Pulsed Laser Deposition of Platinum Nanoparticles as a Catalyst for High-Performance PEM Fuel Cells
Abstract
:1. Introduction
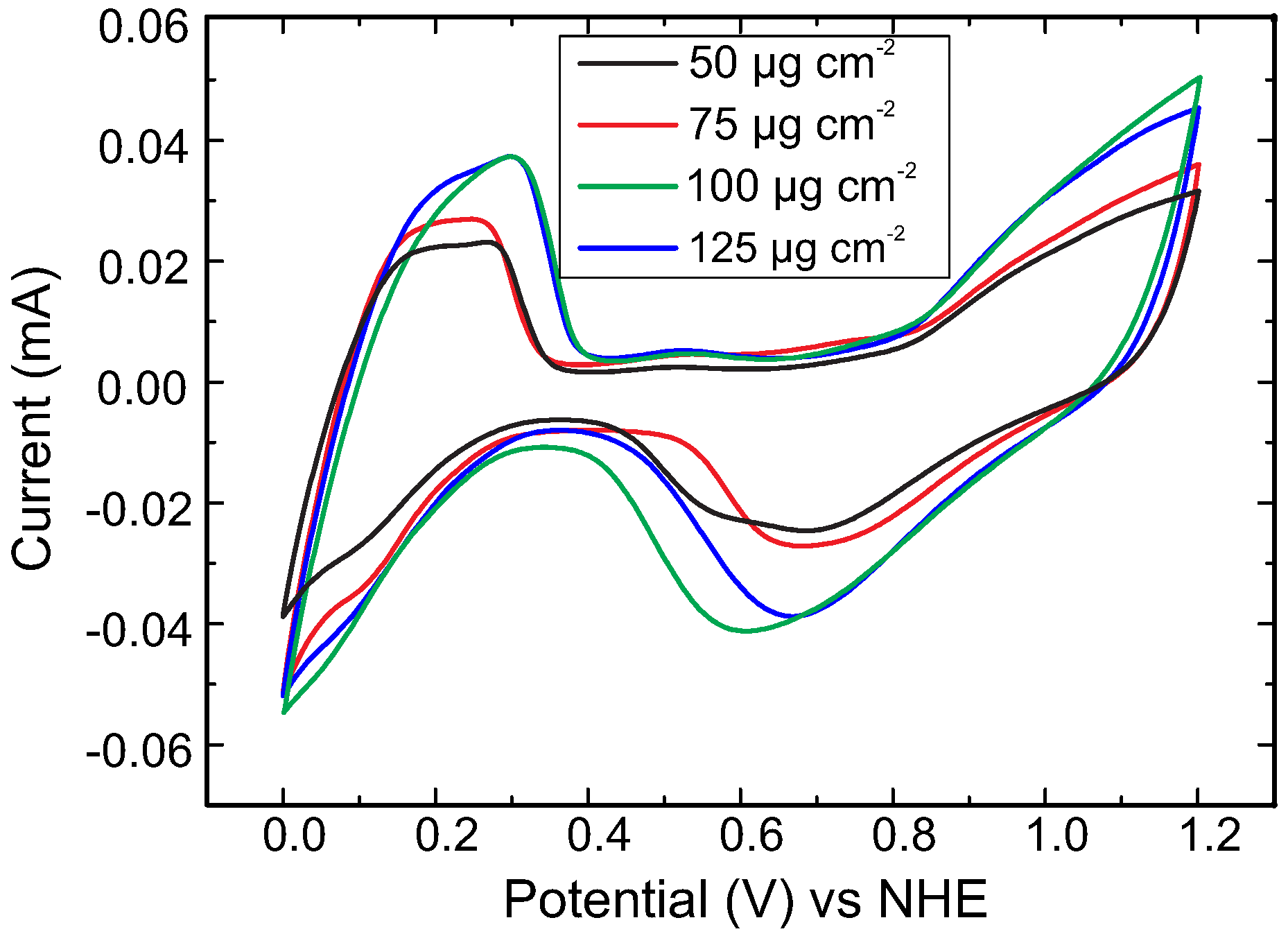
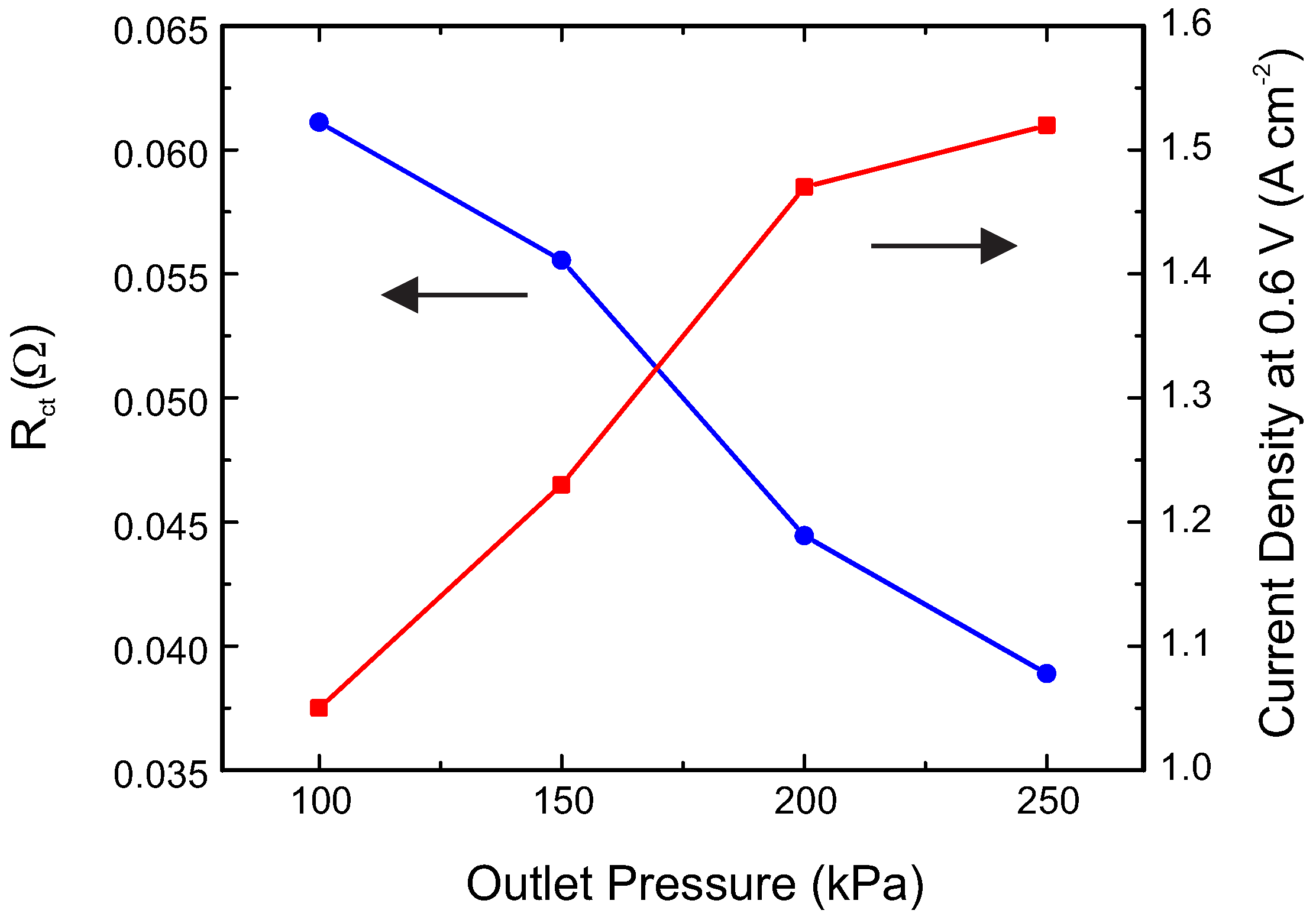
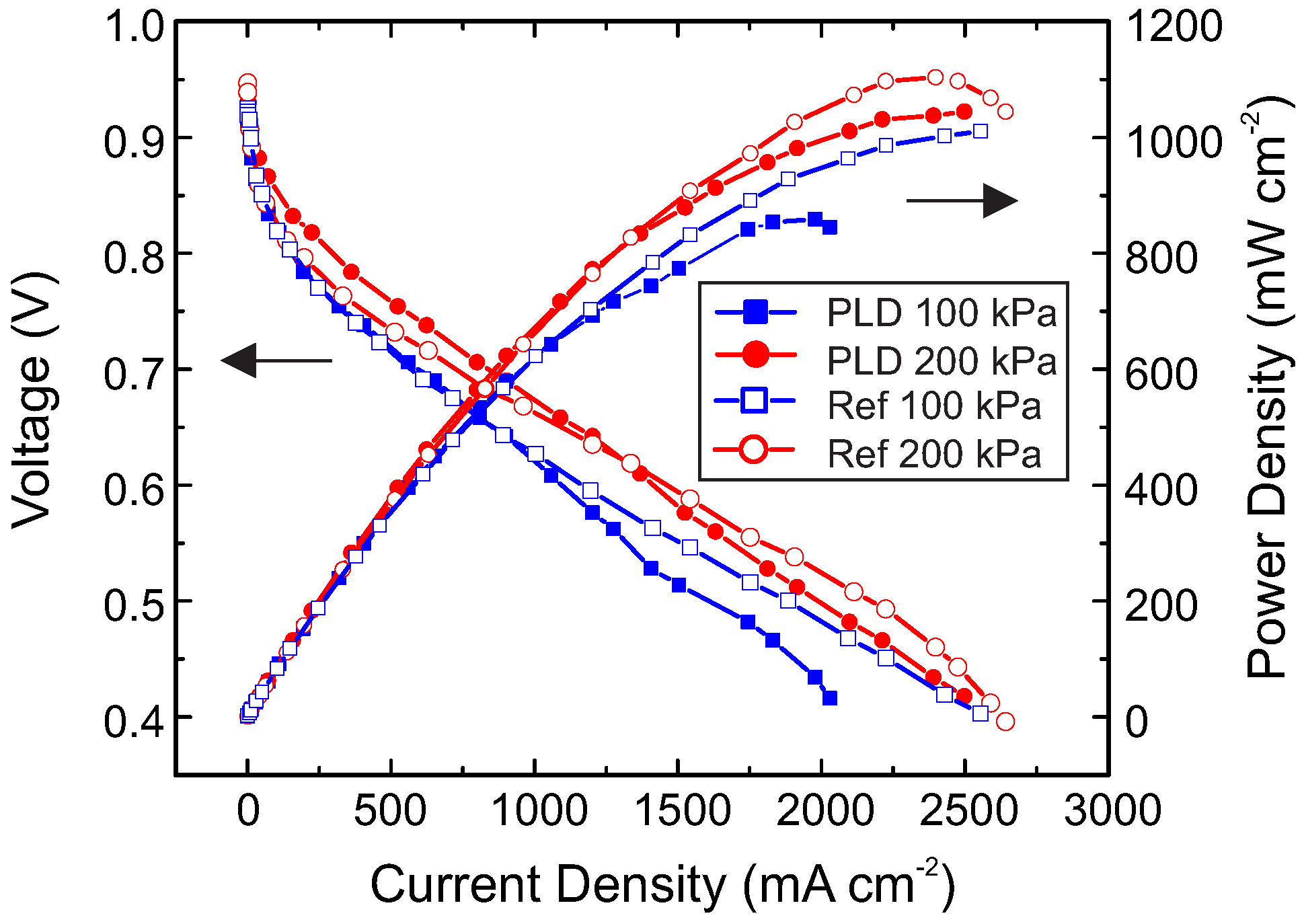
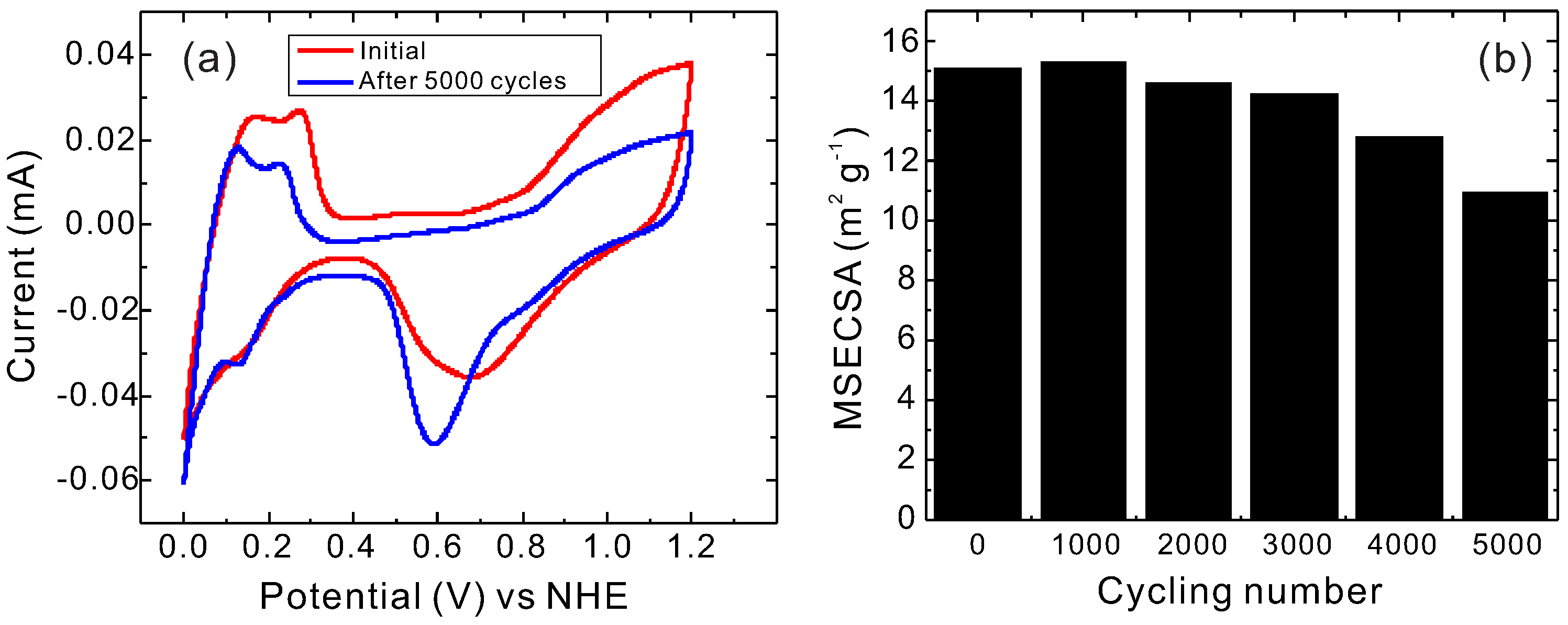
2. Results and Discussion
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Electrochemical Measurements
3.3. Fabrication of MEAs and Measurement of Polarization Curves
3.4. Morphology Characterizations
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Brouzgou, A.; Song, S.Q.; Tsiakaras, P. Low and non-platinum electrocatalysts for PEMFCs: Current status, challenges and prospects. Appl. Catal. B Environ. 2012, 127, 371–388. [Google Scholar] [CrossRef]
- Cuynet, S.; Caillard, A.; Lecas, T.; Bigarrè, J.; Buvat, P.; Brault, P. Deposition of Pt inside fuel cell electrodes using high power impulse magnetron sputtering. J. Phys. D Appl. Phys. 2014, 47, 272001. [Google Scholar] [CrossRef]
- Cavarroc, M.; Ennadjaoui, A.; Mougenot, M.; Brault, P.; Escalier, R.; Tessier, Y.; Durand, J.; Roualdès, S.; Sauvage, T.; Coutanceau, C. Performance of plasma sputtered fuel cell electrodes with ultra-low Pt loadings. Electrochem. Commun. 2009, 11, 859–861. [Google Scholar] [CrossRef]
