abdolmaleki, A.; Adariani, S.R. Copper-cationic salphen catalysts for the oxidation of cyclohexene by oxygen. Catal. Commun. 2015, 59, 97–100. [Google Scholar] [CrossRef]
- Yin, C.X.; Yang, Z.H.; Li, B.; Zhang, F.M.; Wang, J.Q.; Ou, E.C. Allylic oxidation of cyclohexene with molecular oxygen using cobalt resinate as catalyst. Catal. Lett. 2009, 131, 440–443. [Google Scholar] [CrossRef]
- Cai, X.; Wang, H.; Zhang, Q.; Tong, J.; Lei, Z. Magnetically recyclable core-shell Fe3O4@chitosan-schiff base complexes as efficient catalysts for aerobic oxidation of cyclohexene under mild conditions. J. Mol. Catal. A 2014, 383, 217–224. [Google Scholar] [CrossRef]
- Yang, D.X.; Jiang, T.; Wu, T.B.; Zhang, P.; Han, H.L.; Han, B.X. Highly selective oxidation of cyclohexene to 2-cyclohexene-1-one in water using molecular oxygen over Fe-Co-g-C3N4. Catal. Sci. Technol. 2016, 6, 193–200. [Google Scholar] [CrossRef]
- Silva, F.P.; Jacinto, M.J.; Landers, R.; Rossi, L.M. Selective allylic oxidation of cyclohexene by a magnetically recoverable cobalt oxide catalyst. Catal. Lett. 2011, 141, 432–437. [Google Scholar] [CrossRef]
- Wang, L.; Wang, H.; Hapala, P.; Zhu, L.; Ren, L.; Meng, X.; Lewis, J.P.; Xiao, F.S. Superior catalytic properties in aerobic oxidation of olefins over Au nanoparticles on pyrrolidone-modified SBA-15. J. Catal. 2011, 281, 30–39. [Google Scholar] [CrossRef]
- Donoeva, B.G.; Ovoshchnikov, D.S.; Golovko, V.B. Establishing a au nanoparticle size effect in the oxidation of cyclohexene using gradually changing Au catalysts. ACS Catal. 2013, 3, 2986–2991. [Google Scholar] [CrossRef]
- Ghiaci, M.; Dorostkar, N.; Victoria Martinez-Huerta, M.; Fierro, J.L.G.; Moshiri, P. Synthesis and characterization of gold nanoparticles supported on thiol functionalized chitosan for solvent-free oxidation of cyclohexene with molecular oxygen. J. Mol. Catal. A 2013, 379, 340–349. [Google Scholar] [CrossRef]
- Zhou, L.; Lu, T.; Xu, J.; Chen, M.; Zhang, C.; Chen, C.; Yang, X.; Xu, J. Synthesis of hierarchical MeAPO-5 molecular sieves-catalysts for the oxidation of hydrocarbons with efficient mass transport. Microporous Mesoporous Mater. 2012, 161, 76–83. [Google Scholar] [CrossRef]
- Sang, X.X.; Zhang, J.L.; Wu, T.B.; Zhang, B.X.; Ma, X.; Peng, L.; Han, B.X.; Kang, X.C.; Liu, C.C.; Yang, G.Y. Room-temperature synthesis of mesoporous CuO and its catalytic activity for cyclohexene oxidation. RSC Adv. 2015, 5, 67168–67174. [Google Scholar] [CrossRef]
- Nejad, M.S.; Ghasemi, G.; Martinez-Huerta, M.V.; Ghiaci, M. Synthesis and characterization of Au nanocatalyst on modifed bentonite and silica and their applications for solvent free oxidation of cyclohexene with molecular oxygen. J. Mol. Catal. A 2015, 406, 118–126. [Google Scholar] [CrossRef]
- Cao, Y.; Yu, H.; Peng, F.; Wang, H. Selective allylic oxidation of cyclohexene catalyzed by nitrogen-doped carbon nanotubes. ACS Catal. 2014, 4, 1617–1625. [Google Scholar] [CrossRef]
