Hybrids of Gold Nanoparticles with Core-Shell Hyperbranched Polymers: Synthesis, Characterization, and Their High Catalytic Activity for Reduction of 4-Nitrophenol
Abstract
:1. Introduction
2. Results and Discussion
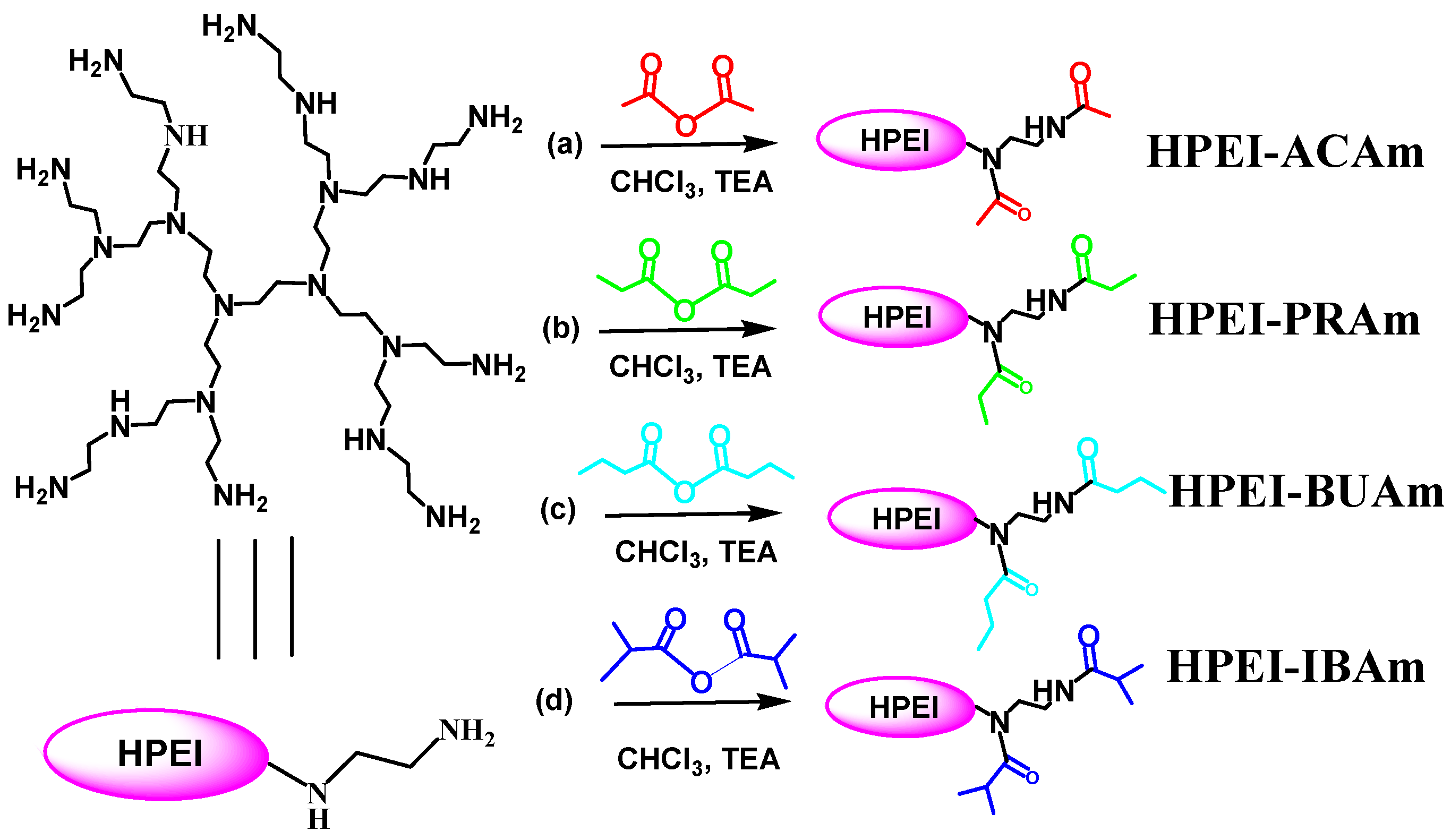
2.1. Synthesis of Hyperbranched PEIs with Different Shells of Amide
| Polymer | Mn of HPEI Core | Degree of Functionalization a (%) | Mn(NMR) b (×104) | General Formula |
|---|---|---|---|---|
| 1 | 10000 | 80 | 1.570 | HPEI10K-ACAm0.80 |
| 2 | 10000 | 82 | 1.785 | HPEI10K-PRAm0.82 |
| 3 | 10000 | 83 | 1.993 | HPEI10K-BUAm0.83 |
| 4 | 10000 | 80 | 1.950 | HPEI10K-IBAm0.80 |
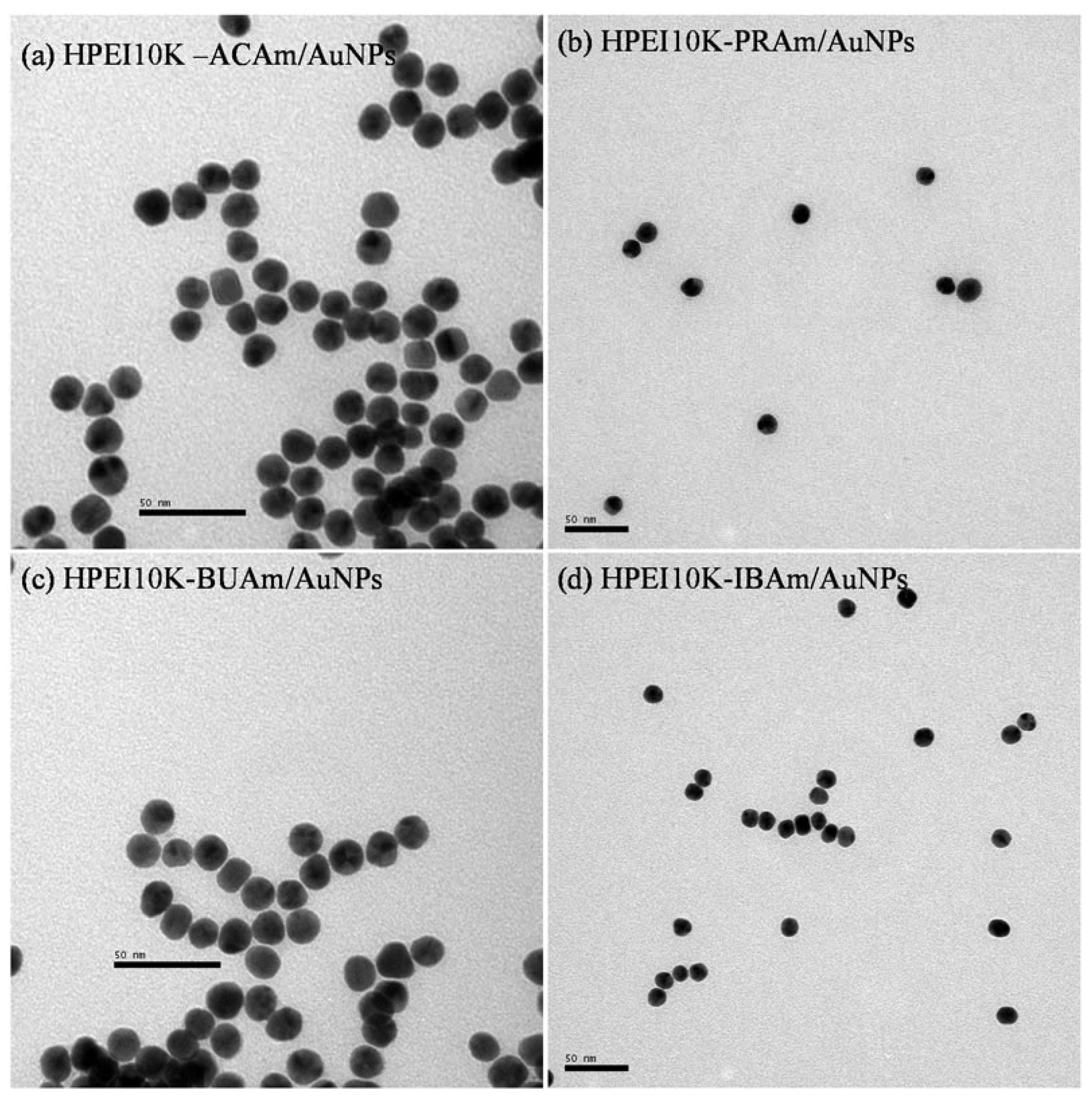
2.2. Preparation of AuNPs and HPEI10K-XXAm Composites
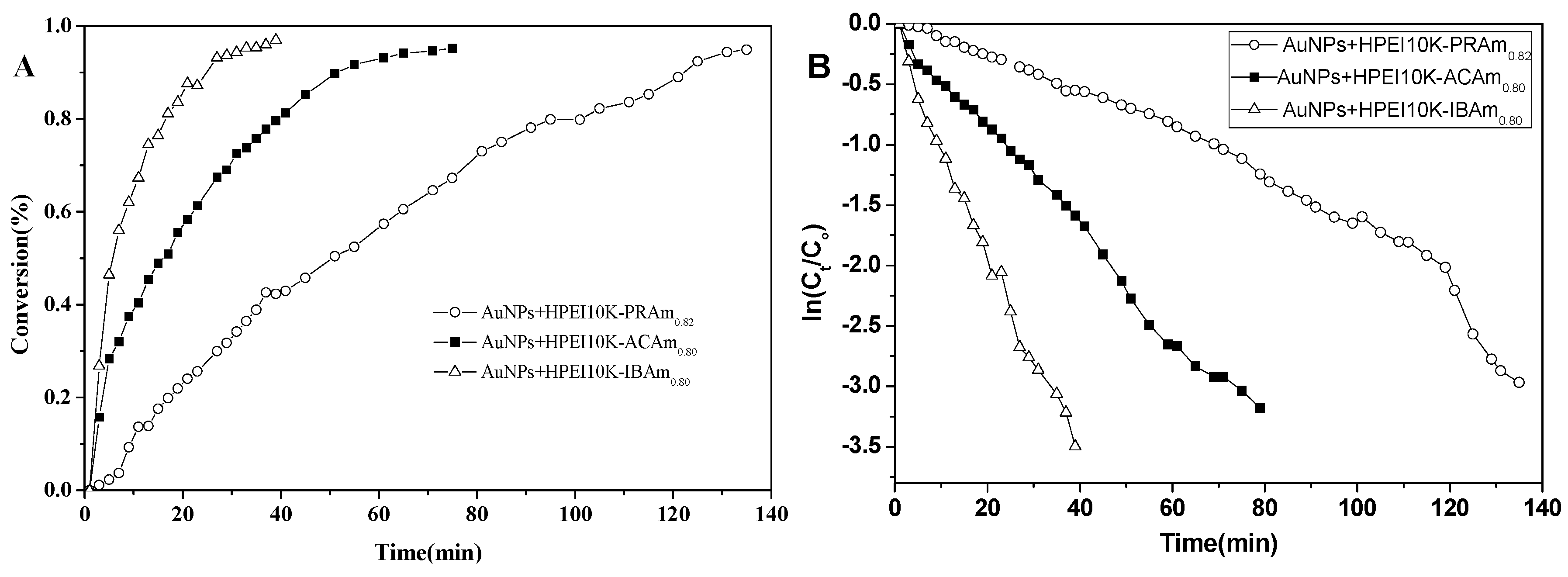
2.3. Comparison of the Catalytic Activity of AuNPs Capping with Different Protectors HPEI10K-XXAm
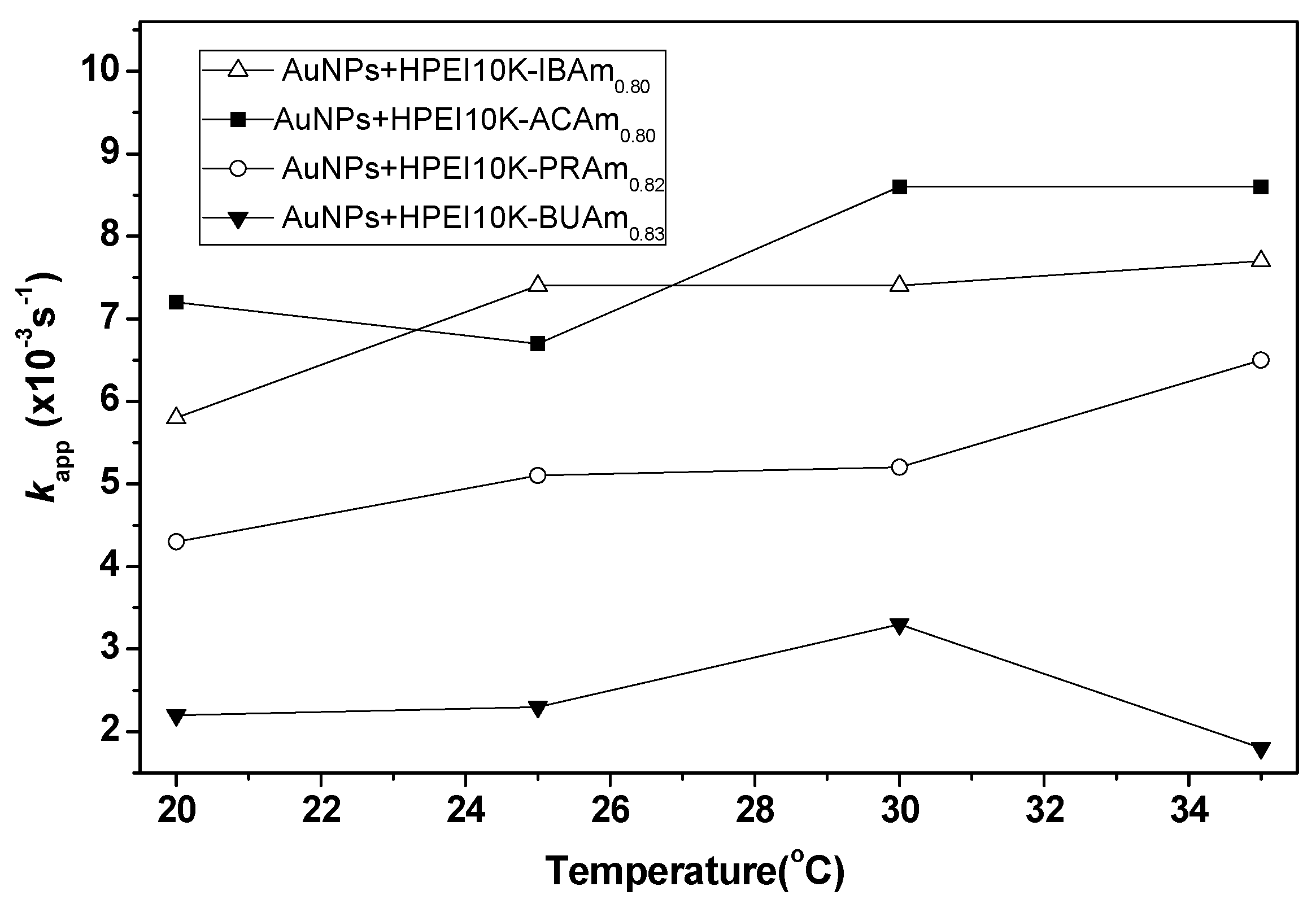
2.4. Effect of Temperature on the Catalytic Activity of the Reduction of 4-Nitrophenolate

2.5. Effect of Gold Composites Concentration on the Catalytic Activity of the Reduction of 4-Nitrophenolate

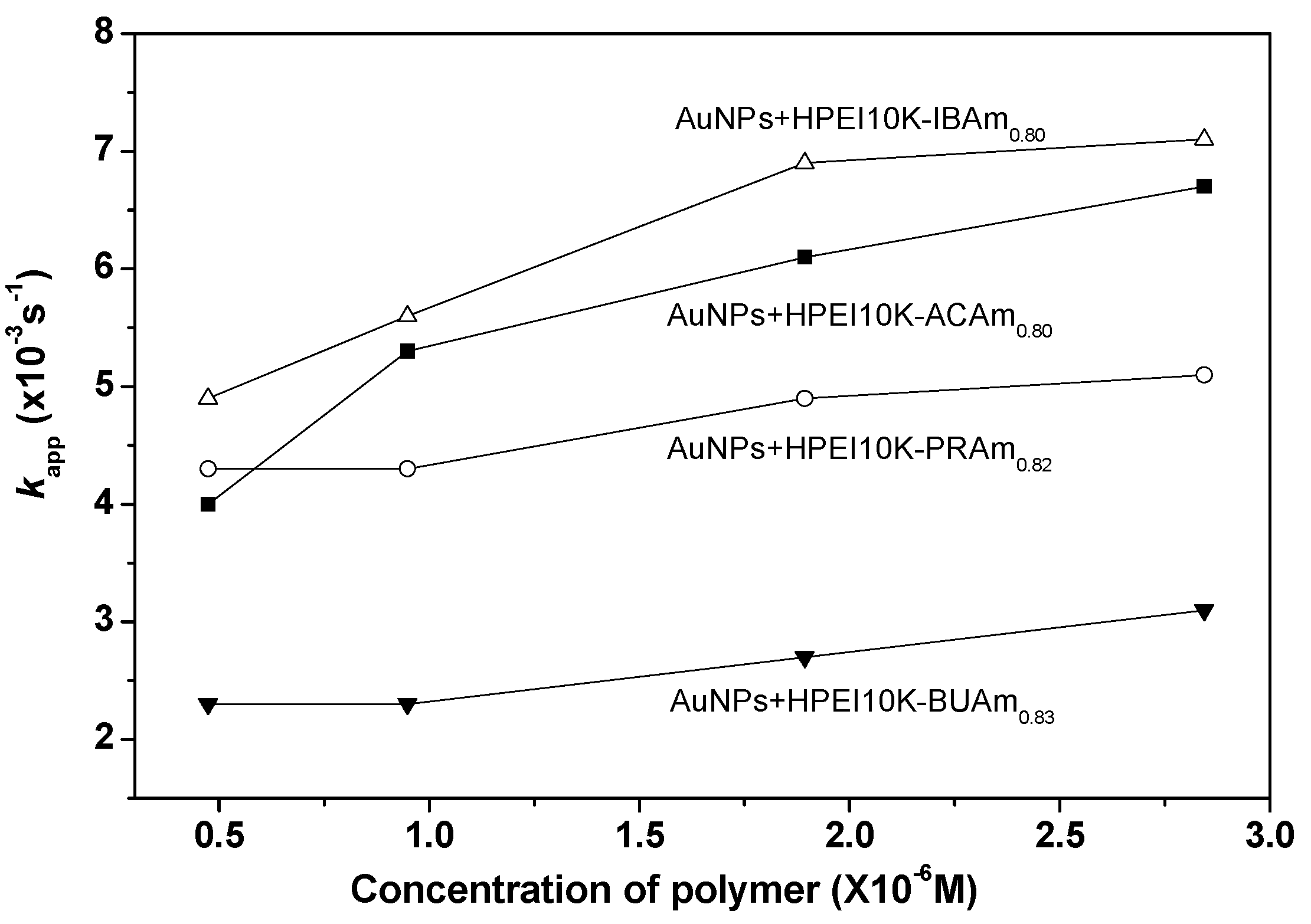
2.6. Effect of HPEI10K-XXAm Polymers Concentration on the Catalytic Activity of the Reduction of 4-Nitrophenolate
| Concentration of AuNPs (×10−6 M) | Concentration of Polymer (×10−6 M) a | k (×10−3) | TOF | ||