- Cogenli, M.S.; Mukerjee, S.; Yurtcan, A.B. Membrane electrode assembly with ultra low platinum loading for cathode electrode of PEM fuel cell by using sputter deposition. Fuel Cells 2015, 15, 288–297. [Google Scholar] [CrossRef]
- Raso, M.A.; Carrillo, I.; Mora, E.; Navarro, E.; Garcia, M.A.; Leo, T.J. Electrochemical study of platinum deposited by electron beam evaporation for application as fuel cell electrodes. Int. J. Hydrog. Energy 2014, 39, 5301–5308. [Google Scholar] [CrossRef]
- Saha, M.S.; Gullà, A.F.; Allen, R.J.; Mukerjee, S. High performance polymer electrolyte fuel cells with ultra-low Pt loading electrodes prepared by dual ion-beam assisted deposition. Electrochim. Acta 2006, 51, 4680–4692. [Google Scholar] [CrossRef]
- Cunningham, N.; Irissou, E.; Lefèvre, M.; Denis, M.C.; Guay, D.; Dodelet, J.P. PEMFC anode with very low Pt loadings using pulsed laser deposition. Electrochem. Solid-State Lett. 2003, 6, A125–A128. [Google Scholar] [CrossRef]
- Mròz, W.; Budner, B.; Tokarz, W.; Piela, P.; Pawlowski, M.L.K. Ultra-low-loading pulsed-laser-deposited platinum catalyst films for polymer electrolyte membrane fuel cells. J. Power Sources 2015, 273, 885–893. [Google Scholar] [CrossRef]
- Hayre, R.O.; Lee, S.J.; Cha, S.W.; Prinz, F.B. A sharp peak in the performance of sputtered platinum fuel cells at ultra-low platinum loading. J. Power Sources 2002, 109, 483–493. [Google Scholar]
