An Alumina-Supported Ni-La-Based Catalyst for Producing Synthetic Natural Gas
Abstract
:1. Introduction
2. Results and Discussion
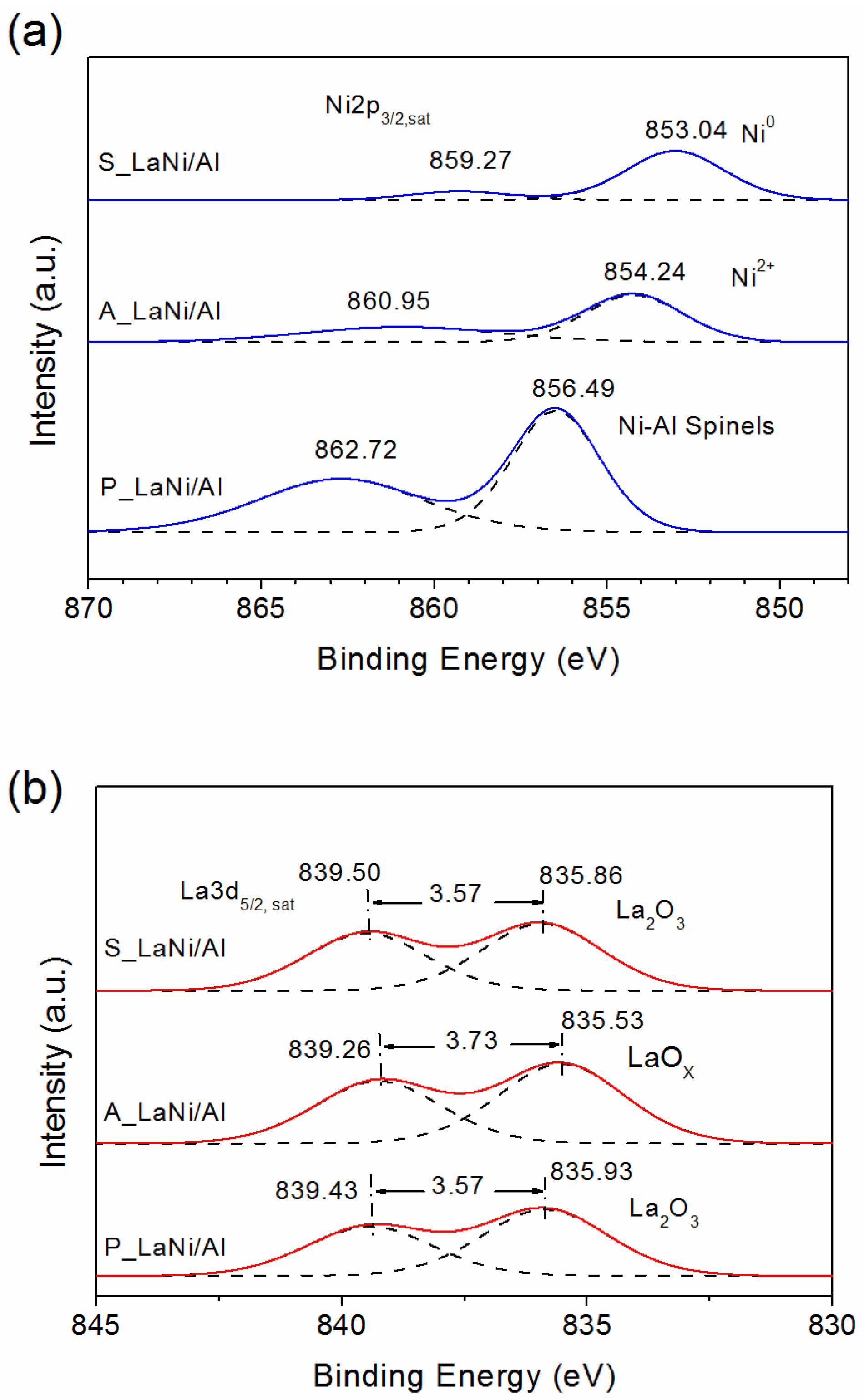
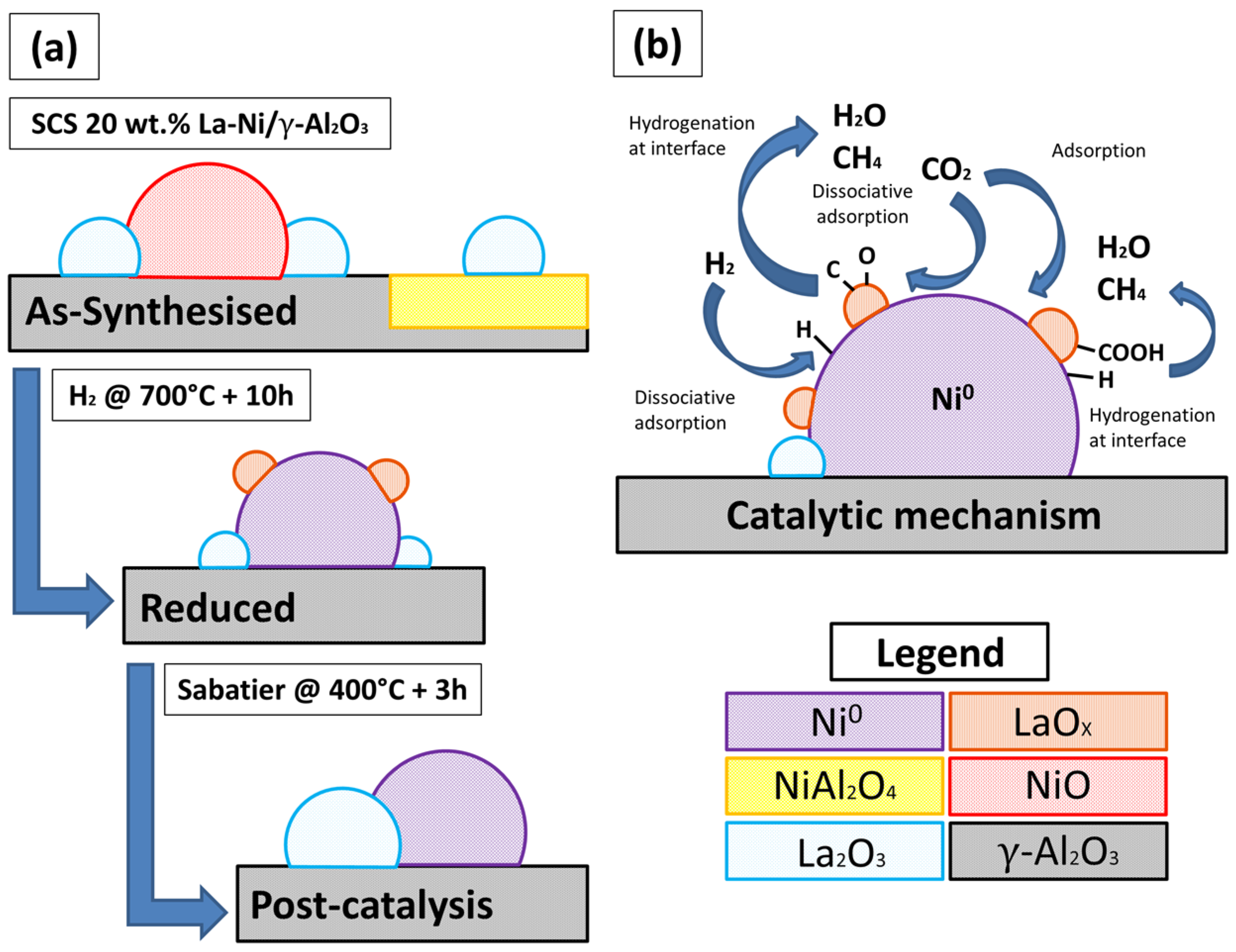
2.1. As-Prepared Catalyst (P_LaNi/Al)
2.2. Reduced Catalyst (A_LaNi/Al)
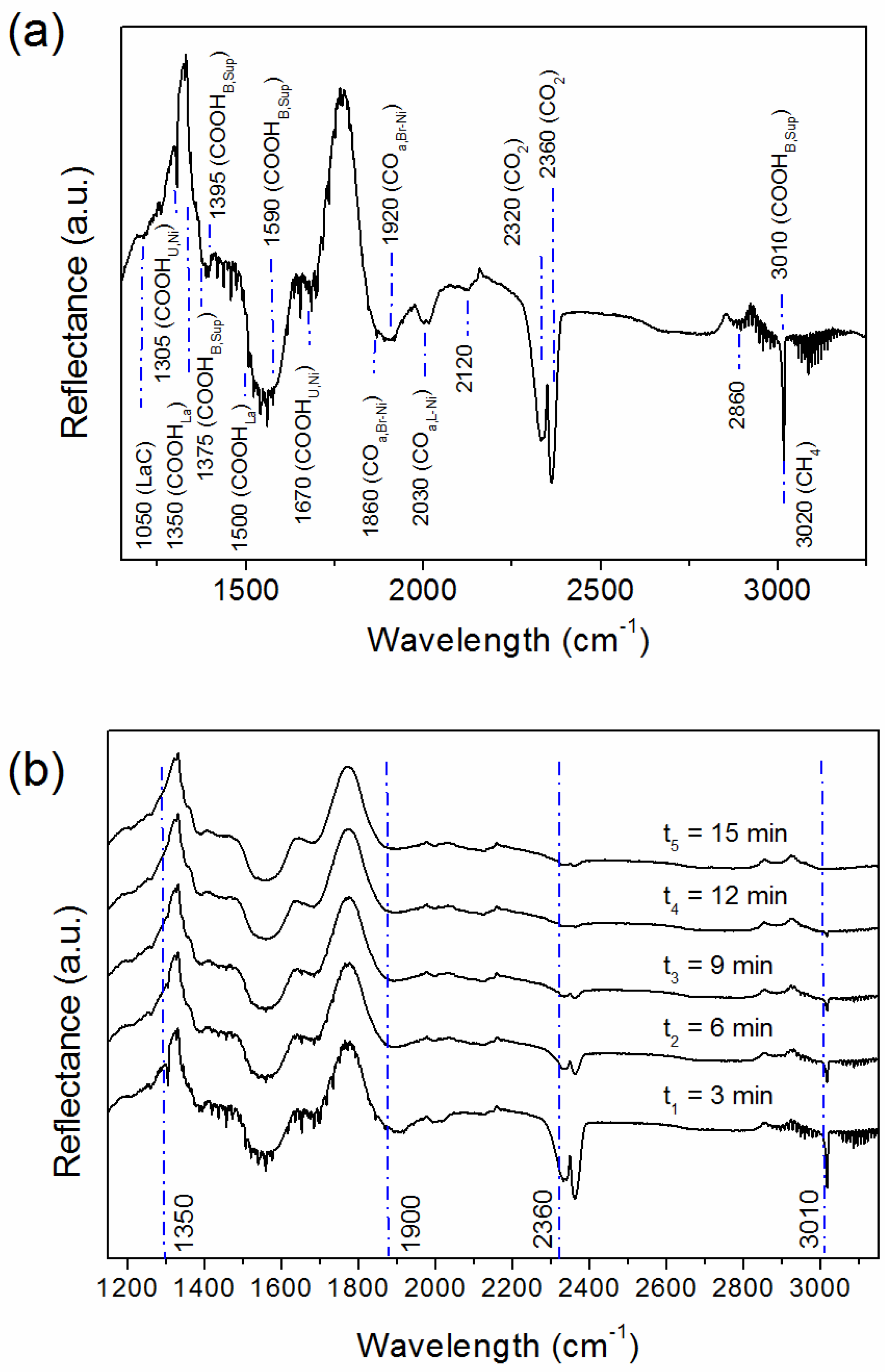
2.3. Spent Catalyst (S_LaNi/Al)
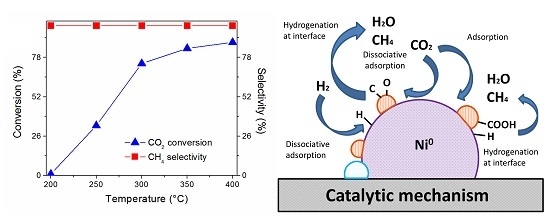
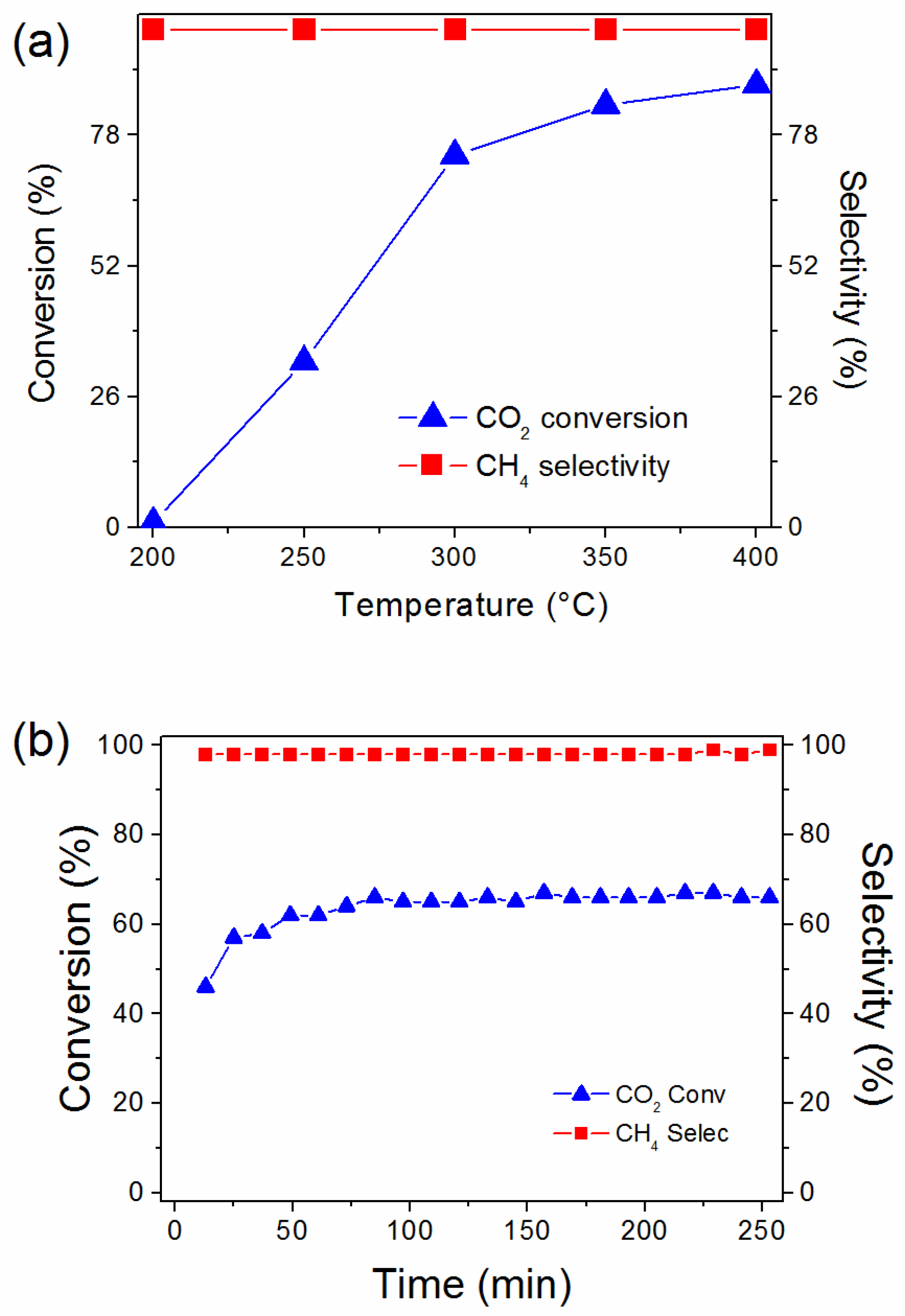
2.4. Catalyst Performance
2.5. Reaction Routes
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Catalyst Characterisation
3.3. Catalytic Assessment
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Susan, S. Climate Change 2007—The Physical Science Basis: Working Group I Contribution to the Fourth Assessment Report of the IPCC; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Tans, P.; Keeling, R. Trends in Atmospheric Carbon Dioxide; NOAA Earth System Research Laboratory: Boulder, CO, USA, 2014. [Google Scholar]
- Wang, W.; Wang, S.; Ma, X.; Gong, J. Recent advances in catalytic hydrogenation of carbon dioxide. Chem. Soc. Rev. 2011, 40, 3703–3727. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Jinlong, G. Methanation of carbon dioxide: an overview. Front. Chem. Sci. Eng. 2011, 5, 2–10. [Google Scholar] [CrossRef]
- Centi, G.; Perathoner, S. Opportunities and prospects in the chemical recycling of carbon dioxide to fuels. Catal. Today 2009, 148, 191–205. [Google Scholar] [CrossRef]
