Electrocatalytic Oxidation of Cellulose to Gluconate on Carbon Aerogel Supported Gold Nanoparticles Anode in Alkaline Medium
Abstract
:1. Introduction
2. Results and Discussion
2.1. Dissolution of Cellulose

2.2. Characterization of Electrocatalytic Anode
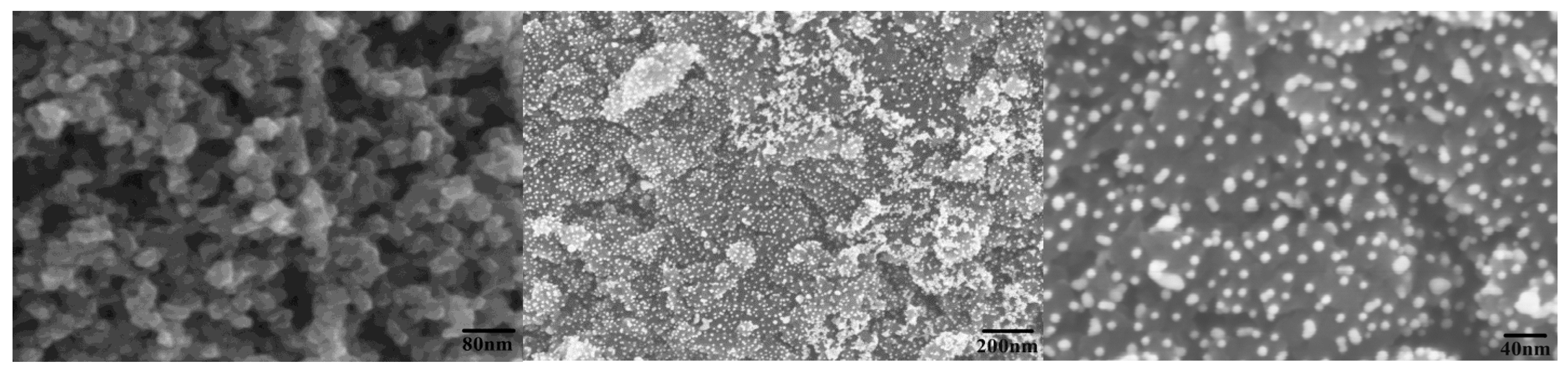
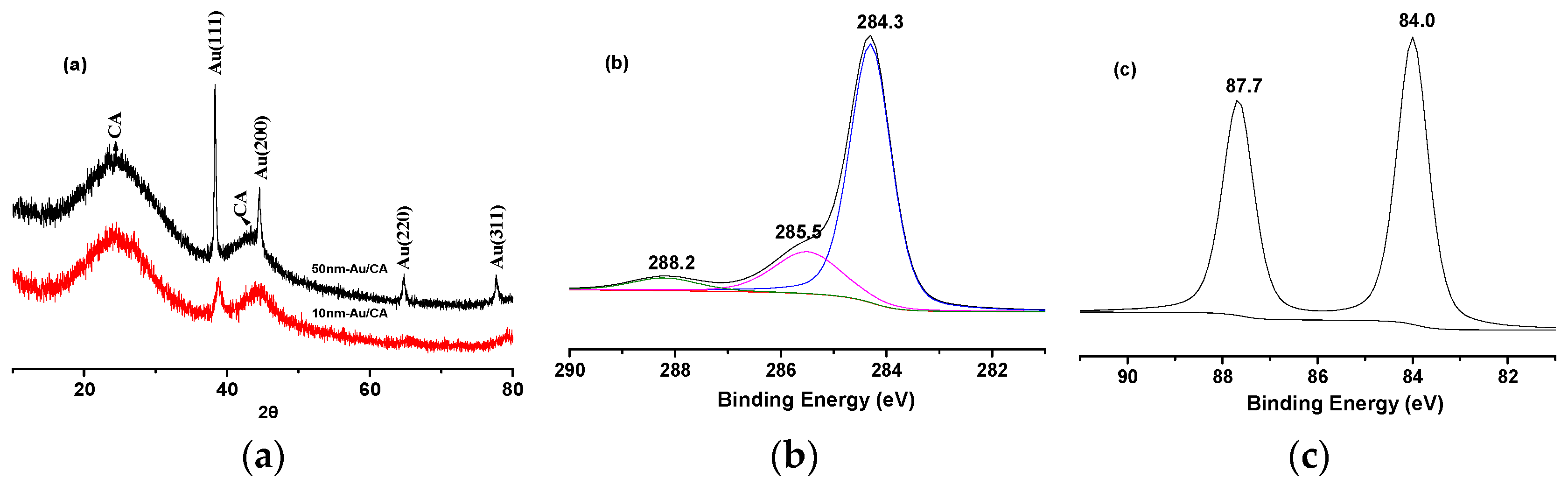
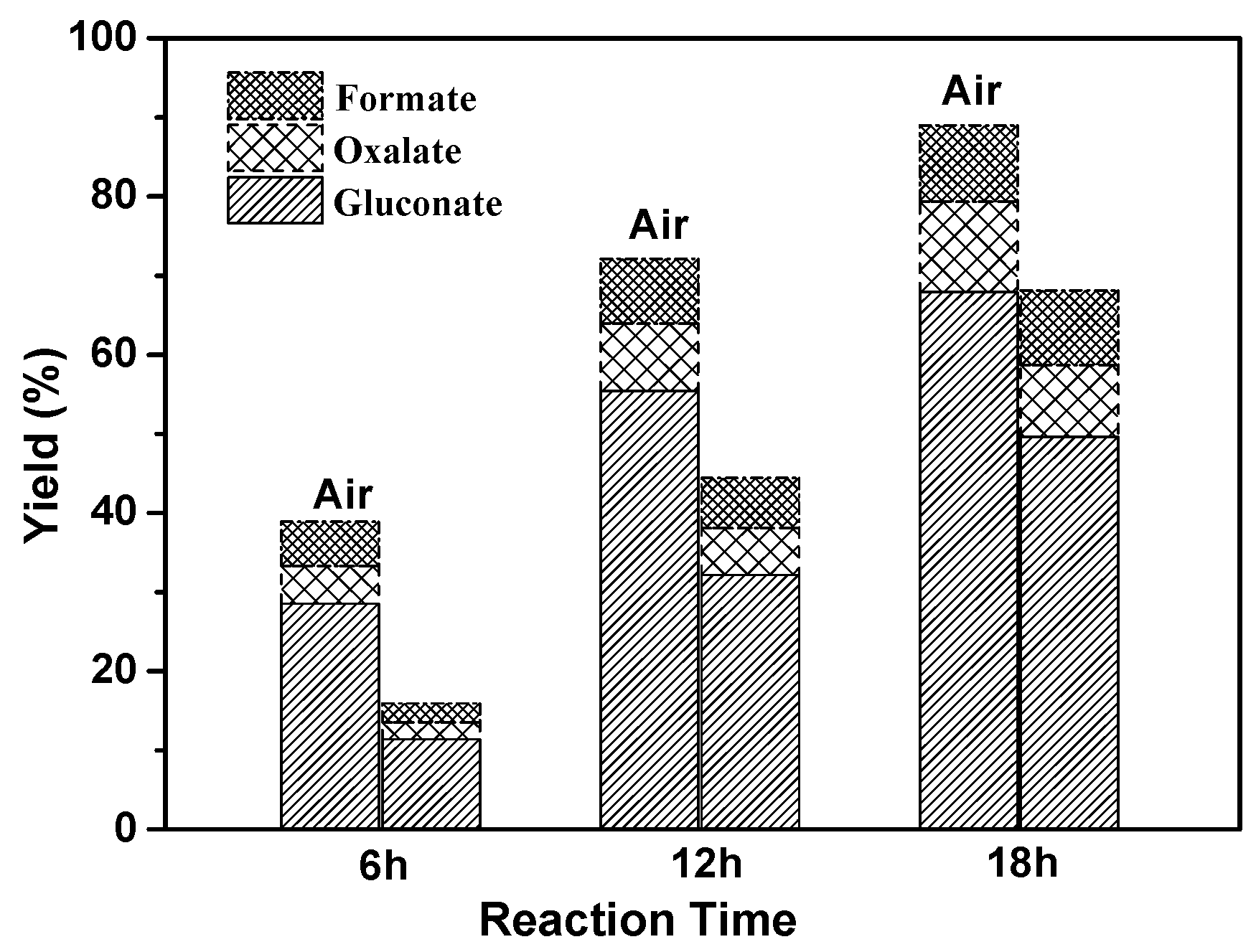
2.3. Electrochemical Oxidation of Cellulose
| Electrocatalyst | Yield (%) | |||||
|---|---|---|---|---|---|---|
| Gluconate | Glycolate | Acetate | Oxalate | Formate | Sum Yields | |
| Au/graphite | 32.7 | 5.4 | 4.3 | 6.5 | 3.4 | 52.3 |
| 50 nm-Au/CA | 46.9 | 7.9 | 6.1 | 10.5 | 8.7 | 80.1 |
| Au/CA | 67.8 | almost no | almost no | 11.5 | 9.6 | 88.9 |
| Structure Properties | CA a | CA | Au/CA | Au/Graphite |
|---|---|---|---|---|
| BET surface area (cm2 g−1) | 747 | 1004 | 499 | 5.78 |
| Pore volume (m3 g−1) | 0.40 | 0.52 | 0.45 | - |


3. Experimental Section
3.1. Materials
3.2. Pretreatment of Cellulose
3.3. Preparation of Au/CA Electrode
3.4. Catalyst Characterization
3.5. Electro-Oxidation Reaction
3.6. Products Analysis
4. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Besson, M.; Gallezot, P.; Pinel, C. Conversion of Biomass into Chemicals over Metal Catalysts. Chem. Rev. 2014, 114, 1827–1870. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xiao, F.S. Nanoporous catalysts for biomass conversion. Green Chem. 2015, 17, 24–39. [Google Scholar] [CrossRef]
- Krochta, J.M.; Hudson, J.S. Alkaline thermochemical degradeation of rice straw to organic acids. Agric. Wastes 1985, 14, 243–254. [Google Scholar] [CrossRef]
- An, D.L.; Ye, A.H.; Deng, W.P.; Zhang, Q.H.; Wang, Y. Selective Conversion of Cellobiose and Cellulose into Gluconic Acid in Water in the Presence of Oxygen, Catalyzed by Polyoxometalate-Supported Gold Nanoparticles. Chem. Eur. J. 2012, 18, 2938–2947. [Google Scholar] [CrossRef] [PubMed]
- Saxena, R.C.; Adhikari, D.K.; Goyal, H.B. Biomass-based energy fuel through biochemical routes: A review. Renew. Sustain. Energy Rev. 2009, 13, 167–178. [Google Scholar] [CrossRef]
- Deng, W.P.; Zhang, Q.H.; Wang, Y. Catalytic transformations of cellulose and cellulose-derived carbohydrates into organic acids. Catal. Taday 2014, 234, 31–41. [Google Scholar] [CrossRef]