- Nakakubo, T.; Shibata, M.; Yasuda, K. Membrane electrode assembly for proton exchange membrane fuel cells prepared by sputter deposition in air and transfer method. J. Electrochem. Soc. 2005, 152, A2316–A2322. [Google Scholar] [CrossRef]
- Huang, T.W.; Qayyum, H.; Lin, G.R.; Chen, S.Y.; Tseng, C.J. Production of high-performance and improved-durability Pt-catalyst/support for proton-exchange-membrane fuel cells with pulsed laser deposition. J. Phys. D Appl. Phys. 2016, 49, 255601. [Google Scholar] [CrossRef]
- Riabinina, D.; Irissou, E.; Drogoff, B.L.; Chaker, M.; Guay, D. Influence of pressure on the Pt nanoparticle growth modes during pulsed laser ablation. J. Appl. Phys. 2010, 108, 034322-1–034322-6. [Google Scholar] [CrossRef]
- Debe, M.K. Electrocatalyst approaches and challenges for automotive fuel cells. Nature 2012, 486, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, E.; Taylor, S.; Rabis, A.; Levecque, P.; Conrad, O.; Kotz, R.; Schmidt, T.J. The effect of platinum nanoparticle distribution on oxygen electroreduction activity and selectivity. ChemCatChem 2014, 6, 1410–1418. [Google Scholar] [CrossRef]
- Yuan, X.; Wang, H.; Sun, J.C.; Zhang, J. AC impedance technique in PEM fuel cell diagnosis-A review. Int. J. Hydrog. Energy 2007, 32, 4365–4380. [Google Scholar] [CrossRef]