|---|---|---|---|---|---|
| kAC b | kIB c | TOFAC d | TOFIB e | ||
| 5.69 | 4.26 | 0.760 | 1.80 | 14.7 | 35.8 |
| 8.50 | 1.98 | 2.80 | 38.5 | 55.6 | |
| 12.2 | 6.07 | 2.60 | 5.10 | 24.6 | 46.7 |
| 9.10 | 4.20 | 9.60 | 38.9 | 90.0 | |
2.7. Effect of HPEI10K-XXAm Polymers Concentration on the Catalytic Activity of the Reduction of 4-Nitrophenolate by 8-nm Gold Nanoparticles
2.8. Effect of Temperature on the Catalytic Activity of the Reduction of 4-Nitrophenolate by 8-nm Gold Nanoparticles
3. Experimental Section
3.1. Chemicals
3.2. Nomenclature
3.3. Synthesis of HPEIs with Different Shells
3.4. Preparation of Citrate Protected AuNPs
3.5. Preparation of AuNPs Coated with Polymers
3.6. Preparation of Small Particle Size AuNPs Coated with Polymers
3.7. Catalytic Reduction of 4-Nitrophenol by NaBH4
3.8. Characterization of AuNPs and Its Composites
4. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Munnik, P.; de Jongh, P.E.; de Jong, K.P. Recent developments in the synthesis of supported catalysts. Chem. Rev. 2015, 115, 6687–6718. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cui, X.; Shi, F.; Deng, Y. Nano-gold catalysis in fine chemical synthesis. Chem. Rev. 2012, 112, 2467–2505. [Google Scholar] [CrossRef] [PubMed]
