Application, Deactivation, and Regeneration of Heterogeneous Catalysts in Bio-Oil Upgrading
Abstract
:1. Introduction
2. Bio-Oil Properties and Compositions
3. Catalytic Cracking
3.1. Catalysts in Catalytic Cracking
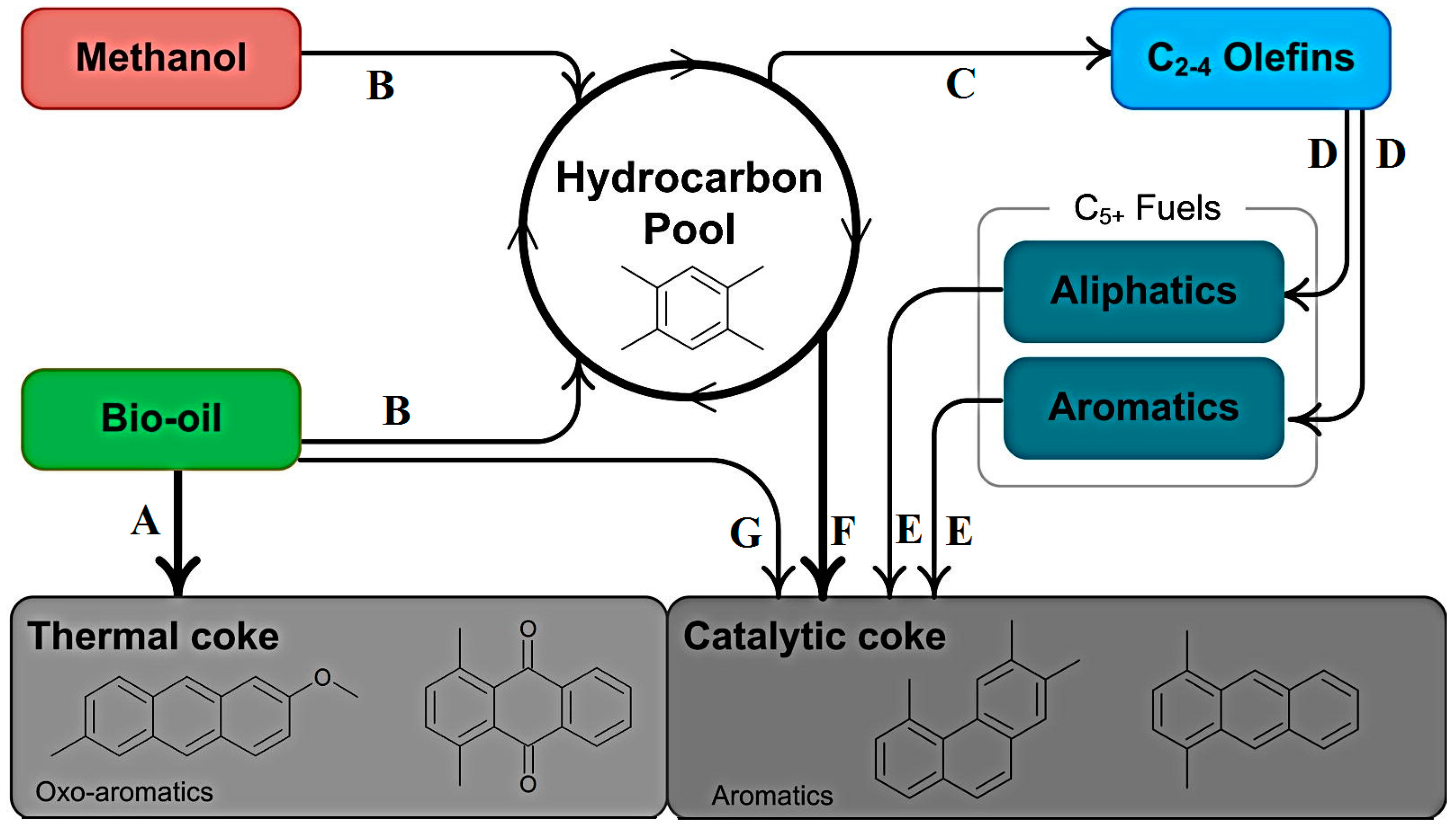
3.1.1. Zeolite Catalysts
3.1.2. Oxides Catalysts
3.2. Catalyst Deactivation in Catalytic Cracking
3.3. Reducing Catalyst Deactivation in Catalytic Cracking
3.4. Catalyst Regeneration and Recycling in Catalytic Cracking
4. Hydrodeoxygenation
4.1. Catalysts Used in Bio-Oil HDO
4.1.1 Sulfided Catalysts
4.1.2. Noble Metal Catalysts
4.1.3. Transition Metal Catalysts
4.1.4. Other Catalysts
4.2. Catalyst Deactivation in Bio-Oil HDO
4.3. Reducing Catalyst Deactivation in Bio-Oil HDO
4.4. Catalyst Regeneration and Recycle in Bio-Oil HDO
5. Summary and Outlook
- Improve understanding of catalyst deactivation and regeneration mechanisms during bio-oil upgrading processes.
- Develop effective catalysts with higher stability and better regeneration properties.
- Integrate upgrading approaches of bio-oil catalytic cracking and/or hydrodeoxygenation with petroleum refining technologies to improve bio-oil upgrading efficiency.
- Explore new biomass resources or genetically engineered biomass to improve the yield and quality of bio-oils produced.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mohr, S.H.; Wang, J.; Ellem, G.; Ward, J.; Giurco, D. Projection of world fossil fuels by country. Fuel 2015, 141, 120–135. [Google Scholar] [CrossRef]
- Zhang, H.; Carlson, T.R.; Xiao, R.; Huber, G.W. Catalytic fast pyrolysis of wood and alcohol mixtures in a fluidized bed reactor. Green Chem. 2012, 14, 98–110. [Google Scholar] [CrossRef]
- Jahirul, M.I.; Rasul, M.G.; Chowdhury, A.A.; Ashwath, N. Biofuels production through biomass pyrolysis—A technological review. Energies 2012, 5, 4952–5001. [Google Scholar] [CrossRef]
- Masnadi, M.S.; Grace, J.R.; Bi, X.T.; Lim, C.J.; Ellis, N. From fossil fuels towards renewables: Inhibitory and catalytic effects on carbon thermochemical conversion during co-gasification of biomass with fossil fuels. Appl. Energy 2015, 140, 196–209. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U.; Steele, P.H. Pyrolysis of wood/biomass for bio-oil: A critical review. Energy Fuels 2006, 20, 848–889. [Google Scholar] [CrossRef]
- Chhiti, Y.; Kemiha, M. Thermal conversion of biomass, pyrolysis and gasification: A review. Int. J. Eng. Sci. 2013, 2, 75–85. [Google Scholar]
- Gorgens, J.F.; Carrier, M.; Garcia-Aparicio, M.P. Biomass conversion to bioenergy products. In Bioenergy from Wood: Sustainable Production in the Tropics; Seifert, T., Ed.; Springer: London, UK, 2013; pp. 158–159. [Google Scholar]
- Goyal, H.B.; Seal, D.; Saxena, R.C. Bio-fuels from thermochemical conversion of renewable resources: A review. Renew. Sustain. Energy Rev. 2008, 12, 504–517. [Google Scholar] [CrossRef]
- Vamvuka, D. Bio-oil, solid and gaseous biofuels from biomass pyrolysis processes—An overview. Int. J. Energy Res. 2011, 35, 835–862. [Google Scholar] [CrossRef]
- Cao, Y.; Pawłowski, A. Sewage sludge-to-energy approaches based on anaerobic digestion and pyrolysis: Brief overview and energy efficiency assessment. Renew. Sustain. Energy Rev. 2012, 16, 1657–1665. [Google Scholar] [CrossRef]
- Lu, Q.; Li, W.Z.; Zhu, X.F. Overview of fuel properties of biomass fast pyrolysis oils. Energy Convers. Manag. 2009, 50, 1376–1383. [Google Scholar] [CrossRef]
- Heo, H.S.; Park, H.J.; Park, Y.K.; Ryu, C.; Suh, D.J.; Suh, Y.W.; Yim, J.W.; Kim, S.S. Bio-oil production from fast pyrolysis of waste furniture sawdust in a fluidized bed. Bioresour. Technol. 2010, 101, S91–S96. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.S.; Kim, J.Y.; Kim, K.H.; Lee, S.; Choi, D.; Choi, I.G.; Choi, J.W. The effect of storage duration on bio-oil properties. J. Anal. Appl. Pyrolysis 2012, 95, 118–125. [Google Scholar] [CrossRef]
- Xu, J.; Jiang, J.; Sun, Y.; Lu, Y. Bio-oil upgrading by means of ethyl ester production in reactive distillation to remove water and to improve storage and fuel characteristics. Biomass Bioenergy 2008, 32, 1056–1061. [Google Scholar]
- Oasmaa, A.; Kuoppala, E. Fast pyrolysis of forestry residue. 3. Storage stability of liquid fuel. Energy Fuels 2003, 17, 1075–1084. [Google Scholar] [CrossRef]
- Kim, S.J.; Jung, S.H.; Kim, J.S. Fast pyrolysis of palm kernel shells: Influence of operation parameters on the bio-oil yield and the yield of phenol and phenolic compounds. Bioresour. Technol. 2010, 101, 9294–9300. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.-L. Bio-oil from fast pyrolysis of rice husk: Yields and related properties and improvement of the pyrolysis system. J. Anal. Appl. Pyrolysis 2007, 80, 30–35. [Google Scholar]
- DeSisto, W.J.; Hill, N.; Beis, S.H.; Mukkamala, S.; Joseph, J.; Baker, C.; Ong, T.H.; Stemmler, E.A.; Wheeler, M.C.; Frederick, B.G.; et al. Fast pyrolysis of pine sawdust in a fluidized-bed reactor. Energy Fuels 2010, 24, 2642–2651. [Google Scholar] [CrossRef]
- Greenhalf, C.E.; Nowakowski, D.J.; Harms, A.B.; Titiloye, J.O.; Bridgwater, A.V. A comparative study of straw, perennial grasses and hardwoods in terms of fast pyrolysis products. Fuel 2013, 108, 216–230. [Google Scholar] [CrossRef]
- Trinh, T.N.; Jensen, P.A.; Dam-Johansen, K.; Knudsen, N.O.; Sørensen, H.R.; Hvilsted, S. Comparison of lignin, macroalgae, wood, and straw fast pyrolysis. Energy Fuels 2013, 27, 1399–1409. [Google Scholar] [CrossRef]
- Yanik, J.; Stahl, R.; Troeger, N.; Sinag, A. Pyrolysis of algal biomass. J. Anal. Appl. Pyrolysis 2013, 103, 134–141. [Google Scholar] [CrossRef]
- Abdullah, N.; Sulaiman, F.; Gerhauser, H. Characterisation of oil palm empty fruit bunches for fuel application. J. Phys. Sci. 2011, 22, 1–24. [Google Scholar]
- Ryu, Y.; Lee, Y.; Nam, J. Performance and emission characteristics of additives-enhanced heavy fuel oil in large two-stroke marine diesel engine. Fuel 2016, 182, 850–856. [Google Scholar] [CrossRef]