- Hunt, A.J.; Sin, E.H.K.; Marriott, R.; Clark, J.H. Generation, capture, and utilization of industrial carbon dioxide. ChemSusChem 2010, 3, 306–322. [Google Scholar] [CrossRef] [PubMed]
- Zhilyaeva, N.A.; Volnina, E.A.; Kukina, M.A.; Frolov, V.M. Carbon dioxide hydrogenation catalysts (a review). Petrol. Chem. 2002, 42, 367–386. [Google Scholar]
- Chang, F.-W.; Kuo, M.-S.; Tsay, M.-T.; Hsieh, M.-C. Hydrogenation of CO2 over nickel catalysts on rice husk ash-alumina prepared by incipient wetness impregnation. Appl. Catal. A Gen. 2003, 247, 309–320. [Google Scholar] [CrossRef]
- Ibraeva, Z.A.; Nekrasov, N.V.; Gudkov, B.S.; Yakerson, V.I.; Beisembaeva, Z.T.; Golosman, E.Z.; Kiperman, S.L. Kinetics of methanation of carbon dioxide on a nickel catalyst. Theor. Exp. Chem. 1991, 26, 584–588. [Google Scholar] [CrossRef]
- Yamasaki, M.; Habazaki, H.; Yoshida, T.; Akiyama, E.; Kawashima, A.; Asami, K.; Hashimoto, K.; Komori, M.; Shimamura, K. Compositional dependence of the CO2 methanation activity of Ni/ZrO2 catalysts prepared from amorphous NiZr alloy precursors. Appl. Catal. A Gen. 1997, 163, 187–197. [Google Scholar] [CrossRef]
- Weatherbee, G.D.; Bartholomew, C.H. Hydrogenation of CO2 on group VIII metals. I. Specific activity of Ni/SiO2. J. Catal. 1981, 68, 67–76. [Google Scholar]
- Solymosi, F.; Erdöhelyi, A.; Bánsági, T. Methanation of CO2 on supported rhodium catalyst. J. Catal. 1981, 68, 371–382. [Google Scholar] [CrossRef]
- Lunde, P.J.; Kester, F.L. Rates of methane formation from carbon dioxide and hydrogen over a ruthenium catalyst. J. Catal. 1973, 30, 423–429. [Google Scholar] [CrossRef]