- Zhang, J.; Song, C.; Zhang, J.; Baker, R.; Zhang, L. Understanding the effects of backpressure on PEM fuel cell reactions and performance. J. Electroanal. Chem. 2013, 688, 130–136. [Google Scholar] [CrossRef]
- Zhang, J.; Li, H.; Zhang, J. Effect of operating backpressure on PEM fuel cell performance. ECS Trans. 2009, 19, 65–76. [Google Scholar]
- Onana, F.F.; Guillet, N.; AlMayouf, A.M. Modified pulse electrodeposition of Pt nanocatalyst as high-performance electrode for PEMFC. J. Power Sources 2014, 271, 401–405. [Google Scholar] [CrossRef]
- Yu, H.; Roller, J.M.; Mustain, W.E.; Maric, R. Influence of the ionomer/carbon ratio for low-Pt loading catalyst layer prepared by reactive spray deposition technology. J. Power Sources 2015, 283, 84–94. [Google Scholar] [CrossRef]
- Khan, A.; Nath, B.K.; Chutia, J. Nanopillar structured platinum with enhanced catalytic utilization for electrochemical reactions in PEMFC. Electrochim. Acta 2014, 146, 171–177. [Google Scholar] [CrossRef]
- Su, H.N.; Liao, S.J.; Shu, T.; Gao, H.L. Performance of an ultra-low platinum loading membrane electrode assembly prepared by a novel catalyst-sprayed membrane technique. J. Power Sources 2010, 195, 756–761. [Google Scholar] [CrossRef]
- Shu, T.; Dang, D.; Xu, D.W.; Chen, R.; Liao, S.J.; Hsieh, C.T.; Su, A.; Song, H.Y.; Du, L. High-performance MEA prepared by direct deposition of platinum on the gas diffusion layer using an atomic layer deposition technique. Electrochim. Acta 2015, 177, 168–173. [Google Scholar] [CrossRef]