- Hashmi, A.S.K. Gold-catalyzed organic reactions. Chem. Rev. 2007, 107, 3180–3211. [Google Scholar] [CrossRef] [PubMed]
- Daniel, M.-C.; Astruc, D. Gold nanoparticles: Assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem. Rev. 2004, 104, 293–346. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-Y.; Cheng, F.; Liu, Y.; Liu, H.-J.; Chen, Y. Preparation and characterization of novel thermoresponsive gold nanoparticles and their responsive catalysis properties. J. Mater. Chem. 2010, 20, 360–368. [Google Scholar] [CrossRef]
- Astruc, D.; Boisselier, E.; Ornelas, C. Dendrimers designed for functions: From physical, photophysical, and supramolecular properties to applications in sensing, catalysis, molecular electronics, photonics, and nanomedicine. Chem. Rev. 2010, 110, 1857–1959. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Crooks, R.M. Homogeneous hydrogenation catalysis with monodisperse, dendrimer-encapsulated pd and pt nanoparticles. Angew. Chem. Int. Ed. 1999, 38, 364–366. [Google Scholar] [CrossRef]
- Esumi, K.; Isono, R.; Yoshimura, T. Preparation of PAMAM- and PPI-metal (silver, platinum, and palladium) nanocomposites and their catalytic activities for reduction of 4-nitrophenol. Langmuir 2004, 20, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xu, S.; Kumacheva, E. Polymer microgels: Reactors for semiconductor, metal, and magnetic nanoparticles. J. Am. Chem. Soc. 2004, 126, 7908–7914. [Google Scholar] [CrossRef] [PubMed]