- Novák, É.; Fodor, K.; Szailer, T.; Oszkó, A.; Erdöhelyi, A. CO2 hydrogenation on Rh/TiO2 previously reduced at different temperatures. Top. Catal. 2002, 20, 107–117. [Google Scholar] [CrossRef]
- Park, J.-N.; McFarland, E.W. A highly dispersed Pd–Mg/SiO2 catalyst active for methanation of CO2. J. Catal. 2009, 266, 92–97. [Google Scholar] [CrossRef]
- Solymosi, F.; Erdöhelyi, A. Hydrogenation of CO2 to CH4 over alumina-supported noble metals. J. Mol. Catal. 1980, 8, 471–474. [Google Scholar] [CrossRef]
- Ando, H.; Fujiwara, M.; Matsumura, Y.; Miyamura, H.; Souma, Y. Methanation of carbon dioxide over LaNi4X type catalysts. Energy Convers. Manag. 1995, 36, 653–656. [Google Scholar] [CrossRef]
- Ando, H.; Fujiwara, M.; Matsumura, Y.; Tanaka, M.; Souma, Y. Catalytic hydrogenation of carbon dioxide over LaNi5 activated during the reaction. J. Mol. Catal. A Chem. 1999, 144, 117–122. [Google Scholar] [CrossRef]
- Scheffer, B.; Heijeinga, J.J.; Moulijn, J.A. An electron spectroscopy and X-ray diffraction study of nickel oxide/alumina and nickel-oxide-tungsten trioxide/alumina catalysts. J. Phys. Chem. 1987, 91, 4752–4759. [Google Scholar] [CrossRef]
- Bartholomew, C.H.; Pannell, R.B. The stoichiometry of hydrogen and carbon monoxide chemisorption on alumina and silica supported nickel. J. Catal. 1980, 65, 390–401. [Google Scholar] [CrossRef]
- Kirumakki, S.R.; Shpeizer, B.G.; Sagar, G.V.; Chary, K.V.R.; Clearfield, A. Hydrogenation of naphthalene over NiO/SiO2–Al2O3 catalysts: Structure–activity correlation. J. Catal. 2006, 242, 319–331. [Google Scholar] [CrossRef]