- Yuan, Y.; Smith, J.A.; Goenaga, G.; Liu, D.J.; Luo, Z.; Liu, J. Platinum decorated aligned carbon nanotubes: electrocatalyst for improved performance of proton exchange membrane fuel cells. J. Power Sources 2011, 196, 6160–6167. [Google Scholar] [CrossRef]
- Hamel, C.; Garbarino, S.; Irissou, E.; Laplante, F.; Chaker, M.; Guay, D. Influence of the velocity of Pt ablated species on the structural and electrocatalytic properties of Pt thin films. Int. J. Hydrog. Energy 2010, 35, 8486–8493. [Google Scholar] [CrossRef]
- Greszler, T.A.; Caulk, D.; Sinha, P. The impact of platinum loading on oxygen transport resistance. J. Electrochem. Soc. 2012, 159, F831–F840. [Google Scholar] [CrossRef]
- Weber, A.Z.; Kusoglu, A. Unexplained transport resistances for low-loaded fuel-cell catalyst layers. J. Mater. Chem. A 2014, 2, 17207–17211. [Google Scholar] [CrossRef]
- Kongkanand, A.; Mathias, M.F. The priority and challenge of high-power performance of low-platinum proton-exchange membrane fuel cells. J. Phys. Chem. Lett. 2016, 7, 1127–1137. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Lin, G.; Shih, A.J.; Hu, S.J. Assembly pressure and membrane swelling in PEM fuel cells. J. Power Sources 2009, 192, 544–551. [Google Scholar] [CrossRef]