- Amaniampong, P.N.; Li, K.; Jia, X.; Wang, B.; Borgna, A.; Yang, Y. Titania-Supported Gold Nanoparticles as Efficient Catalysts for the Oxidation of Cellobiose to Organic Acids in Aqueous Medium. ChemCatChem 2014, 6, 2105–2114. [Google Scholar] [CrossRef]
- Cantero, D.A.; Martinez, C.; Bermejo, M.D.; Cocero, M.J. Simultaneous and selective recovery of cellulose and hemicellulose fractions from wheat bran by supercritical water hydrolysis. Green Chem. 2015, 17, 610–618. [Google Scholar] [CrossRef]
- Tan, X.S.; Deng, W.P.; Liu, M.; Zhang, Q.H.; Wang, Y. Carbon nanotube-supported gold nanoparticles as efficient catalysts for selective oxidation of cellobiose into gluconic acid in aqueous medium. Chem. Commun. 2009, 7179–7181. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.C.; Bauzer, M.M.; Nobe, K. Electrochemical Generated Acid Catalysis of Cellulose Hydrolysis. J. Electrochem. Soc. 1986, 135, 83–87. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Peng, Y.; Yin, X.L.; Liu, Z.H.; Li, G. Degradation of lignin to BHT by electrochemical catalysis on Pb/PbO2 anode in alkaline solution. J. Chem. Technol. Biotechnol. 2014, 89, 1954–1960. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, Q.; Fan, H.X.; Li, Y.; Li, G. Electrochemical control of the conversion of cellulose oligosaccharides into glucose. J. Ind. Eng. Chem. 2014, 20, 3487–3492. [Google Scholar] [CrossRef]
- Xin, L.; Zhang, Z.Y.; Qi, J.; Chadderdon, D.; Li, W.Z. Electrocatalytic oxidation of ethylene glycol (EG) on supported Pt and Au catalysts in alkaline media: Reaction pathway investigation in three-electrode cell and fuel cell reactors. Appl. Catal. B 2012, 125, 85–94. [Google Scholar] [CrossRef]
- Kwon, Y.; Kleijn, S.E.F.; Schouten, K.J.P.; Koper, M.T.M. Cellobiose hydrolysis and decomposition by electrochemical generation of acid and hydroxyl radicals. ChemSusChem 2012, 5, 1935–1943. [Google Scholar] [CrossRef] [PubMed]
- Meng, D.F.; Li, G.; Liu, Z.H.; Yang, F. Study of depolymerization of cotton cellulose by Pb/PbO2 anode electrochemical catalysis in sulfuric acid solution. Polym. Degrad. Stab. 2011, 96, 1173–1178. [Google Scholar] [CrossRef]