- Li, C.; Chen, Y.-W. Temperature-programmed-reduction studies of nickel oxide/alumina catalysts: Effects of the preparation method. Thermochim. Acta 1995, 256, 457–465. [Google Scholar] [CrossRef]
- Poncelet, G.; Centeno, M.A.; Molina, R. Characterization of reduced α-alumina-supported nickel catalysts by spectroscopic and chemisorption measurements. Appl. Catal. A Gen. 2005, 288, 232–242. [Google Scholar] [CrossRef]
- Tsai, W.; Schwarz, J.A.; Driscoll, C.T. Differential cation exchange capacity (DCEC) of nickel supported on silica-aluminas. J. Catal. 1982, 78, 88–95. [Google Scholar] [CrossRef]
- Barros, B.S.; Melo, D.M.A.; Libs, S.; Kiennemann, A. CO2 reforming of methane over La2NiO4/α-Al2O3 prepared by microwave assisted self-combustion method. Appl. Catal. A Gen. 2010, 378, 69–75. [Google Scholar] [CrossRef]
- Sánchez-Sánchez, M.C.; Navarro, R.M.; Fierro, J.L.G. Ethanol steam reforming over Ni/La–Al2O3 catalysts: Influence of lanthanum loading. Catal. Today 2007, 129, 336–345. [Google Scholar] [CrossRef]
- Yang, R.; Li, X.; Wu, J.; Zhang, X.; Zhang, Z. Promotion Effects of copper and lanthanum oxides on nickel/gamma-alumina catalyst in the hydrotreating of crude 2-ethylhexanol. J. Phys. Chem. C 2009, 113, 17787–17794. [Google Scholar] [CrossRef]
- Scheffer, B.; Molhoek, P.; Moulijn, J.A. Temperature-programmed reduction of NiOWO3/Al2O3 hydrodesulphurization catalysts. Appl. Catal. 1989, 46, 11–30. [Google Scholar] [CrossRef]