- Bönnemann, H.; Brijoux, W.; Tilling, A.S.; Siepen, K. Application of heterogeneous colloid catalysts for the preparation of fine chemicals. Top. Catal. 1997, 4, 217–227. [Google Scholar] [CrossRef]
- Zhao, M.; Sun, L.; Crooks, R.M. Preparation of cu nanoclusters within dendrimer templates. J. Am. Chem. Soc. 1998, 120, 4877–4878. [Google Scholar] [CrossRef]
- Mecking, S.; Thomann, R.; Frey, H.; Sunder, A. Preparation of catalytically active palladium nanoclusters in compartments of amphiphilic hyperbranched polyglycerols. Macromolecules 2000, 33, 3958–3960. [Google Scholar] [CrossRef]
- Aymonier, C.; Schlotterbeck, U.; Antonietti, L.; Zacharias, P.; Thomann, R.; Tiller, J.C.; Mecking, S. Hybrids of silver nanoparticles with amphiphilic hyperbranched macromolecules exhibiting antimicrobial properties. Chem. Commun. 2002, 3018, 3018–3019. [Google Scholar] [CrossRef]
- Mornet, S.; Vasseur, S.; Grasset, F.; Duguet, E. Magnetic nanoparticle design for medical diagnosis and therapy. J. Mater. Chem. 2004, 14, 2161–2175. [Google Scholar] [CrossRef]