- Delidovich, I.V.; Moroz, B.L.; Taran, O.P.; Gromov, N.K.; Pyrjaev, P.A.; Prosvirin, I.P.; Bukhtiyarov, V.I.; Parmon, V.N. Aerobic selective oxidation of glucose to gluconate catalyzed by Au/Al2O3 and Au/C: Impact of the mass-transfer processes on the overall kinetics. Chem. Eng. J. 2013, 223, 921–931. [Google Scholar]
- Pasta, M.; Mantia, F.L.; Cui, Y. Mechanism of glucose electrochemical oxidation on gold surface. Electrochem. Acta 2010, 55, 5561–5568. [Google Scholar] [CrossRef]
- Duchemin, B.J.C. Mercerisation of cellulose in aqueous NaOH at low concentrations. Green Chem. 2015, 17, 3941–3947. [Google Scholar] [CrossRef]
- Sugano, Y.; Vestergaard, M.; Yoshikawa, H.; Saito, M.; Tamiya, E. Direct Electrochemical Oxidation of Cellulose: A Cellulose-Based Fuel Cell System. Electroanalysis 2010, 22, 1688–1694. [Google Scholar] [CrossRef]
- Zhang, J.Z.; Liu, X.; Hedhili, N.J.; Zhu, Y.H.; Han, Y. Highly Selective and Complete Conversion of Cellobiose to Gluconic Acid over Au/Cs2HPW12O40 Nanocomposite Catalyst. ChemCatChem 2011, 3, 1294–1298. [Google Scholar] [CrossRef]
- Biella, S.; Prati, L.; Rossi, M. Selective oxidation of D-glucose on gold catalyst. J. Catal. 2002, 206, 242–247. [Google Scholar] [CrossRef]
- Wu, M.F.; Jin, Y.N.; Zhao, G.H.; Li, M.F.; Li, D.M. Electrosorption-promoted Photodegradation of Opaque Wastewater on A Novel TiO2/Carbon Aerogel Electrode. Environ. Sci. Technol. 2010, 44, 1780–1785. [Google Scholar] [CrossRef] [PubMed]
- Komanoya, T.; Kobayashi, H.; Hara, K.; Chun, W.J.; Fukuoka, A. Catalysis and characterization of carbon-supported ruthenium for cellulose hydrolysis. Appl. Catal. A 2011, 407, 188–194. [Google Scholar] [CrossRef]
- Deng, W.P.; Zhang, Q.H.; Wang, Y. Catalytic transformations of cellulose and its derived carbohydrates into 5-hydroxymethylfurfural, levulinic acid, and lactic acid. Sci. China Chem. 2015, 58, 29–46. [Google Scholar] [CrossRef]
- Yan, L.F.; Qi, X.Y. Degradation of Cellulose to Organic Acids in its Homogeneous Alkaline Aqueous Solution. ACS Sustain. Chem. Eng. 2014, 2, 897–901. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, Q.; Wei, H.; Wang, J.Q.; Cho, M.; Cho, H.; Terasaki, O.; Wan, Y. Aggregation-Free Gold Nanoparticles in Ordered Mesoporous Carbons: Toward Highly Active and Stable Heterogeneous Catalysts. J. Am. Chem. Soc. 2013, 135, 11849–11860. [Google Scholar] [CrossRef] [PubMed]