- Mordovin, V.P.; Kasimtsev, A.V.; Alekhin, V.P.; Zhigunov, V.V. Industrial technologies for production of LaNi5-based hydride materials. In Hydrogen Materials Science and Chemistry of Carbon Nanomaterials; Veziroglu, T.N., Zaginaichenko, S., Schur, D., Baranowski, B., Shpak, A., Skorokhod, V., Kale, A., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 407–414. [Google Scholar]
- Bunch, A.Y.; Wang, X.; Ozkan, U.S. Hydrodeoxygenation of benzofuran over sulfided and reduced Ni–Mo/γ-Al2O3 catalysts: Effect of H2S. J. Mol. Catal. A Chem. 2007, 270, 264–272. [Google Scholar] [CrossRef]
- Damyanova, S.; Daza, L.; Fierro, J.L.G. Surface and catalytic properties of lanthanum-promoted Ni/sepiolite catalysts for styrene hydrogenation. J. Catal. 1996, 159, 150–161. [Google Scholar] [CrossRef]
- Légaré, P.; Fritsch, A. XPS study of transition metal/alumina model catalysts: Equilibrium and energy referencing. Surf. Interface Anal. 1990, 15, 698–700. [Google Scholar] [CrossRef]
- Salvati, L.; Makovsky, L.E.; Stencel, J.M.; Brown, F.R.; Hercules, D.M. Surface spectroscopic study of tungsten-alumina catalysts using X-ray photoelectron, ion scattering, and raman spectroscopies. J. Phys. Chem. 1981, 85, 3700–3707. [Google Scholar] [CrossRef]
- Tanabe, T.; Asaki, Z. Formation mechanism of LaNi5 in the reduction-diffusion process. Metall. Mater. Trans. B 1998, 29, 331–338. [Google Scholar] [CrossRef]