- Park, J.; Jang, J.H.; Park, S. Effect of fuel temperature on heavy fuel oil spray characteristics in a common-rail fuel injection system for marine engines. Ocean Eng. 2015, 104, 580–589. [Google Scholar] [CrossRef]
- Elkasabi, Y.; Mullen, C.A.; Pighinelli, A.L.; Boateng, A.A. Hydrodeoxygenation of fast-pyrolysis bio-oils from various feedstocks using carbon-supported catalysts. Fuel Process. Technol. 2014, 123, 11–18. [Google Scholar] [CrossRef]
- Zhou, M.; Xiao, G.; Wang, K.; Jiang, J. Catalytic conversion of aqueous fraction of bio-oil to alcohols over CNT-supported catalysts. Fuel 2016, 180, 749–758. [Google Scholar] [CrossRef]
- Wang, S.; Cai, Q.; Chen, J.; Zhang, L.; Zhu, L.; Luo, Z. Co-cracking of bio-oil model compound mixtures and ethanol over different metal oxide-modified HZSM-5 catalysts. Fuel 2015, 160, 534–543. [Google Scholar] [CrossRef]
- Luo, G.; Resende, F.L. In-situ and ex-situ upgrading of pyrolysis vapors from beetle-killed trees. Fuel 2016, 166, 367–375. [Google Scholar] [CrossRef]
- Gamliel, D.P.; Du, S.; Bollas, G.M.; Valla, J.A. Investigation of in situ and ex situ catalytic pyrolysis of miscanthus× giganteus using a PyGC–MS microsystem and comparison with a bench-scale spouted-bed reactor. Bioresour. Technol. 2015, 191, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wei, L.; Crandall, Z.; Julson, J.; Gu, Z. Combining Mo–Cu/HZSM-5 with a two-stage catalytic pyrolysis system for pine sawdust thermal conversion. Fuel 2015, 150, 656–663. [Google Scholar] [CrossRef]
- Heo, H.S.; Park, H.J.; Dong, J.I.; Park, S.H.; Kim, S.; Suh, D.J.; Suhd, Y.W.; Kim, S.S.; Park, Y.K. Fast pyrolysis of rice husk under different reaction conditions. J. Ind. Eng. Chem. 2010, 16, 27–31. [Google Scholar] [CrossRef]
- Mullen, C.A.; Boateng, A.A.; Goldberg, N.M.; Lima, I.M.; Laird, D.A.; Hicks, K.B. Bio-oil and bio-char production from corn cobs and stover by fast pyrolysis. Biomass Bioenergy 2010, 34, 67–74. [Google Scholar] [CrossRef]
- Ren, S.; Ye, X.P.; Borole, A.P.; Kim, P.; Labbé, N. Analysis of switchgrass-derived bio-oil and associated aqueous phase generated in a semi-pilot scale auger pyrolyzer. J. Anal. Appl. Pyrolysis 2016, 119, 97–103. [Google Scholar] [CrossRef]
- Obeid, W.; Salmon, E.; Lewan, M.D.; Hatcher, P.G. Hydrous pyrolysis of Scenedesmus algae and algaenan-like residue. Org. Geochem. 2015, 85, 89–101. [Google Scholar] [CrossRef]
- Venderbosch, R.H.; Prins, W. Fast pyrolysis technology development. Biofuels Bioprod. Biorefin. 2010, 4, 178–208. [Google Scholar] [CrossRef]
- Oasmaa, A.; Meier, D. Norms and standards for fast pyrolysis liquids: 1. Round robin test. J. Anal. Appl. Pyrolysis 2005, 73, 323–334. [Google Scholar] [CrossRef]
- Fan, Y.; Cai, Y.; Li, X.; Yu, N.; Yin, H. Catalytic upgrading of pyrolytic vapors from the vacuum pyrolysis of rape straw over nanocrystalline HZSM-5 zeolite in a two-stage fixed-bed reactor. J. Anal. Appl. Pyrolysis 2014, 108, 185–195. [Google Scholar] [CrossRef]
- Murata, K.; Liu, Y.; Inaba, M.; Takahara, I. Catalytic fast pyrolysis of jatropha wastes. J. Anal. Appl. Pyrolysis 2012, 94, 75–82. [Google Scholar] [CrossRef]
- Cheng, S.; Wei, L.; Zhao, X.; Kadis, E.; Julson, J. Conversion of Prairie Cordgrass to Hydrocarbon Biofuel over Co-Mo/HZSM-5 Using a Two-Stage Reactor System. Energy Technol. 2016, 4, 706–713. [Google Scholar] [CrossRef]
- Cheng, S.; Wei, L.; Zhao, X.; Huang, Y.; Raynie, D.; Qiu, C.; Kiratu, J.; Yu, Y. Directly catalytic upgrading bio-oil vapor produced by prairie cordgrass pyrolysis over Ni/HZSM-5 using a two stage reactor. AIMS Energy 2015, 3, 227–240. [Google Scholar]
- Cheng, S.; Wei, L.; Zhao, X. Development of a bifunctional Ni/HZSM-5 catalyst for converting prairie cordgrass to hydrocarbon biofuel. Energy Sour. Part A 2016, 38, 2433–2437. [Google Scholar] [CrossRef]
- Cai, Y.; Fan, Y.; Li, X.; Chen, L.; Wang, J. Preparation of refined bio-oil by catalytic transformation of vapors derived from vacuum pyrolysis of rape straw over modified HZSM-5. Energy 2016, 102, 95–105. [Google Scholar] [CrossRef]
- Veses, A.; Puértolas, B.; Callén, M.S.; García, T. Catalytic upgrading of biomass derived pyrolysis vapors over metal-loaded ZSM-5 zeolites: Effect of different metal cations on the bio-oil final properties. Microporous Mesoporous Mater. 2015, 209, 189–196. [Google Scholar] [CrossRef]
- Widayatno, W.B.; Guan, G.; Rizkiana, J.; Du, X.; Hao, X.; Zhang, Z.; Abudula, A. Selective catalytic conversion of bio-oil over high-silica zeolites. Bioresour. Technol. 2015, 179, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wei, L.; Cheng, S.; Huang, Y.; Yu, Y.; Julson, J. Catalytic cracking of camelina oil for hydrocarbon biofuel over ZSM-5-Zn catalyst. Fuel Process. Technol. 2015, 139, 117–126. [Google Scholar] [CrossRef]
- Zhao, X.; Wei, L.; Cheng, S.; Cao, Y.; Julson, J.; Gu, Z. Catalytic cracking of carinata oil for hydrocarbon biofuel over fresh and regenerated Zn/Na-ZSM-5. Appl. Catal. A 2015, 507, 44–55. [Google Scholar] [CrossRef]
- Biswas, S.; Sharma, D.K. Effect of different catalysts on the cracking of Jatropha oil. J. Anal. Appl. Pyrolysis 2014, 110, 346–352. [Google Scholar] [CrossRef]
- Wang, L.; Lei, H.; Bu, Q.; Ren, S.; Wei, Y.; Zhu, L.; Zhang, X.; Liu, Y.; Yadavalli, G.; Lee, J.; Chen, S.; Tang, J. Aromatic hydrocarbons production from ex situ catalysis of pyrolysis vapor over Zinc modified ZSM-5 in a packed-bed catalysis coupled with microwave pyrolysis reactor. Fuel 2014, 129, 78–85. [Google Scholar] [CrossRef]
- Mihalcik, D.J.; Mullen, C.A.; Boateng, A.A. Screening acidic zeolites for catalytic fast pyrolysis of biomass and its components. J. Anal. Appl. Pyrolysis 2011, 92, 224–232. [Google Scholar] [CrossRef]
- Widayatno, W.B.; Guan, G.; Rizkiana, J.; Yang, J.; Hao, X.; Tsutsumi, A.; Abudula, A. Upgrading of bio-oil from biomass pyrolysis over Cu-modified β-zeolite catalyst with high selectivity and stability. Appl. Catal. B 2016, 186, 166–172. [Google Scholar] [CrossRef]