- Frederix, F.; Friedt, J.-M.; Choi, K.-H.; Laureyn, W.; Campitelli, A.; Mondelaers, D.; Maes, G.; Borghs, G. Biosensing based on light absorption of nanoscaled gold and silver particles. Anal. Chem. 2003, 75, 6894–6900. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-Y.; Cheng, F.; Liu, Y.; Li, W.-G.; Chen, Y.; Pan, H.; Liu, H.-J. Thermoresponsive gold nanoparticles with adjustable lower critical solution temperature as colorimetric sensors for temperature, ph and salt concentration. J. Mater. Chem. 2010, 20, 278–284. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, L.; Liu, J.-S.; Liu, X. Simultaneous enrichment, separation and detection of mercury (II) ions using cloud point extraction and colorimetric sensor based on thermoresponsive hyperbranched polymer-gold nanocomposite. Anal. Methods 2015. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Li, Z.; Liu, J.; Xu, L.; Liu, X. An unusual red-to-brown colorimetric sensing method for ultrasensitive silver(I) ion detection based on a non-aggregation of hyperbranched polyethylenimine derivative stabilized gold nanoparticles. Analyst 2015, 140, 5335–5343. [Google Scholar] [CrossRef] [PubMed]
- Praharaj, S.; Nath, S.; Ghosh, S.K.; Kundu, S.; Pal, T. Immobilization and recovery of au nanoparticles from anion exchange resin: Resin-bound nanoparticle matrix as a catalyst for the reduction of 4-nitrophenol. Langmuir 2004, 20, 9889–9892. [Google Scholar] [CrossRef] [PubMed]