- Yasuda, N.; Tsuchiya, T.; Sasaki, S.; Okinaka, N.; Akiyama, T. Self-ignition combustion synthesis of LaNi5 at different hydrogen pressures. Int. J. Hydrog. Energy 2011, 36, 8604–8609. [Google Scholar] [CrossRef]
- Zhang, Z.; Verykios, X.E. Carbon dioxide reforming of methane to synthesis gas over Ni/La2O3 catalysts. Appl. Catal. A Gen. 1996, 138, 109–133. [Google Scholar] [CrossRef]
- Fleisch, T.H.; Hicks, R.F.; Bell, A.T. An XPS study of metal-support interactions on PdSiO2 and PdLa2O3. J. Catal. 1984, 87, 398–413. [Google Scholar] [CrossRef]
- Abelló, S.; Berrueco, C.; Montané, D. High-loaded nickel–alumina catalyst for direct CO2 hydrogenation into synthetic natural gas (SNG). Fuel 2013, 113, 598–609. [Google Scholar] [CrossRef]
- Liu, H.; Zou, X.; Wang, X.; Lu, X.; Ding, W. Effect of CeO2 addition on Ni/Al2O3 catalysts for methanation of carbon dioxide with hydrogen. J. Nat. Gas Chem. 2012, 21, 703–707. [Google Scholar] [CrossRef]
- González-Cortés, S.L.; Imbert, F.E. Fundamentals, properties and applications of solid catalysts prepared by solution combustion synthesis (SCS). Appl. Catal. A Gen. 2013, 452, 117–131. [Google Scholar] [CrossRef]
- Barrault, J.; Guilleminot, A.; Percheron-Guegan, A.; Paul-Boncour, V.; Achard, J.C. Olefin hydrogenation over some LaNi5−xMx intermetallic systems. Appl. Catal. 1986, 22, 263–271. [Google Scholar] [CrossRef]
- Inui, T. Highly effective conversion of carbon dioxide to valuable compounds on composite catalysts. Catal. Today 1996, 29, 329–337. [Google Scholar] [CrossRef]