- French, R.; Czernik, S. Catalytic pyrolysis of biomass for biofuels production. Fuel Process. Technol. 2010, 91, 25–32. [Google Scholar] [CrossRef]
- Liu, S.; Xie, Q.; Zhang, B.; Cheng, Y.; Liu, Y.; Chen, P.; Ruan, R. Fast microwave-assisted catalytic co-pyrolysis of corn stover and scum for bio-oil production with CaO and HZSM-5 as the catalyst. Bioresour. Technol. 2016, 204, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Heo, H.S.; Jeon, J.K.; Kim, J.; Ryoo, R.; Jeong, K.E.; Park, Y.K. Highly valuable chemicals production from catalytic upgrading of radiata pine sawdust-derived pyrolytic vapors over mesoporous MFI zeolites. Appl. Catal. B 2010, 95, 365–373. [Google Scholar] [CrossRef]
- Yoo, M.L.; Park, Y.H.; Park, Y.K.; Park, S.H. Catalytic Pyrolysis of Wild Reed over a Zeolite-Based Waste Catalyst. Energies 2016, 9, 201. [Google Scholar] [CrossRef]
- Wang, D.; Xiao, R.; Zhang, H.; He, G. Comparison of catalytic pyrolysis of biomass with MCM-41 and CaO catalysts by using TGA–FTIR analysis. J. Anal. Appl. Pyrolysis 2010, 89, 171–177. [Google Scholar] [CrossRef]
- Stefanidis, S.D.; Kalogiannis, K.G.; Iliopoulou, E.F.; Lappas, A.A.; Pilavachi, P.A. In-situ upgrading of biomass pyrolysis vapors: catalyst screening on a fixed bed reactor. Bioresour. Technol. 2011, 102, 8261–8267. [Google Scholar] [CrossRef] [PubMed]
- Stefanidis, S.D.; Karakoulia, S.A.; Kalogiannis, K.G.; Iliopoulou, E.F.; Delimitis, A.; Yiannoulakis, H.; Zampetakis, T.; Lappas, A.A.; Triantafyllidis, K.S. Natural magnesium oxide (MgO) catalysts: A cost-effective sustainable alternative to acid zeolites for the in situ upgrading of biomass fast pyrolysis oil. Appl. Catal. B 2016, 196, 155–173. [Google Scholar] [CrossRef]
- Lim, X.Y.; Andresen, J.M. Pyro-catalytic deoxgenated bio-oil from palm oil empty fruit bunch and fronds with boric oxide in a fixed-bed reactor. Fuel Process. Technol. 2011, 92, 1796–1804. [Google Scholar] [CrossRef]
- Imran, A.; Bramer, E.A.; Seshan, K.; Brem, G. High quality bio-oil from catalytic flash pyrolysis of lignocellulosic biomass over alumina-supported sodium carbonate. Fuel Process. Technol. 2014, 127, 72–79. [Google Scholar] [CrossRef]
- Karnjanakom, S.; Bayu, A.; Hao, X.; Kongparakul, S.; Samart, C.; Abudula, A.; Guan, G. Selectively catalytic upgrading of bio-oil to aromatic hydrocarbons over Zn, Ce or Ni-doped mesoporous rod-like alumina catalysts. J. Mol. Catal. A Chem. 2016, 421, 235–244. [Google Scholar] [CrossRef]
- Lu, Q.; Zhang, Z.F.; Dong, C.Q.; Zhu, X.F. Catalytic upgrading of biomass fast pyrolysis vapors with nano metal oxides: an analytical Py-GC/MS study. Energies 2010, 3, 1805–1820. [Google Scholar] [CrossRef]
- Zheng, Y.; Chen, D.; Zhu, X. Aromatic hydrocarbon production by the online catalytic cracking of lignin fast pyrolysis vapors using Mo2N/γ-Al2O3. J. Anal. Appl. Pyrolysis 2013, 104, 514–520. [Google Scholar] [CrossRef]
- Zhou, L.; Yang, H.; Wu, H.; Wang, M.; Cheng, D. Catalytic pyrolysis of rice husk by mixing with zinc oxide: characterization of bio-oil and its rheological behavior. Fuel Process. Technol. 2013, 106, 385–391. [Google Scholar] [CrossRef]
- Veses, A.; Aznar, M.; López, J.M.; Callén, M.S.; Murillo, R.; García, T. Production of upgraded bio-oils by biomass catalytic pyrolysis in an auger reactor using low cost materials. Fuel 2015, 141, 17–22. [Google Scholar] [CrossRef]
- Aysu, T.; Sanna, A. Nannochloropsis algae pyrolysis with ceria-based catalysts for production of high-quality bio-oils. Bioresour. Technol. 2015, 194, 108–116. [Google Scholar] [CrossRef] [PubMed]
- De Rezende Pinho, A.; de Almeida, M.B.; Mendes, F.L.; Ximenes, V.L.; Casavechia, L.C. Co-processing raw bio-oil and gasoil in an FCC unit. Fuel Process. Technol. 2015, 131, 159–166. [Google Scholar] [CrossRef]
- Zhang, Z.B.; Lu, Q.; Ye, X.N.; Li, W.T.; Hu, B.; Dong, C.Q. Production of phenolic-rich bio-oil from catalytic fast pyrolysis of biomass using magnetic solid base catalyst. Energy Convers. Manag. 2015, 106, 1309–1317. [Google Scholar] [CrossRef]
- Bu, Q.; Lei, H.; Wang, L.; Yadavalli, G.; Wei, Y.; Zhang, X.; Zhu, L.; Liu, Y. Biofuel production from catalytic microwave pyrolysis of Douglas fir pellets over ferrum-modified activated carbon catalyst. J. Anal. Appl. Pyrolysis 2015, 112, 74–79. [Google Scholar] [CrossRef]
- Uzun, B.B.; Sarioğlu, N. Rapid and catalytic pyrolysis of corn stalks. Fuel Process. Technol. 2009, 90, 705–716. [Google Scholar] [CrossRef]
- Kim, J.W.; Joo, S.H.; Seo, B.; Kim, S.S.; Shin, D.H.; Park, S.H.; Jeon, J.K.; Park, Y.K. Bio-Oil Upgrading via Catalytic Pyrolysis of Waste Mandarin Residue Over SBA-15 Catalysts. J. Nanosci. Nanotechnol. 2013, 13, 2566–2572. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chen, J.; Cai, Q.; Zhang, F.; Wang, Y.; Ru, B.; Wang, Q. The effect of mild hydrogenation on the catalytic cracking of bio-oil for aromatic hydrocarbon production. Int. J. Hydrog. Energy 2016, 41, 16385–16393. [Google Scholar] [CrossRef]
- Zhao, X.; Wei, L.; Cheng, S.; Julson, J.; Anderson, G.; Muthukumarappan, K.; Qiu, C. Development of hydrocarbon biofuel from sunflower seed and sunflower meat oils over ZSM-5. J. Renew. Sustain. Energy 2016, 8, 013109. [Google Scholar] [CrossRef]
- Vitolo, S.; Bresci, B.; Seggiani, M.; Gallo, M.G. Catalytic upgrading of pyrolytic oils over HZSM-5 zeolite: behaviour of the catalyst when used in repeated upgrading–regenerating cycles. Fuel 2001, 80, 17–26. [Google Scholar] [CrossRef]
- Wang, J.; Zhong, Z.; Ding, K.; Xue, Z. Catalytic fast pyrolysis of mushroom waste to upgraded bio-oil products via pre-coked modified HZSM-5 catalyst. Bioresour. Technol. 2016, 212, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez, M.; Valle, B.; Bilbao, J.; Gayubo, A.G.; Castaño, P. Effect of operating conditions on the coke nature and HZSM-5 catalysts deactivation in the transformation of crude bio-oil into hydrocarbons. Catal. Today 2012, 195, 106–113. [Google Scholar] [CrossRef]