- Bauer, F.; Denneler, S.; Willert-Porada, M. Influence of temperature and humidity on the mechanical properties of Nafion 117 polymer electrolyte membrane. J. Polym. Sci. B Polym. Phys. 2005, 43, 786–795. [Google Scholar] [CrossRef]
- Eason, R. Pulsed Laser Deposition of Thin Films: Applications-Led Growth of Functional Materials, 1st ed.; John Wiley and Sons, Inc.: Hoboken, NJ, USA, 2006. [Google Scholar]
- Rodrìguez, J.M.D.; Meliàn, J.A.H.; Peña, J.P. Determination of the real surface area of Pt electrodes by hydrogen adsorption using cyclic voltammetry. J. Chem. Educ. 2000, 77, 1195–1197. [Google Scholar] [CrossRef]
- Wang, X.X.; Tan, Z.H.; Zeng, M.; Wang, J.N. Carbon nanocages: A new support material for Pt catalyst with remarkably high durability. Sci. Rep. 2014, 4, 4437. [Google Scholar] [CrossRef] [PubMed]
- Tsai, B.T.; Tseng, C.J.; Liu, Z.S.; Wang, C.H.; Lee, C.I.; Yang, C.C.; Lo, S.K. Effects of flow field design on the performance of a PEM fuel cell with metal foam as the flow distributor. Int. J. Hydrog. Energy 2012, 37, I3060–I3066. [Google Scholar] [CrossRef]
- Tseng, C.J.; Tsai, B.T.; Liu, Z.S.; Cheng, T.C.; Chang, W.C.; Lo, S.K. A PEM fuel cell with metal foam as flow distributor. Energy Convers. Manag. 2012, 62, 14–21. [Google Scholar] [CrossRef]