- Campbell, C.T.; Parker, S.C.; Starr, D.E. The effect of size-dependent nanoparticle energetics on catalyst sintering. Science 2002, 298, 811–814. [Google Scholar] [CrossRef] [PubMed]
- Moisan, S.; Martinez, V.; Weisbecker, P.; Cansell, F.; Mecking, S.; Aymonier, C. General approach for the synthesis of organic-inorganic hybrid nanoparticles mediated by supercritical CO2. J. Am. Chem. Soc. 2007, 129, 10602–10606. [Google Scholar] [CrossRef] [PubMed]
- Poupart, R.; Le Droumaguet, B.; Guerrouache, M.; Carbonnier, B. Copper nanoparticles supported on permeable monolith with carboxylic acid surface functionality: Stability and catalytic properties under reductive conditions. Mater. Chem. Phys. 2015, 163, 446–452. [Google Scholar] [CrossRef]
- Bronstein, L.M.; Shifrina, Z.B. Dendrimers as encapsulating, stabilizing, or directing agents for inorganic nanoparticles. Chem. Rev. 2011, 111, 5301–5344. [Google Scholar] [CrossRef] [PubMed]
- Cho, T.J.; Zangmeister, R.A.; MacCuspie, R.I.; Patri, A.K.; Hackley, V.A. Newkome-type dendron-stabilized gold nanoparticles: Synthesis, reactivity, and stability. Chem. Mater. 2011, 23, 2665–2676. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Fan, Y.; Yuan, Y.; Chen, Y.; Cheng, F.; Jiang, S.