- Valle, B.; Castaño, P.; Olazar, M.; Bilbao, J.; Gayubo, A.G. Deactivating species in the transformation of crude bio-oil with methanol into hydrocarbons on a HZSM-5 catalyst. J. Catal. 2012, 285, 304–314. [Google Scholar] [CrossRef]
- Guisnet, M.; Costa, L.; Ribeiro, F.R. Prevention of zeolite deactivation by coking. J. Mol. Catal. A Chem. 2009, 305, 69–83. [Google Scholar] [CrossRef]
- Guisnet, M.; Magnoux, P. Organic chemistry of coke formation. Appl. Catal. A 2001, 212, 83–96. [Google Scholar] [CrossRef]
- Valle, B.; Gayubo, A.G.; Aguayo, A.T.; Olazar, M.; Bilbao, J. Selective production of aromatics by crude bio-oil valorization with a nickel-modified HZSM-5 zeolite catalyst. Energy Fuels 2010, 24, 2060–2070. [Google Scholar] [CrossRef]
- Gayubo, A.G.; Valle, B.; Aguayo, A.T.; Olazar, M.; Bilbao, J. Olefin production by catalytic transformation of crude bio-oil in a two-step process. Ind. Eng. Chem. Res. 2009, 49, 123–131. [Google Scholar] [CrossRef]
- Cerqueira, H.S.; Caeiro, G.; Costa, L.; Ribeiro, F.R. Deactivation of FCC catalysts. J. Mol. Catal. A Chem. 2008, 292, 1–13. [Google Scholar] [CrossRef]
- Graça, I.; Carmo, A.M.; Lopes, J.M.; Ribeiro, M.F. Improving HZSM-5 resistance to phenolic compounds for the bio-oils/FCC feedstocks co-processing. Fuel 2015, 140, 484–494. [Google Scholar] [CrossRef]
- Fogassy, G.; Thegarid, N.; Schuurman, Y.; Mirodatos, C. From biomass to bio-gasoline by FCC co-processing: effect of feed composition and catalyst structure on product quality. Energy Environ. Sci. 2011, 4, 5068–5076. [Google Scholar] [CrossRef]
- Li, X.; Li, J.; Zhou, G.; Feng, Y.; Wang, Y.; Yu, G.; Deng, S.; Huang, J.; Wang, B. Enhancing the production of renewable petrochemicals by co-feeding of biomass with plastics in catalytic fast pyrolysis with ZSM-5 zeolites. Appl. Catal. A 2014, 481, 173–182. [Google Scholar] [CrossRef]
- Zhu, X.; Mallinson, R.G.; Resasco, D.E. Role of transalkylation reactions in the conversion of anisole over HZSM-5. Appl. Catal. A 2010, 379, 172–181. [Google Scholar] [CrossRef]
- Sharma, R.K.; Bakhshi, N.N. Catalytic upgrading of pyrolysis oil. Energy Fuels 1993, 7, 306–314. [Google Scholar] [CrossRef]
- Sharma, R.K.; Bakhshi, N.N. Catalytic conversion of fast pyrolysis oil to hydrocarbon fuels over HZSM-5 in a dual reactor system. Biomass Bioenergy 1993, 5, 445–455. [Google Scholar] [CrossRef]
- Gayubo, A.G.; Valle, B.; Aguayo, A.T.; Olazar, M.; Bilbao, J. Pyrolytic lignin removal for the valorization of biomass pyrolysis crude bio-oil by catalytic transformation. J. Chem. Technol. Biotechnol. 2010, 85, 132–144. [Google Scholar] [CrossRef]
- Valle, B.; Gayubo, A.G.; Alonso, A.; Aguayo, A.T.; Bilbao, J. Hydrothermally stable HZSM-5 zeolite catalysts for the transformation of crude bio-oil into hydrocarbons. Appl. Catal. B 2010, 100, 318–327. [Google Scholar] [CrossRef]
- Argyle, M.D.; Bartholomew, C.H. Heterogeneous catalyst deactivation and regeneration: A review. Catalysts 2015, 5, 145–269. [Google Scholar] [CrossRef]
- Luo, Y.; Guda, V.K.; Steele, P.H.; Mitchell, B.; Yu, F. Hydrodeoxygenation of oxidized distilled bio-oil for the production of gasoline fuel type. Energy Convers. Manag. 2016, 112, 319–327. [Google Scholar] [CrossRef]
- Cheng, S.; Wei, L.; Zhao, X.; Kadis, E.; Cao, Y.; Julson, J.; Gu, Z. Hydrodeoxygenation of prairie cordgrass bio-oil over Ni based activated carbon synergistic catalysts combined with different metals. New Biotechnol. 2016, 33, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Jie, Y.; Li, B.; Kai, X.; Yan, Z.; Li, R. Catalytic hydrodeoxygenation of crude bio-oil over an unsupported bimetallic dispersed catalyst in supercritical ethanol. Fuel Process. Technol. 2016, 148, 19–27. [Google Scholar] [CrossRef]
- Oh, S.; Hwang, H.; Choi, H.S.; Choi, J.W. Investigation of chemical modifications of micro-and macromolecules in bio-oil during hydrodeoxygenation with Pd/C catalyst in supercritical ethanol. Chemosphere 2014, 117, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, P.M.; Gardini, D.; Damsgaard, C.D.; Grunwaldt, J.D.; Jensen, P.A.; Wagner, J.B.; Jensen, A.D. Deactivation of Ni-MoS 2 by bio-oil impurities during hydrodeoxygenation of phenol and octanol. Appl. Catal. A 2016, 523, 159–170. [Google Scholar] [CrossRef]
- Kadarwati, S.; Hu, X.; Gunawan, R.; Westerhof, R.; Gholizadeh, M.; Hasan, M.M.; Li, C.Z. Coke formation during the hydrotreatment of bio-oil using NiMo and CoMo catalysts. Fuel Process. Technol. 2016, 155, 261–268. [Google Scholar] [CrossRef]
- Hong, Y.K.; Lee, D.W.; Eom, H.J.; Lee, K.Y. The catalytic activity of Sulfided Ni/W/TiO2 (anatase) for the hydrodeoxygenation of Guaiacol. J. Mol. Catal. A Chem. 2014, 392, 241–246. [Google Scholar] [CrossRef]
- Oh, S.; Kim, U.J.; Choi, I.G.; Choi, J.W. Solvent effects on improvement of fuel properties during hydrodeoxygenation process of bio-oil in the presence of Pt/C. Energy 2016, 113, 116–123. [Google Scholar] [CrossRef]
- Huang, Y.; Wei, L.; Zhao, X.; Cheng, S.; Julson, J.; Cao, Y.; Gu, Z. Upgrading pine sawdust pyrolysis oil to green biofuels by HDO over zinc-assisted Pd/C catalyst. Energy Convers. Manag. 2016, 115, 8–16. [Google Scholar] [CrossRef]
- Dwiatmoko, A.A.; Zhou, L.; Kim, I.; Choi, J.W.; Suh, D.J.; Ha, J.M. Hydrodeoxygenation of lignin-derived monomers and lignocellulose pyrolysis oil on the carbon-supported Ru catalysts. Catal. Today 2016, 265, 192–198. [Google Scholar] [CrossRef]
- Wang, Y.; He, T.; Liu, K.; Wu, J.; Fang, Y. From biomass to advanced bio-fuel by catalytic pyrolysis/hydro-processing: hydrodeoxygenation of bio-oil derived from biomass catalytic pyrolysis. Bioresour. Technol. 2012, 108, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.H.; Park, R.S.; Kim, H.; Park, S.H.; Jung, S.C.; Jeon, J.K.; Kim, S.C.; Park, Y.K. Hydrodeoxygenation of guaiacol over Pt loaded zeolitic materials. J. Ind. Eng. Chem. 2016, 37, 18–21. [Google Scholar] [CrossRef]