- Plowman, B.J.; O’Mullane, A.P.; Bhargava, S.K. The active site behaviour of electrochemically synthesised gold nanomaterials. Faraday Discuss. 2011, 152, 43–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, C.Y.; Xue, W.J.; Li, J.J.; Xing, W.; Hao, Z.P. Mesoporous carbon-confined Au catalysts with superior activity for selective oxidation of glucose to gluconic acid. Green Chem. 2013, 15, 1035–1041. [Google Scholar] [CrossRef]
- Ruffo, M.P.R.; Falletta, E.; Mari, C.M.; Pina, C.D. Alkaline glucose oxidation on nanostructured gold electrodes. Gold Bullet. 2010, 43, 57–64. [Google Scholar]
- Wu, C.; Yan, P.T.; Zhang, R.J.; Jin, J.L.; Zhang, X.H.; Kang, H.M. Comparative study of HNO3 activation effect on porous carbons having different porous characteristics. J. Appl. Electrochem. 2015, 45, 849–856. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, J.; Chen, C.; Huang, Y.; Wang, A.; Zhang, T. Graphene oxide for cellulose hydrolysis: How it works as a highly active catalyst? Chem. Commun. 2014, 50, 3439–3442. [Google Scholar] [CrossRef] [PubMed]
- Delidovich, I.; Palkovits, R. Impacts of acidity and textural properties of oxidized carbon materials on their catalytic activity for hydrolysis of cellobiose. Microporous Mesoporouws Mater. 2016, 219, 317–321. [Google Scholar] [CrossRef]
- Sugano, Y.; Latonen, R.M.; Akieh-Pirkanniemi, M.; Bobacka, J.; Ivaska, A. Electrocatalytic Oxidation of Cellulose at a Gold Electrode. ChemSusChem 2014, 7, 2240–2247. [Google Scholar] [CrossRef] [PubMed]
- Qi, P.Y.; Chen, S.S.; Chen, J.; Zheng, J.W.; Zheng, X.L.; Yuan, Y.Z. Catalysis and Reactivation of Ordered Mesoporous Carbon-Supported Gold Nanoparticles for the Base-Free Oxidation of Glucose to Gluconic Acid. ACS Catal. 2015, 5, 2659–2670. [Google Scholar] [CrossRef]
- Ishimotoa, T.; Hamatakea, Y.; Azunob, H.; Kishidab, T.; Koyamaa, M. Theoretical study of support effect of Au catalyst for glucose oxidation of alkaline fuel cell anode. Appl. Surf. Sci. 2015, 324, 76–81. [Google Scholar] [CrossRef]
- Burke, L.D.; Nugent, P.F. The electrochemistry of gold: II The electrocatalytic behavior of the metal in aqueous media. Gold Bullet. 1998, 31, 39–50. [Google Scholar] [CrossRef]