- Hao, J.M.; Wang, J.Y.; Wang, Q.; Yu, Y.C.; Cai, S.X.; Zhao, F.Y. Catalytic oxidation of cyclohexane over Ti-Zr-Co catalysts. Appl. Catal. A 2009, 368, 29–34. [Google Scholar] [CrossRef]
- Hao, J.M.; Liu, B.Z.; Cheng, H.Y.; Wang, Q.; Wang, J.Y.; Cai, S.X.; Zhao, F.Y. Cyclohexane oxidation on a novel Ti70Zr10Co20 catalyst containing quasicrystal. Chem. Commun. 2009, 3460–3462. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Cheng, H.Y.; Sun, L.S.; Liang, F.; Zhang, C.; Ying, Z.; Lin, W.W.; Zhao, F.Y. Synthesis of acetophenone from aerobic catalytic oxidation of ethylbenzene over Ti-Zr-Co alloy catalyst: Influence of annealing conditions. Appl. Catal. A 2016, 512, 9–14. [Google Scholar] [CrossRef]
- Dapurkar, S.E.; Kawanami, H.; Komura, K.; Yokoyama, T.; Ikushima, Y. Solvent-free allylic oxidation of cycloolefins over mesoporous CrMCM-41 molecular sieve catalyst at 1 atm dioxygen. Appl. Catal. A 2008, 346, 112–116. [Google Scholar] [CrossRef]
- Hermans, I.; Jacobs, P.A.; Peeters, J. Understanding the autoxidation of hydrocarbons at the molecular level and consequences for catalysis. J. Mol. Catal. A 2006, 251, 221–228. [Google Scholar] [CrossRef]
- Kim, W.J.; Kelton, K.F. Icosahedral-phase formation and stability in Ti Zr Co alloys. Philos. Mag. Lett. 1996, 74, 439–448. [Google Scholar] [CrossRef]
- Kim, D.H.; Lee, S.Y.; Jin, J.E.; Kim, G.T.; Lee, D.J. Electrical conductivity enhancement of metallic single-walled carbon nanotube networks by CoO decoration. Phys. Chem. Chem. Phys. 2014, 16, 6980–6985. [Google Scholar] [CrossRef] [PubMed]
- Barreca, D.; Massignan, C.; Daolio, S.; Fabrizio, M.; Piccirillo, C.; Armelao, L.; Tondello, E. Composition and microstructure of cobalt oxide thin films obtained from a novel cobalt(ii) precursor by chemical vapor deposition. Chem. Mater. 2001, 12, 588–593. [Google Scholar] [CrossRef]
- Dupin, J.C.; Gonbeau, D.; Vinatier, P.; Levasseur, A. Systematic XPS studies of metal oxides, hydroxides and peroxides. Phys. Chem. Chem. Phys. 2000, 2, 1319–1324. [Google Scholar] [CrossRef]
- Todorova, S.; Kolev, H.; Holgado, J.P.; Kadinov, G.; Bonev, C.; Pereñíguez, R.; Caballero, A. Complete n-hexane oxidation over supported Mn-Co catalysts. Appl. Catal. B 2010, 94, 46–54. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, T.; Cheng, H.; Lin, W.; Zhang, C.; Yu, Y.; Zhao, F. Aerobic Catalytic Oxidation of Cyclohexene over TiZrCo Catalysts. Catalysts 2016, 6, 24. https://doi.org/10.3390/catal6020024
Liu T, Cheng H, Lin W, Zhang C, Yu Y, Zhao F. Aerobic Catalytic Oxidation of Cyclohexene over TiZrCo Catalysts. Catalysts. 2016; 6(2):24. https://doi.org/10.3390/catal6020024
Chicago/Turabian StyleLiu, Tong, Haiyang Cheng, Weiwei Lin, Chao Zhang, Yancun Yu, and Fengyu Zhao. 2016. "Aerobic Catalytic Oxidation of Cyclohexene over TiZrCo Catalysts" Catalysts 6, no. 2: 24. https://doi.org/10.3390/catal6020024