- Hellinger, M.; Baier, S.; Mortensen, P.M.; Kleist, W.; Jensen, A.D.; Grunwaldt, J.D. Continuous Catalytic Hydrodeoxygenation of Guaiacol over Pt/SiO2 and Pt/H-MFI-90. Catalysts 2015, 5, 1152–1166. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Zhang, C.; Zhai, Y.; Liu, Y.; Zhang, R.; Tang, X. Upgrading of bio-oil using supercritical 1-Butanol over a Ru/C heterogeneous catalyst: role of the solvent. Energy Fuels 2014, 28, 4611–4621. [Google Scholar] [CrossRef]
- Huynh, T.M.; Armbruster, U.; Atia, H.; Bentrup, U.; Phan, B.M.Q.; Eckelt, R.; Nguyen, L.H.; Nguyen, D.A.; Martin, A. Upgrading of bio-oil and subsequent co-processing under FCC conditions for fuel production. React. Chem. Eng. 2016, 1, 239–251. [Google Scholar] [CrossRef]
- Ahmadi, S.; Yuan, Z.; Rohani, S.; Xu, C.C. Effects of nano-structured CoMo catalysts on hydrodeoxygenation of fast pyrolysis oil in supercritical ethanol. Catal. Today 2016, 269, 182–194. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, T.; Ma, L.; Zhang, Q.; Jiang, T. Hydrotreatment of bio-oil over Ni-based catalyst. Bioresour. Technol. 2013, 127, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Shafaghat, H.; Rezaei, P.S.; Daud, W.M.A.W. Catalytic hydrodeoxygenation of simulated phenolic bio-oil to cycloalkanes and aromatic hydrocarbons over bifunctional metal/acid catalysts of Ni/HBeta, Fe/HBeta and NiFe/HBeta. J. Ind. Eng. Chem. 2016, 35, 268–276. [Google Scholar] [CrossRef]
- Wang, L.; Ye, P.; Yuan, F.; Li, S.; Ye, Z. Liquid phase in-situ hydrodeoxygenation of bio-derived phenol over Raney Ni and Nafion/SiO2. Int. J. Hydrog. Energy 2015, 40, 14790–14797. [Google Scholar] [CrossRef]
- Sankaranarayanan, T.M.; Berenguer, A.; Ochoa-Hernández, C.; Moreno, I.; Jana, P.; Coronado, J.M.; Serrano, D.P.; Pizarro, P. Hydrodeoxygenation of anisole as bio-oil model compound over supported Ni and Co catalysts: Effect of metal and support properties. Catal. Today 2015, 243, 163–172. [Google Scholar] [CrossRef]
- Ambursa, M.M.; Ali, T.H.; Lee, H.V.; Sudarsanam, P.; Bhargava, S.K.; Hamid, S.B.A. Hydrodeoxygenation of dibenzofuran to bicyclic hydrocarbons using bimetallic Cu–Ni catalysts supported on metal oxides. Fuel 2016, 180, 767–776. [Google Scholar] [CrossRef]
- Mortensen, P.M.; Grunwaldt, J.D.; Jensen, P.A.; Jensen, A.D. Influence on nickel particle size on the hydrodeoxygenation of phenol over Ni/SiO2. Catal. Today 2016, 259, 277–284. [Google Scholar] [CrossRef]
- Yang, Y.; Ochoa-Hernández, C.; Pizarro, P.; Víctor, A.; Coronado, J.M.; Serrano, D.P. Ce-promoted Ni/SBA-15 catalysts for anisole hydrotreating under mild conditions. Appl. Catal. B 2016, 197, 206–213. [Google Scholar] [CrossRef]
- Xu, Y.; Li, Y.; Wang, C.; Wang, C.; Ma, L.; Wang, T.; Zhang, X.; Zhang, Q. In-situ hydrogenation of model compounds and raw bio-oil over Ni/CMK-3 catalyst. Fuel Process. Technol. 2016. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, T.; Ma, L.; Zhang, Q.; Liang, W. Upgrading of the liquid fuel from fast pyrolysis of biomass over MoNi/γ-Al2O3 catalysts. Appl. Energy 2010, 87, 2886–2891. [Google Scholar] [CrossRef]
- Martínez, N.; García, R.; Fierro, J.L.G.; Wheeler, C.; Austin, R.N.; Gallagher, J.R.; Miller, J.T.; Krause, T.R.; Escalona, N.; Sepúlveda, C. Effect of Cu addition as a promoter on Re/SiO2 catalysts in the hydrodeoxygenation of 2-methoxyphenol as a model bio oil compound. Fuel 2016, 186, 112–121. [Google Scholar] [CrossRef]
- Tran, N.T.; Uemura, Y.; Chowdhury, S.; Ramli, A. Vapor-phase hydrodeoxygenation of guaiacol on Al-MCM-41 supported Ni and Co catalysts. Appl. Catal. A 2016, 512, 93–100. [Google Scholar] [CrossRef]
- Sun, J.; Karim, A.M.; Zhang, H.; Kovarik, L.; Li, X.S.; Hensley, A.J.; McEwen, J.S.; Wang, Y. Carbon-supported bimetallic Pd–Fe catalysts for vapor-phase hydrodeoxygenation of guaiacol. J. Catal. 2013, 306, 47–57. [Google Scholar] [CrossRef]
- Lai, Q.; Zhang, C.; Holles, J.H. Hydrodeoxygenation of Guaiacol over Ni@ Pd and Ni@ Pt Bimetallic Overlayer Catalysts. Appl. Catal. A 2016, 528, 1–13. [Google Scholar] [CrossRef]
- Qiu, S.; Xu, Y.; Weng, Y.; Ma, L.; Wang, T. Efficient Hydrogenolysis of Guaiacol over Highly Dispersed Ni/MCM-41 Catalyst Combined with HZSM-5. Catalysts 2016, 6, 134. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, T.; Ma, L.; Zhang, Q.; Yu, Y.; Liu, Q. Characterization and catalytic properties of Ni and NiCu catalysts supported on ZrO2–SiO2 for guaiacol hydrodeoxygenation. Catal. Commun. 2013, 33, 15–19. [Google Scholar] [CrossRef]
- Han, Y.; McIlroy, D.N.; McDonald, A.G. Hydrodeoxygenation of pyrolysis oil for hydrocarbon production using nanospring based catalysts. J. Anal. Appl. Pyrolysis 2016, 117, 94–105. [Google Scholar] [CrossRef]
- Duan, P.; Zhang, C.; Wang, F.; Fu, J.; Lü, X.; Xu, Y.; Shi, X. Activated carbons for the hydrothermal upgrading of crude duckweed bio-oil. Catal. Today 2016, 274, 73–81. [Google Scholar] [CrossRef]
- Le, T.A.; Ly, H.V.; Kim, J.; Kim, S.S.; Choi, J.H.; Woo, H.C.; Othman, M.R. Hydrodeoxygenation of 2-furyl methyl ketone as a model compound in bio-oil from pyrolysis of Saccharina Japonica Alga in fixed-bed reactor. Chem. Eng. J. 2014, 250, 157–163. [Google Scholar] [CrossRef]
- Ly, H.V.; Im, K.; Go, Y.; Galiwango, E.; Kim, S.S.; Kim, J.; Choi, J.H.; Woo, H.C. Spray pyrolysis synthesis of γ-Al2O3 supported metal and metal phosphide catalysts and their activity in the hydrodeoxygenation of a bio-oil model compound. Energy Convers. Manag. 2016, 127, 545–553. [Google Scholar] [CrossRef]
- Hakim, S.H.; Shanks, B.H.; Dumesic, J.A. Catalytic upgrading of the light fraction of a simulated bio-oil over CeZrO x catalyst. Appl. Catal. B 2013, 142, 368–376. [Google Scholar] [CrossRef]
- Wang, W.; Yang, S.; Qiao, Z.; Liu, P.; Wu, K.; Yang, Y. Preparation of Ni–W–P–B amorphous catalyst for the hydrodeoxygenation of p-cresol. Catal. Commun. 2015, 60, 50–54. [Google Scholar] [CrossRef]
- Peroni, M.; Mancino, G.; Baráth, E.; Gutiérrez, O.Y.; Lercher, J.A. Bulk and γ-Al2O3-supported Ni2P and MoP for hydrodeoxygenation of palmitic acid. Appl. Catal. B 2016, 180, 301–311. [Google Scholar] [CrossRef]