- Doyle, R.; Lyons, M.E. The mechanism of oxygen evolution at superactivated gold electrodes in aqueous alkaline solution. J. Solid State Electrochem. 2014, 18, 3271–3286. [Google Scholar] [CrossRef]
- Sun, K.; Kohyama, M.; Tanaka, S.; Takeda, S. Direct O2 Activation on Gold/Metal Oxide Catalysts through a Unique Double Linear O–Au–O Structure. ChemCatChem 2013, 5, 2217–2222. [Google Scholar] [CrossRef]
- Sun, K.; Kohyama, M.; Tanaka, S.; Takeda, S. Theoretical Study of Atomic Oxygen on Gold Surface by Hückel Theory and DFT Calculations. J. Phys. Chem. A 2012, 116, 9568–9573. [Google Scholar] [CrossRef] [PubMed]
- Genies, L.; Bultel, Y.; Faure, R.; Durand, R. Impedance study of the oxygen reduction reaction on platinum nanoparticles in alkaline media. Electrochimica Acta 2003, 48, 3879–3890. [Google Scholar] [CrossRef]
- Ge, X.M.; Sumboja, A.; Wuu, D.; An, T.; Li, B.; Goh, F.W.T.; Hor, T.S.A.; Zong, Y.; Liu, Z.L. Oxygen Reduction in Alkaline Media: From Mechanisms to Recent Advances of Catalysts. ACS Catal. 2015, 5, 4643–4667. [Google Scholar] [CrossRef]
- Shinagawa, T.; Garcia-Esparza, A.T.; Takanabe, K. Mechanistic Switching by Hydronium Ion Activity for Hydrogen Evolution and Oxidation over Polycrystalline Platinum Disk and Platinum/Carbon Electrodes. ChemElectroChem 2014, 1, 1497–1507. [Google Scholar] [CrossRef]
- Jin, F.M.; Yun, J.; Li, G.M.; Kishita, A.; Tohji, K.; Enomoto, H. Hydrothermal conversion of carbohydrate biomass into formic acid at mild temperatures. Green Chem. 2008, 10, 612–615. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, H.; Wu, M.; Zhao, G. Electrocatalytic Oxidation of Cellulose to Gluconate on Carbon Aerogel Supported Gold Nanoparticles Anode in Alkaline Medium. Catalysts 2016, 6, 5. https://doi.org/10.3390/catal6010005
Xiao H, Wu M, Zhao G. Electrocatalytic Oxidation of Cellulose to Gluconate on Carbon Aerogel Supported Gold Nanoparticles Anode in Alkaline Medium. Catalysts. 2016; 6(1):5. https://doi.org/10.3390/catal6010005
Chicago/Turabian StyleXiao, Hanshuang, Meifen Wu, and Guohua Zhao. 2016. "Electrocatalytic Oxidation of Cellulose to Gluconate on Carbon Aerogel Supported Gold Nanoparticles Anode in Alkaline Medium" Catalysts 6, no. 1: 5. https://doi.org/10.3390/catal6010005