-C. Amphiphilic hyperbranched copolymers bearing a hyperbranched core and a dendritic shell as novel stabilizers rendering gold nanoparticles with an unprecedentedly long lifetime in the catalytic reduction of 4-nitrophenol. J. Mater. Chem. 2012, 22, 21173–21182. [Google Scholar] [CrossRef]
- Liu, X.; Cheng, F.; Liu, H.; Chen, Y. Unusual salt effect on the lower critical solution temperature of hyperbranched thermoresponsive polymers. Soft Matter 2008, 4, 1991–1994. [Google Scholar] [CrossRef]
- Kimling, J.; Maier, M.; Okenve, B.; Kotaidis, V.; Ballot, H.; Plech, A. Turkevich method for gold nanoparticle synthesis revisited. J. Phys. Chem. B 2006, 110, 15700–15707. [Google Scholar] [CrossRef] [PubMed]
- Mayya, K.S.; Caruso, F. Phase transfer of surface-modified gold nanoparticles by hydrophobization with alkylamines. Langmuir 2003, 19, 6987–6993. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, G.; Zhang, W.; Jiang, X.; Zheng, P.; Shi, L.; Dong, A. Responsive catalysis of thermoresponsive micelle-supported gold nanoparticles. J. Mol. Catal. A 2007, 266, 233–238. [Google Scholar] [CrossRef]
- Panigrahi, S.; Basu, S.; Praharaj, S.; Pande, S.; Jana, S.; Pal, A.; Ghosh, S.K.; Pal, T. Synthesis and size-selective catalysis by supported gold nanoparticles: Study on heterogeneous and homogeneous catalytic process. J. Phys. Chem. C 2007, 111, 4596–4605. [Google Scholar] [CrossRef]