- Boullosa-Eiras, S.; Lødeng, R.; Bergem, H.; Stöcker, M.; Hannevold, L.; Blekkan, E.A. Catalytic hydrodeoxygenation (HDO) of phenol over supported molybdenum carbide, nitride, phosphide and oxide catalysts. Catal. Today 2014, 223, 44–53. [Google Scholar] [CrossRef]
- Yang, Y.; Gilbert, A.; Xu, C.C. Hydrodeoxygenation of bio-crude in supercritical hexane with sulfided CoMo and CoMoP catalysts supported on MgO: a model compound study using phenol. Appl. Catal. A 2009, 360, 242–249. [Google Scholar] [CrossRef]
- Popov, A.; Kondratieva, E.; Mariey, L.; Goupil, J.M.; El Fallah, J.; Gilson, J.P.; Travert, A.; Maugé, F. Bio-oil hydrodeoxygenation: Adsorption of phenolic compounds on sulfided (Co)Mo catalysts. J. Catal. 2013, 297, 176–186. [Google Scholar] [CrossRef]
- Gandarias, I.; Barrio, V.L.; Requies, J.; Arias, P.L.; Cambra, J.F.; Güemez, M.B. From biomass to fuels: Hydrotreating of oxygenated compounds. Int. J. Hydrog. Energy 2008, 33, 3485–3488. [Google Scholar] [CrossRef]
- Horáček, J.; Kubička, D. Bio-oil hydrotreating over conventional CoMo & NiMo catalysts: The role of reaction conditions and additives. Fuel 2016. [Google Scholar] [CrossRef]
- He, Z.; Wang, X. Hydrodeoxygenation of model compounds and catalytic systems for pyrolysis bio-oils upgrading. Catal. Sustain. Energy 2012, 1, 28–52. [Google Scholar] [CrossRef]
- Wildschut, J.; Mahfud, F.H.; Venderbosch, R.H.; Heeres, H.J. Hydrotreatment of fast pyrolysis oil using heterogeneous noble-metal catalysts. Ind. Eng. Chem. Res. 2009, 48, 10324–10334. [Google Scholar] [CrossRef]
- Ardiyanti, A.R.; Gutierrez, A.; Honkela, M.L.; Krause, A.O.I.; Heeres, H.J. Hydrotreatment of wood-based pyrolysis oil using zirconia-supported mono-and bimetallic (Pt, Pd, Rh) catalysts. Appl. Catal. A 2011, 407, 56–66. [Google Scholar] [CrossRef]
- Ardiyanti, A.R.; Khromova, S.A.; Venderbosch, R.H.; Yakovlev, V.A.; Heeres, H.J. Catalytic hydrotreatment of fast-pyrolysis oil using non-sulfided bimetallic Ni-Cu catalysts on a δ-Al2O3 support. Appl. Catal. B 2012, 117, 105–117. [Google Scholar] [CrossRef]
- Leng, S.; Wang, X.; He, X.; Liu, L.; Liu, Y.E.; Zhong, X.; Zhuang, G.; Wang, J.G. NiFe/γ-Al2O3: A universal catalyst for the hydrodeoxygenation of bio-oil and its model compounds. Catal. Commun. 2013, 41, 34–37. [Google Scholar] [CrossRef]
- Olcese, R.N.; Bettahar, M.; Petitjean, D.; Malaman, B.; Giovanella, F.; Dufour, A. Gas-phase hydrodeoxygenation of guaiacol over Fe/SiO2 catalyst. Appl. Catal. B 2012, 115, 63–73. [Google Scholar] [CrossRef]
- Wang, W.; Yang, Y.; Luo, H.; Hu, T.; Liu, W. Amorphous Co–Mo–B catalyst with high activity for the hydrodeoxygenation of bio-oil. Catal. Commun. 2011, 12, 436–440. [Google Scholar] [CrossRef]
- Wang, W.; Yang, Y.; Luo, H.; Liu, W. Characterization and hydrodeoxygenation properties of Co promoted Ni–Mo–B amorphous catalysts: influence of Co content. React. Kinet. Mech. Catal. 2010, 101, 105–115. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Y.; Zhao, L.; Wei, W. Catalytic activities of NiMo carbide supported on SiO2 for the hydrodeoxygenation of ethyl benzoate, acetone, and acetaldehyde. Energy Fuels 2010, 24, 2052–2059. [Google Scholar] [CrossRef]
- Ghampson, I.T.; Sepúlveda, C.; Garcia, R.; Frederick, B.G.; Wheeler, M.C.; Escalona, N.; DeSisto, W.J. Guaiacol transformation over unsupported molybdenum-based nitride catalysts. Appl. Catal. B 2012, 413, 78–84. [Google Scholar] [CrossRef]
- Koike, N.; Hosokai, S.; Takagaki, A.; Nishimura, S.; Kikuchi, R.; Ebitani, K.; Suzuki, Y.; Oyama, S.T. Upgrading of pyrolysis bio-oil using nickel phosphide catalysts. J. Catal. 2016, 333, 115–126. [Google Scholar] [CrossRef]
- Lee, H.; Kim, Y.M.; Lee, I.G.; Jeon, J.K.; Jung, S.C.; Do Chung, J.; Choi, W.G.; Park, Y.K. Recent advances in the catalytic hydrodeoxygenation of bio-oil. Korean J. Chem. Eng. 2015, 1–17. [Google Scholar] [CrossRef]
- Mortensen, P.M.; Grunwaldt, J.D.; Jensen, P.A.; Knudsen, K.G.; Jensen, A.D. A review of catalytic upgrading of bio-oil to engine fuels. Appl. Catal. A 2011, 407, 1–19. [Google Scholar] [CrossRef]
- Gao, D.; Schweitzer, C.; Hwang, H.T.; Varma, A. Conversion of guaiacol on noble metal catalysts: reaction performance and deactivation studies. Ind. Eng. Chem. Res. 2014, 53, 18658–18667. [Google Scholar] [CrossRef]
- Popov, A.; Kondratieva, E.; Goupil, J.M.; Mariey, L.; Bazin, P.; Gilson, J.P.; Travert, A.; Maugé, F. Bio-oils hydrodeoxygenation: adsorption of phenolic molecules on oxidic catalyst supports. J. Phys. Chem. C 2010, 114, 15661–15670. [Google Scholar] [CrossRef]
- Zanuttini, M.S.; Dalla Costa, B.O.; Querini, C.A.; Peralta, M.A. Hydrodeoxygenation of m-cresol with Pt supported over mild acid materials. Appl. Catal. A 2014, 482, 352–361. [Google Scholar] [CrossRef]
- Furimsky, E.; Massoth, F.E. Deactivation of hydroprocessing catalysts. Catal. Today 1999, 52, 381–495. [Google Scholar] [CrossRef]
- Echeandia, S.; Arias, P.L.; Barrio, V.L.; Pawelec, B.; Fierro, J.L.G. Synergy effect in the HDO of phenol over Ni–W catalysts supported on active carbon: Effect of tungsten precursors. Appl. Catal. B 2010, 101, 1–12. [Google Scholar] [CrossRef]
- De La Puente, G.; Gil, A.; Pis, J.J.; Grange, P. Effects of support surface chemistry in hydrodeoxygenation reactions over CoMo/activated carbon sulfided catalysts. Langmuir 1999, 15, 5800–5806. [Google Scholar] [CrossRef]
- Wang, B.; Duan, P.G.; Xu, Y.P.; Wang, F.; Shi, X.L.; Fu, J.; Lu, X.Y. Co-hydrotreating of algae and used engine oil for the direct production of gasoline and diesel fuels or blending components. Energy 2016. [Google Scholar] [CrossRef]
- Elliott, D.C.; Hart, T.R.; Neuenschwander, G.G.; Rotness, L.J.; Olarte, M.V.; Zacher, A.H.; Solantausta, Y. Catalytic hydroprocessing of fast pyrolysis bio-oil from pine sawdust. Energy Fuels 2012, 26, 3891–3896. [Google Scholar] [CrossRef]
- Li, X.; Gunawan, R.; Wang, Y.; Chaiwat, W.; Hu, X.; Gholizadeh, M.; Mourant, D.; Bromly, J.; Li, C.Z. Upgrading of bio-oil into advanced biofuels and chemicals. Part III. Changes in aromatic structure and coke forming propensity during the catalytic hydrotreatment of a fast pyrolysis bio-oil with Pd/C catalyst. Fuel 2014, 116, 642–649. [Google Scholar] [CrossRef]