- Murata, S.; Imanishi, M.; Hasegawa, S.; Namba, R. Vertically aligned carbon nanotube electrodes for high current density operating proton exchange membrane fuel cells. J. Power Sources 2014, 253, 104–113. [Google Scholar] [CrossRef]
- Tseng, C.J.; Lo, S.T.; Lo, S.C.; Chu, P.P. Characterization of Pt-Cu binary catalysts for oxygen reduction for fuel cell applications. Mater. Chem. Phys. 2006, 100, 385–390. [Google Scholar] [CrossRef]
- Su, B.J.; Wang, K.W.; Cheng, T.C.; Tseng, C.J. Preparation of PtSn/C electrocatalysts with improved activity and durability toward oxygen reduction reaction by alcohol-reduction process. Mater. Chem. Phys. 2012, 135, 395–400. [Google Scholar] [CrossRef]
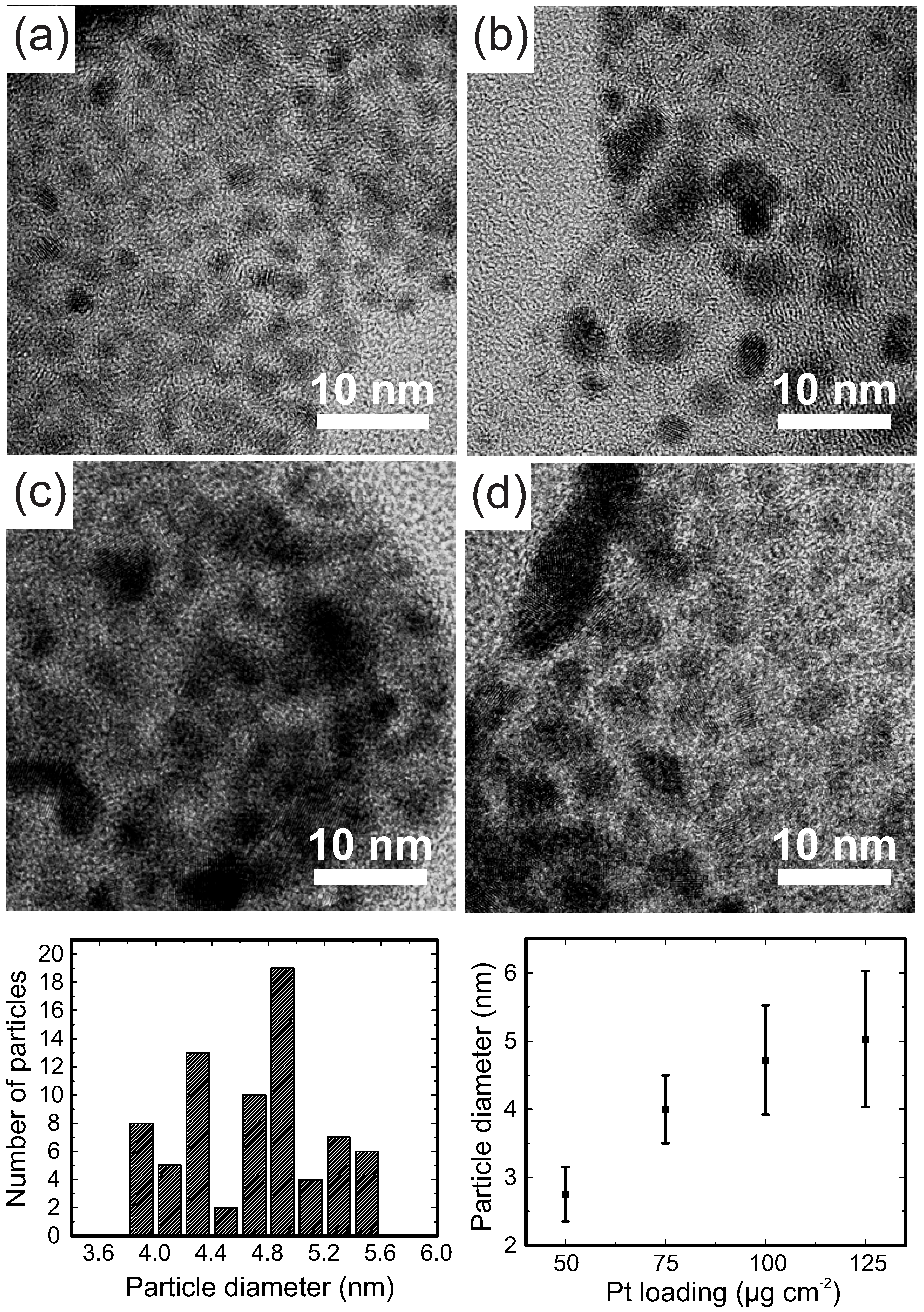
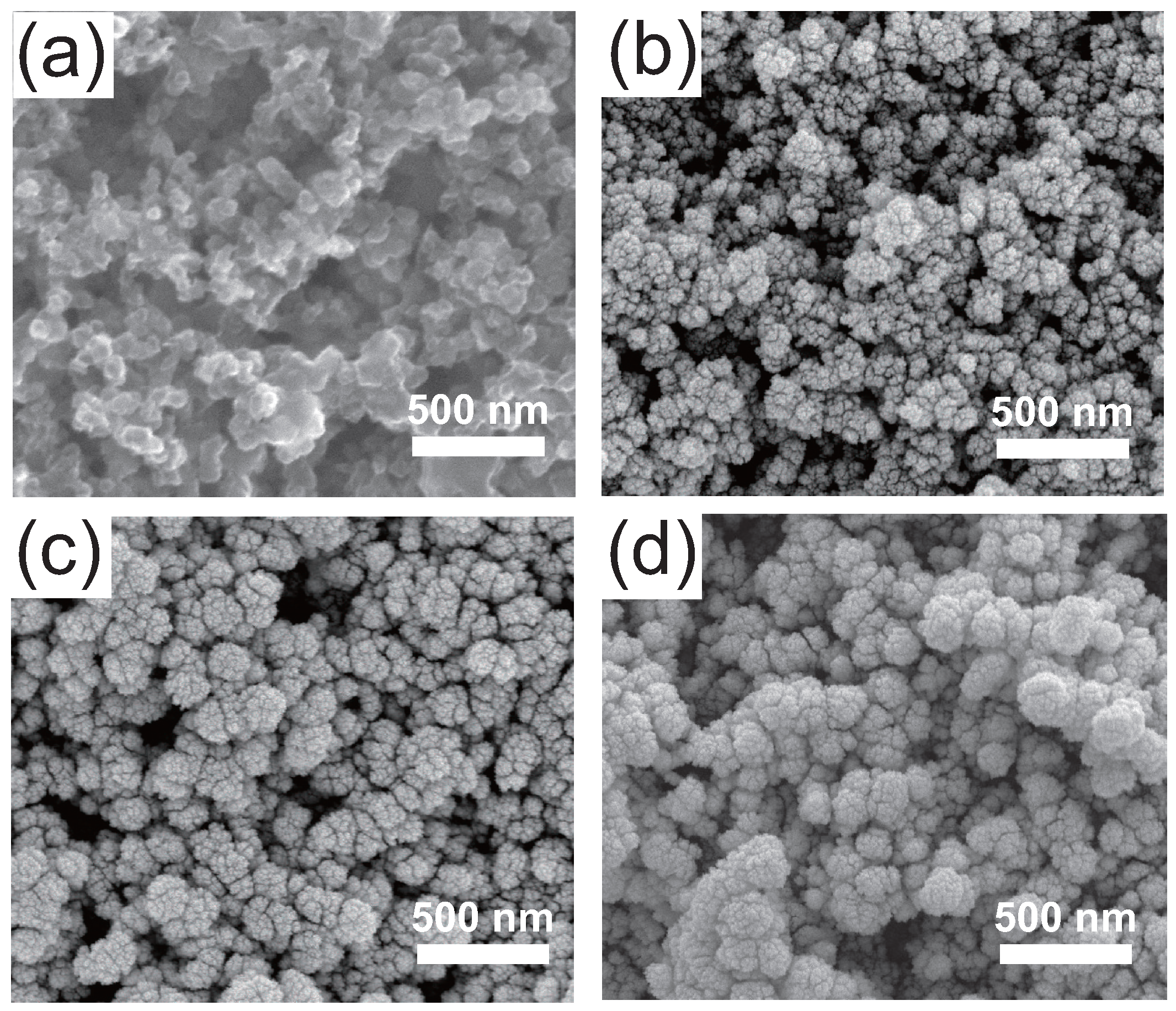
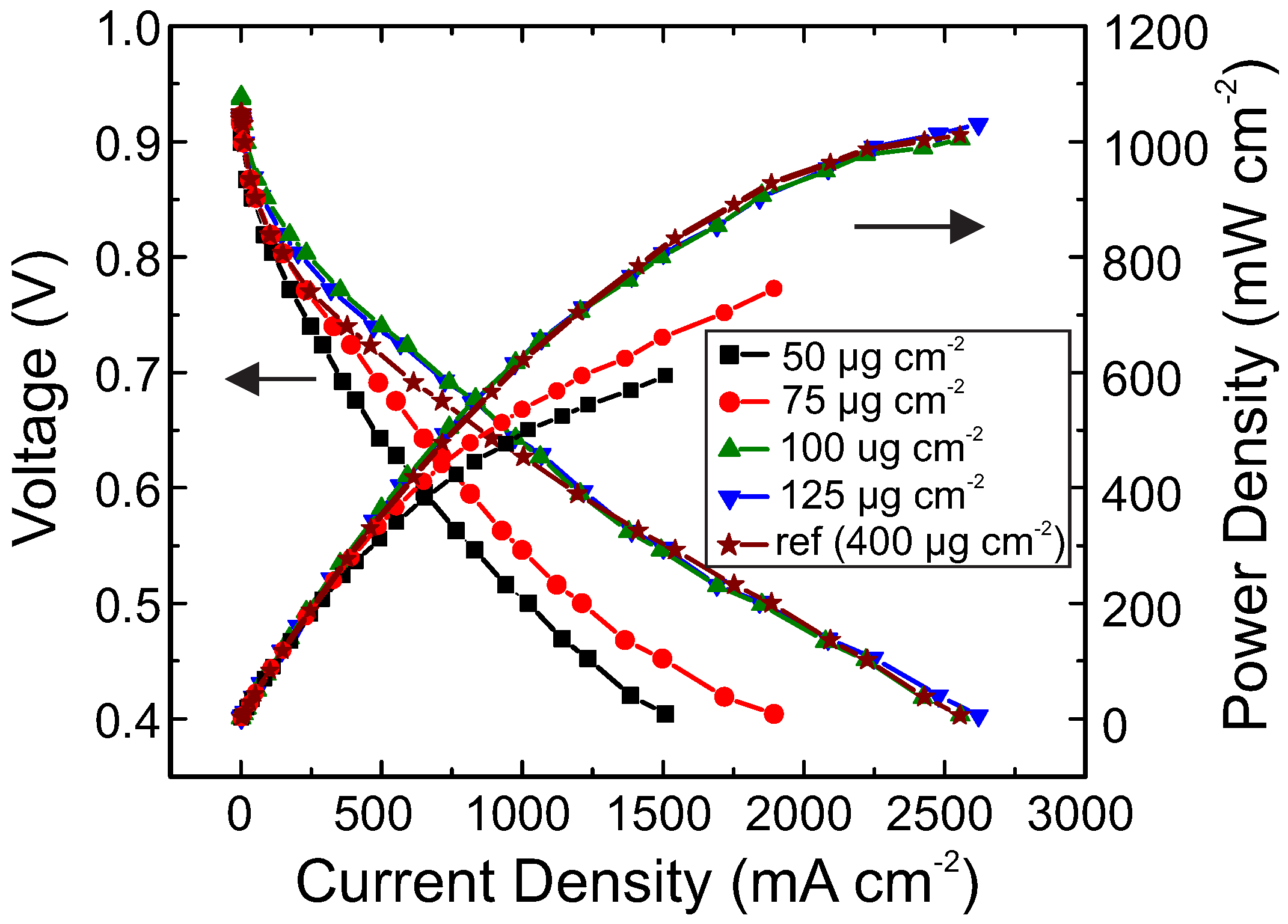


| Cathode Catalyst Deposition Method | Anode Pt Loading * (g·cm) | Cathode Pt Loading (g·cm) | Current Density at 0.6 V (mA·cm) | Power Density at 0.6 V (mW·cm) | Cathode MSPD (kW·g) |
|---|---|---|---|---|---|
| PLD | 200 | 50 | 650 | 390 | 7.8 |
| 200 | 75 | 820 | 490 | 6.5 | |
| 200 | 100 | 1205 | 723 | 7.3 | |
| 200 | 125 | 1216 | 729 | 5.8 | |
| E-TEK | 200 | 400 | 1190 | 710 | 1.7 |
| Deposition Technique | Pt Loading Anode/Cathode (g·cm) | Operating Condition (C/kPa) | Current Density at 0.6 V (mA·cm) | Overall MSPD at 0.6 V (kW·g) | Reference |
|---|---|---|---|---|---|