- Esumi, K.; Miyamoto, K.; Yoshimura, T. Comparison of PAMAM-Au and PPI-Au nanocomposites and their catalytic activity for reduction of 4-nitrophenol. J. Colloid Interface Sci. 2002, 254, 402–405. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, K.; Yoshimura, T.; Esumi, K. Preparation of gold-dendrimer nanocomposites by laser irradiation and their catalytic reduction of 4-nitrophenol. Langmuir 2003, 19, 5517–5521. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Xu, L.; Liu, X.; Cao, M. Hybrids of Gold Nanoparticles with Core-Shell Hyperbranched Polymers: Synthesis, Characterization, and Their High Catalytic Activity for Reduction of 4-Nitrophenol. Catalysts 2016, 6, 3. https://doi.org/10.3390/catal6010003
Liu Y, Xu L, Liu X, Cao M. Hybrids of Gold Nanoparticles with Core-Shell Hyperbranched Polymers: Synthesis, Characterization, and Their High Catalytic Activity for Reduction of 4-Nitrophenol. Catalysts. 2016; 6(1):3. https://doi.org/10.3390/catal6010003
Chicago/Turabian StyleLiu, Yi, Li Xu, Xunyong Liu, and Mengnan Cao. 2016. "Hybrids of Gold Nanoparticles with Core-Shell Hyperbranched Polymers: Synthesis, Characterization, and Their High Catalytic Activity for Reduction of 4-Nitrophenol" Catalysts 6, no. 1: 3. https://doi.org/10.3390/catal6010003