- Jacobson, K.; Maheria, K.C.; Dalai, A.K. Bio-oil valorization: A review. Renew. Sustain. Energy Rev. 2013, 23, 91–106. [Google Scholar] [CrossRef]
- Zhang, S.; Yan, Y.; Li, T.; Ren, Z. Upgrading of liquid fuel from the pyrolysis of biomass. Bioresour. Technol. 2005, 96, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Li, M.L.; Duan, P.G.; Fu, J.; Lü, X.Y.; Xu, Y.P. Co-hydrotreating of used engine oil and the low-boiling fraction of bio-oil blends for the production of liquid fuel. Fuel Process. Technol. 2016, 146, 62–69. [Google Scholar] [CrossRef]
- Chen, W.; Luo, Z.; Yu, C.; Li, G.; Yang, Y.; Zhang, H. Upgrading of bio-oil in supercritical ethanol: Catalysts screening, solvent recovery and catalyst stability study. J. Supercrit. Fluids 2014, 95, 387–393. [Google Scholar] [CrossRef]
| Physicochemical Properties | Bio-Oil | Heavy Fuel Oil |
|---|---|---|
| Water content (wt%) | 12–30 | 0.10 |
| Carbon (wt%) | 41.70–69.50 | 85.60–86.68 |
| Hydrogen (wt%) | 5.70–9.40 | 10.30–12.04 |
| Oxygen (wt%) | 19.40–50.30 | 0.60–0.65 |
| Nitrogen (wt%) | 0–9.80 | 0.60 |
| Sulfur (wt%) | 0–0.77 | 2.50 |
| Ash (wt%) | <0.25 | 0.04 |
| pH | 2.26–4.30 | - |
| Viscosity (Pa·s) | 11.10–62.20@25 °C | 0.23@30 °C |
| Density (g/mL) | 0.98–1.19 | 0.94 |
| Higher heating value (HHV, MJ/kg) | 17.40–32.46 | 44.17 |
| Reference | [12,16,17,18,19,20,21,22,23,24] | [12,16,17,18,19,20,21,22,23,24] |
| Feedstock | Switchgrass (% 1) | Rice Husk (%) | Palm Shells (%) | Pine Sawdust (%) | Algae (%) | Corn Stover (wt% 2) | Switchgrass (wt%) | Algae (wt%) | Pine (wt%) | Hardwood (wt%) | Softwood (wt%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Temperature (°C) | 510 | 450 | 490 | 500 | 500 | 500 | 500 | 360 | 520 | 460 | 510 |
| Phenols | 18.95 | 29.20 | 50.44 | 16.98 | 27.93 | 2.39 | 0.97 | 1.70 | - | 1.40–3.90 | 1.40–3.90 |
| Ketones | 9.86 | 2.80 | - | - | 3.16 | 0.20 | 1.39 | 1.50 | 5.36 | 0.08–0.96 | 0.02–0.73 |
| Aldehydes | 10.26 | 0.00 | 3.42 | - | - | 4.00 | - | - | 9.73 | 1.03–14.36 | 0.52–0.70 |
| Acids | 3.25 | 5.10 | 6.87 | 4.64 | 10.42 | 6.26 | 10.03 | 0.50 | 5.60 | 3.30–21.50 | 2.20–19.00 |
| Esters | 4.23 | - 3 | - | - | - | - | - | - | - | - | |
| Furans | 7.81 | 2.30 | - | - | 6.41 | 0.71 | 3.38 | - | 4.47 | 0.20–1.93 | 0.39–1.83 |
| Ethers | 9.22 | - | 4.51 | - | - | - | - | - | - | - | - |
| Alcohols | 5.66 | 9.40 | 1.01 | - | 0.36 | 7.12 | 0.64 | - | 2.9 | 6.41–7.82 | 1.78–3.17 |
| Others | 30.76 | 51.20 | 33.75 | 78.38 | 51.72 | 79.32 | 83.59 | 96.30 | 71.94 | 49.53–87.58 | 70.67–94.21 |
| Reference | [19] | [31] | [16] | [18] | [21] | [32] | [33] | [34] | [15,35] | [36] | [36] |
| Catalysts | Feedstocks | Temperature (°C) | Reactor Type | Main Products | Product Yield (wt%) | Product Compositions (Peak Area %) | Coke Yield (wt%) | Findings | References |
|---|---|---|---|---|---|---|---|---|---|
| Zeolite catalysts | |||||||||
| HZSM-5, ZnO/HZSM-5, Ga2O3/HZSM-5, CuO/HZSM-5, Mo/HZSM-5, Cu/HZSM-5, Mo–Cu/HZSM-5, β-zeolite, Co/HZSM-5, Co-Mo/HZSM-5 | Rape straw bio-oil, hydroxypropanone, cyclopentanone, acetic acid, phenol, guaiacol, pine sawdust bio-oil, jatropha bio-oil, prairie cordgrass bio-oil | 400–600 | Fixed-bed, quartz tube | Hydrocarbons | 4.58–34.06 (OP 1) | 25.71–96.9 | 0.33–25.07 | HZSM-5 with Si/Al ratio of 50 obtained high oil phase yield. | [27,30,37,38,39] |
| Ni/HZSM-5, P/HZSM-5, Zn/HZSM-5, Ti/ HZSM-5, Mg/ZSM-5, Ni/ZSM-5, Cu/ZSM-5, Ga/ZSM-5, Sn/ZSM-5, Ultrastable-Y, ZSM-5, Beta-zeolite, Zn/ZSM-5, Zn/Na-ZSM-5, ZSM-5 + SiAl, NiMo/SiAl, Zn/ZSM-5 | Prairie cordgrass bio-oil, rape straw bio-oil, pine wood bio-oil, fallopia japonica bio-oil, camelina oil, carinata oil, jatropha oil, douglas fir sawdust bio-oil | 268.9–600 | Fixed-bed | Hydrocarbons | 15.02–82.88 (W 2) | 16–93.69 | 1.1–2.5 | 12% Ni/HZSM-5 yielded the highest amount of gasoline hydrocarbons. Higher reaction temperature is required to maintain zeolite recycle. | [40,41,42,43,44,45,46,47,48] |
| H-Mordenite, H-ZSM-5, H-Y, H-Beta, H-Ferrierite, Cu/β-zeolite, Co/ZSM-5, Fe/ZSM-5, Ni/ZSM-5, Ce/ZSM-5, Ga/ZSM-5, Cu/ZSM-5, Na/ZSM-5, CaO/HZSM-5, | Oak, corn cob, corn stover, switchgrass bio-oil, Japanese knotweed bio-oil, aspen wood bio-oil, cellulose bio-oil, straw lignin bio-oil, corn stover bio-oil | 400–600 | Micro pyrolyzer, fixed-bed, tubular quartz | Hydrocarbons | 1–93.30 | - 3 | 0.71–31.2 | H-ZSM-5 was most effective for producing aromatic hydrocarbons. Cu doping decreased coke deposit on spent catalyst. | [49,50,51,52] |
| Ga/Meso-MFI, HZSM-5, Meso-MFI, HY, waste FCC | Radiata pine sawdust bio-oil, wild reed bio-oil | 500 | Fixed-bed, U-tube quartz | Aromatic hydrocarbons, furans, phenolics | 11.7–15.4 (OP) | 2–42.7 (wt% 4) | 17.3–21.3 | Ga/Meso-MFI increased organic fraction of bio-oil and catalyst resistance to coke deposition. | [53,54] |
| Oxides catalysts | |||||||||
| CaO, MCM-41, MgO, NiO, Al2O3, FCC, ZSM-5, ZrO2/TiO2, Silica alumina, MgO, B2O3, Na2CO3/γ-Al2O3 | Corncob bio-oil, beech wood bio-oil, empty palm oil fruit bunch bio-oil, oil palm fronds bio-oil, woody fiber bio-oil | 400–1000 | Thermogravimetric analyzer , fixed-bed | Aromatic hydrocarbons, phenolics, | 5.46–37.1 (OP) | 0–49.8 | - | ZrO2/TiO2 and ZSM-5 effectively reduced bio-oil oxygen content. B2O3 promoted cleavage of C–O bond. | [55,56,57,58,59] |
| Nano MgO, CaO, TiO2, Fe2O3, NiO, ZnO, Zn/Al2O3, Ce/Al2O3, Ni/Al2O3, Mo2N/γ-Al2O3 | Poplar wood bio-oil, sunflower stalk bio-oil, lignin bio-oil | 500–850 | Fixed-bed, pyroprobe pyrolzer | Hydrocarbons | 6.7–17.5 | - | 5.37–8.59 | CaO greatly reduced acids in bio-oil. | [60,61,62] |
| ZnO, sepiolite, bentonite, attapulgite, red mud, Ce/Al2O3, Ce/ZrO2, Ni–Ce/Al2O3, Ni–Ce/ZrO2, Mg–Ce/Al2O3, Mg–Ce/ZrO2 | Rice husk bio-oil, ,pine woodchips bio-oil, algae bio-oil | 400–600 | Auger, fixed-bed | Hydrocarbons, phenols | 16.77–49.91 (W) | 6.99–67.98 | - | NiCe/Al2O3 produced highest bio-oil yield with the lowest oxygen. | [63,64,65] |