| Pulsed electrodeposition | 400/120 | 80/250 | 1400 | 1.61 | [18] |
| Reactive spray deposition | 75/75 | 80/180 | 1400 | 6.00 | [19] |
| DC magnetron sputtering | 50/100 | 70/100 | 800 | 3.28 | [20] |
| Electrospray | 40/120 | 70/200 | 1200 | 4.50 | [21] |
| Sputtering | 100/60 | 80/100 | 490 | 1.83 | [4] |
| Impulse sputtering | 20/20 | 70/300 | 400 | 6.00 | [2] |
| Atomic layer deposition | 180/500 | 80/100 | 1400 | 1.20 | [22] |
| Pulsed laser deposition | -/7 | 80/240 | 100 | - | [8] |
| Chemical vapor deposition | 200/200 | 80/100 | 1100 | 1.65 | [23] |
| This work | 17/100 | 70/200 | 1450 | 7.43 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qayyum, H.; Tseng, C.-J.; Huang, T.-W.; Chen, S.-y. Pulsed Laser Deposition of Platinum Nanoparticles as a Catalyst for High-Performance PEM Fuel Cells. Catalysts 2016, 6, 180. https://doi.org/10.3390/catal6110180
Qayyum H, Tseng C-J, Huang T-W, Chen S-y. Pulsed Laser Deposition of Platinum Nanoparticles as a Catalyst for High-Performance PEM Fuel Cells. Catalysts. 2016; 6(11):180. https://doi.org/10.3390/catal6110180
Chicago/Turabian StyleQayyum, Hamza, Chung-Jen Tseng, Ting-Wei Huang, and Szu-yuan Chen. 2016. "Pulsed Laser Deposition of Platinum Nanoparticles as a Catalyst for High-Performance PEM Fuel Cells" Catalysts 6, no. 11: 180. https://doi.org/10.3390/catal6110180