- Tsipouriari, V.A.; Verykios, X.E. Carbon and oxygen reaction pathways of CO2 reforming of methane over Ni/La2O3 and Ni/Al2O3 catalysts studied by isotopic tracing techniques. J. Catal. 1999, 187, 85–94. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, H.; Xu, H.; Li, W. The CO2 reforming of CH4 over Ni/La2O3/α-Al2O3 catalysts: The effect of La2O3 contents on the kinetic performance. Appl. Catal. A Gen. 2007, 331, 60–69. [Google Scholar] [CrossRef]
- Martínez, R.; Romero, E.; Guimon, C.; Bilbao, R. CO2 reforming of methane over coprecipitated Ni–Al catalysts modified with lanthanum. Appl. Catal. A Gen. 2004, 274, 139–149. [Google Scholar] [CrossRef]
- Verykios, X.E. Catalytic dry reforming of natural gas for the production of chemicals and hydrogen. Hem. Ind. 2002, 56, 238–255. [Google Scholar] [CrossRef]
- Fujita, S.-I.; Nakamura, M.; Doi, T.; Takezawa, N. Mechanisms of methanation of carbon dioxide and carbon monoxide over nickel/alumina catalysts. Appl. Catal. A Gen. 1993, 104, 87–100. [Google Scholar] [CrossRef]
- Zakumbaeva, G.D.; Urumbaeva, S.U.; Nigmetova, D.T.; Khisametdinov, A.M.; Kuanyshev, A.S. CO2 hydrogenation on iron-containing mono- and bimetallic catalysts. React. Kinet. Catal. Lett. 1987, 34, 123–128. [Google Scholar] [CrossRef]
- Lansink Rotgerink, H.G.J.; Slaa, J.C.; van Ommen, J.G.; Ross, J.R.H. Studies on the promotion of nickel-Alumina coprecipitated catalysts: III. Cerium oxide. Appl. Catal. 1988, 45, 281–290. [Google Scholar] [CrossRef]
- Bartholomew, C.H.; Pannell, R.B.; Butler, J.L. Support and crystallite size effects in CO hydrogenation on nickel. J. Catal. 1980, 65, 335–347. [Google Scholar] [CrossRef]