| Other catalysts | |||||||||
| FCC | Pine woodchip bio-oil, gas oil | 540–560 | Fluidized bed | Gasoline, light cycle oil | - | - | - | Similar product yields were obtained from FCC feed when 10% bio-oil was added. | [66] |
| Fe/AC, K3PO4/Fe3O4 | Douglas fir pellet bio-oil, poplar wood bio-oil | 309–591 | Microwave, quartz tube | Furans, phenols, guaiacols, ketones, ethers | 23.30–45.20 (W) | 48.6–87 | - | Acid-catalyzed ring opening of furans forms aldehydes. | [67,68] |
| Catalysts | Feed Stocks | Temperature (°C) | Pressure (MPa) | Reactor Type | Main Products | Product Yield (wt%) | Product Compositions (Peak Area %) | Coke Yield (wt%) | Findings | References |
|---|---|---|---|---|---|---|---|---|---|---|
| Sulfided catalysts | ||||||||||
| Ni-MoS2/ZrO2, Sulfided NiMo and CoMo catalysts, Ni/W/TiO2 | Phenol, 1-octanol, mallee wood bio-oil, guaiacol | 150–300 | 5–10 | Packed bed, autoclave | Cyclohexane, cyclohexene, octane, octane, alkyl phenols | 16–46 | - 3 | 5.7–28.1 | Cl, S and K in bio-oil deactivated catalyst. | [95,96,97] |
| Noble metal catalysts | ||||||||||
| Pt/C | Miscanthus bio-oil | 250–350 | 3 | Autoclave | Heavy oil, light oil | 48.3–83.3 (W 1) | - | 3.8–16.1 | Ethanol improved bio-oil quality as a co-reactant. | [98] |
| Zn2+-Pd/C | Pine sawdust bio-oil | 150–350 | 1.38–4.14 | Autoclave | Hydrocarbons | 40.28–46.72 (OP 2) | 0.54–6.06 | 0.22–0.41 | Zn2+ and Pd had the synergistic effect for HDO bio-oil upgrading. | [99] |
| Ru/MWCNT, Ru/CARF, Ru/Vulcan carbon, Ru/AC, Ru/Graphite, Pt/carbon, Pd/carbon, Pt/HZSM-5, Pt/Mesoporous Beta, Pt/HBeta, Pt/MMZBeta, Pt/Al-MCM-48,Pt/Si-MCM-48, Pt/ZSM-5, Pt/Al2O3, Pt/SiO2, Pt/H-MFI-90 | Oak chips bio-oil, grass bio-oil, eucalyptus bio-oil, benthamii, bio-oil, equine manure bio-oil, guaiacol, cresol, dibenzofuran, 1-octanol | 50–320 | 4–14.5 | Autoclave or fixed-bed | Hydrocarbons | 0.08–91 | - | - | Ru/MWCNT was highly active for producing alkanes and cycloalkanes. Pt/Mesoporous Beta and Pt/HBeta showed higher guaiacol conversions. | [100,101,102,103] |
| Ru/C | Pine sawdust bio-oil | 250–300 | 2 | Autoclave | Esters, ethers | 42.3–84.6 (W) | 40.5 | 0.2–9.9 | Acids, aldehydes, ketones, phenols and furans contents of upgraded bio-oil decreased significantly. | [104] |
| Transition metal catalysts | ||||||||||
| Ni/AC, Ni-Fe/AC, Ni-Mo/AC, Ni-Cu/AC, Ni–Co/HZSM-5, Ni–Co/HBeta, Ni–Co/HY, Ni–Co/ZrO2 | Prairie cordgrass bio-oil, wood bio-oil | 250–350 | 3.4–5 | Autoclave | Hydrocarbons | 17.35–37.0 (OP) | 16.37–39.42 | 3.09–8.86 | Ni/AC produced the highest content of gasoline range hydrocarbons. 10Ni10Co/HZSM-5 shows the best performance in bio-oil HDO. | [92,105] |
| CoMo/AC, CoMo/γ-Al2O3, CoMo/HZSM-5,CoMo/MCM-41, CoMo/SBA-15, Ru/C | Sawdust bio-oil | 300–350 | 20.7–22.5 | Autoclave | Heavy oil, light oil | 55.0–66.6 (W) | - | <1–10 | CoMo/MCM-41 had better resistance to coke deposition than other CoMo catalysts. | [106] |
| Raney Ni, nafion/SiO2, nano Ni/SiO, Ni/HBeta, Fe/HBeta, NiFe/HBeta, Ni/HZSM-5, Ni/ZSM-5, Co/ZSM-5, Ni/SBA-15, Co/SBA-15, Ni/Al-SBA-15, Co/Al-SBA-15, Ni/Ce-SBA-15, Cu–Ni/TiO2, Cu–Ni/ZrO2, Cu–Ni/CeO2 | Phenol, o-cresol, anisole, dibenzo furan | 160–340 | 0.7–10 | Autoclave, Fixed-bed | Hydrocarbons | 3.1–92.4 | - | - | Raney Ni was effective for hydrogenation and Nafion/SiO2 was effective for dehydration. Small nickel particles favored deoxygenation and large particles favored hydrogenation. | [107,108,109,110,111,112,113] |
| Ni/CMK-3, MoNi/γ-Al2O3 | Pine sawdust bio-oil | 100–230 | 0.1–3 | Autoclave | Alcohols, esters | - | 48.21–51.14 | - | Hydrogen donors provided hydrogen for in-situ hydrogenation. Mo addition inhibited the formation of NiAl2O4. | [114,115] |
| Co/Al-MCM-41, Ni/Al-MCM-41, NiCo/Al-MCM-41, Cu/C, Fe/C, Pd/C, Pt/C, PdFe/C, Ru/C, Ni@Pd/silica alumina, Ni@Pt/silica alumina, CuRe/SiO2, Ni/MCM-41, HZSM-5, Ni/ZrO2–SiO2, NiCu/ZrO2–SiO2 | Guaiacol | 120–450 | 0.1–5 | Fixed-bed, autoclave | Hydrocarbons | 0.1–95.4 | - | - | Co was active in HDO via C–O hydrogenolysis and Ni favored C–C hydrogenolysis. Pd-Fe catalyst was active and selective for HDO. Overlayer catalysts enhanced deoxygenation activity. | [116,117,118,119,120,121] |
| Other catalysts | ||||||||||
| Ni-NS, Ru-NS, Ni–Al2O3, Ni/SiO2–Al2O3, Pine wood AC, coconut shell AC, bamboo stem AC, apricot pit AC, peach pit AC, coal AC, | Pine bio-oil, duckweed bio-oil | 300–400 | 4.1–6 | Autoclave | Hydrocarbons | 51.6–98.8 (W) | 46.37–89.37 | 5.3–10.1 | NS catalysts showed promising effect for upgrading bio-oils to biofuels. | [122,123] |
| CoP/Al2O3, CoMoP/γ-Al2O3, MoP/γ-Al2O3, Ni2P/γ-Al2O3, Ni/γ-Al2O3, Co/γ-Al2O3, Ni2P/γ-Al2O3, CoP/γ-Al2O3, CeZrOx | 2-furyl methyl ketone, acetic acid, acetol, furfural | 250–400 | 0.1 | Fixed-bed | Methyl cyclopentane, cyclohexane, pyruvaldehyde, 1,2-propylene glycol | 11–100 | - | - | CoP/Al2O3 had higher selectivity to methyl cyclopentane. | [124,125,126] |
| CoMo/MgO, CoMoP/MgO, Ni–W–P–B, Ni2P/γ-Al2O3, MoP/γ-Al2O3, Mo2C/TiO2, MoP/TiO2, Mo2N/TiO2, MoO3/TiO2 | Phenol, p-cresol, palmitic acid | 180–450 | 2.5–5 | Micro-reactor, autoclave, fixed-bed | Benzene, toluene, pentadecane, cyclohexyl-aromatics | - | 13.23–64.23 | - | CoMoP/MgO showed superior activity in phenol HDO. | [127,128,129,130] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, S.; Wei, L.; Zhao, X.; Julson, J. Application, Deactivation, and Regeneration of Heterogeneous Catalysts in Bio-Oil Upgrading. Catalysts 2016, 6, 195. https://doi.org/10.3390/catal6120195
Cheng S, Wei L, Zhao X, Julson J. Application, Deactivation, and Regeneration of Heterogeneous Catalysts in Bio-Oil Upgrading. Catalysts. 2016; 6(12):195. https://doi.org/10.3390/catal6120195
Chicago/Turabian StyleCheng, Shouyun, Lin Wei, Xianhui Zhao, and James Julson. 2016. "Application, Deactivation, and Regeneration of Heterogeneous Catalysts in Bio-Oil Upgrading" Catalysts 6, no. 12: 195. https://doi.org/10.3390/catal6120195