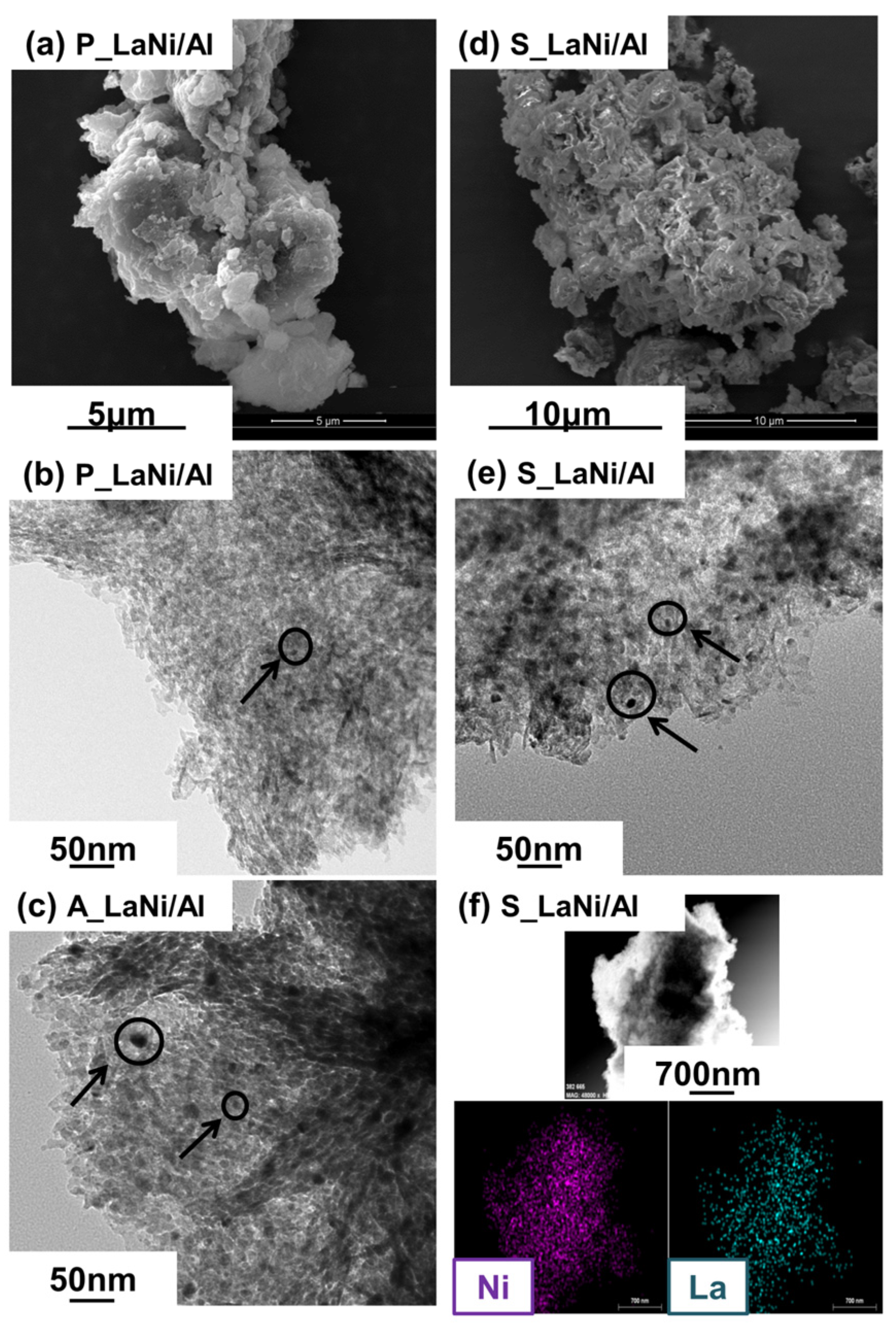

| Materials | Particle Size (nm) | Ni Crystallite Size (nm) | |
|---|---|---|---|
| TEM | XRD | H2 Pulse (% Disp.) | |
| P_LaNi/Al | 1–6 | - | - |
| A_LaNi/Al | 4–20 | 5 | 37 (2.58) |
| S_LaNi/Al | 4–9 | 6 | - |
| Ni/Al | - | - | 41 (2.36) |
| Materials | Ni2p3/2 (Sat) | La3p5/2 (Sat) | ||||
|---|---|---|---|---|---|---|
| BE (eV) | Species | Ni/Al | BE (eV) | Species | La/Al | |
| P_LaNi/Al | 856.49 | Ni-Al surface spinels | 0.081 | 835.86 | La2O3 | 0.022 |
| 862.72 | 839.43 | |||||
| A_LaNi/Al | 854.24 | Ni2+ | 0.028 | 835.53 | LaOx | 0.022 |
| 860.95 | 839.26 | |||||
| S_LaNi/Al | 853.04 | Metallic | 0.029 | 835.93 | La2O3 | 0.020 |
| 859.27 | Ni | 839.50 | ||||
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivero-Mendoza, D.E.; Stanley, J.N.G.; Scott, J.; Aguey-Zinsou, K.-F. An Alumina-Supported Ni-La-Based Catalyst for Producing Synthetic Natural Gas. Catalysts 2016, 6, 170. https://doi.org/10.3390/catal6110170
Rivero-Mendoza DE, Stanley JNG, Scott J, Aguey-Zinsou K-F. An Alumina-Supported Ni-La-Based Catalyst for Producing Synthetic Natural Gas. Catalysts. 2016; 6(11):170. https://doi.org/10.3390/catal6110170
Chicago/Turabian StyleRivero-Mendoza, Daniel E., Jessica N. G. Stanley, Jason Scott, and Kondo-François Aguey-Zinsou. 2016. "An Alumina-Supported Ni-La-Based Catalyst for Producing Synthetic Natural Gas" Catalysts 6, no. 11: 170. https://doi.org/10.3390/catal6110170