High Efficient Hydrogenation of Lignin-Derived Monophenols to Cyclohexanols over Pd/γ-Al2O3 under Mild Conditions
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalyst Characterization
| Sample | Surface Area (m2/g) | Pore Volumes (cm3/g) | Average pore diameter (Å) | Pd a (wt. %) |
|---|---|---|---|---|
| γ-Al2O3 | 230 | 0.471 | 82.0 | - |
| 1Pd/γ-Al2O3 b | 223 | 0.468 | 83.1 | 0.71 |
| 2Pd/γ-Al2O3 | 218 | 0.463 | 84.7 | 1.79 |
| 3Pd/γ-Al2O3 | 197 | 0.423 | 86.0 | 3.05 |
| 5Pd/γ-Al2O3 | 174 | 0.403 | 92.5 | 4.54 |
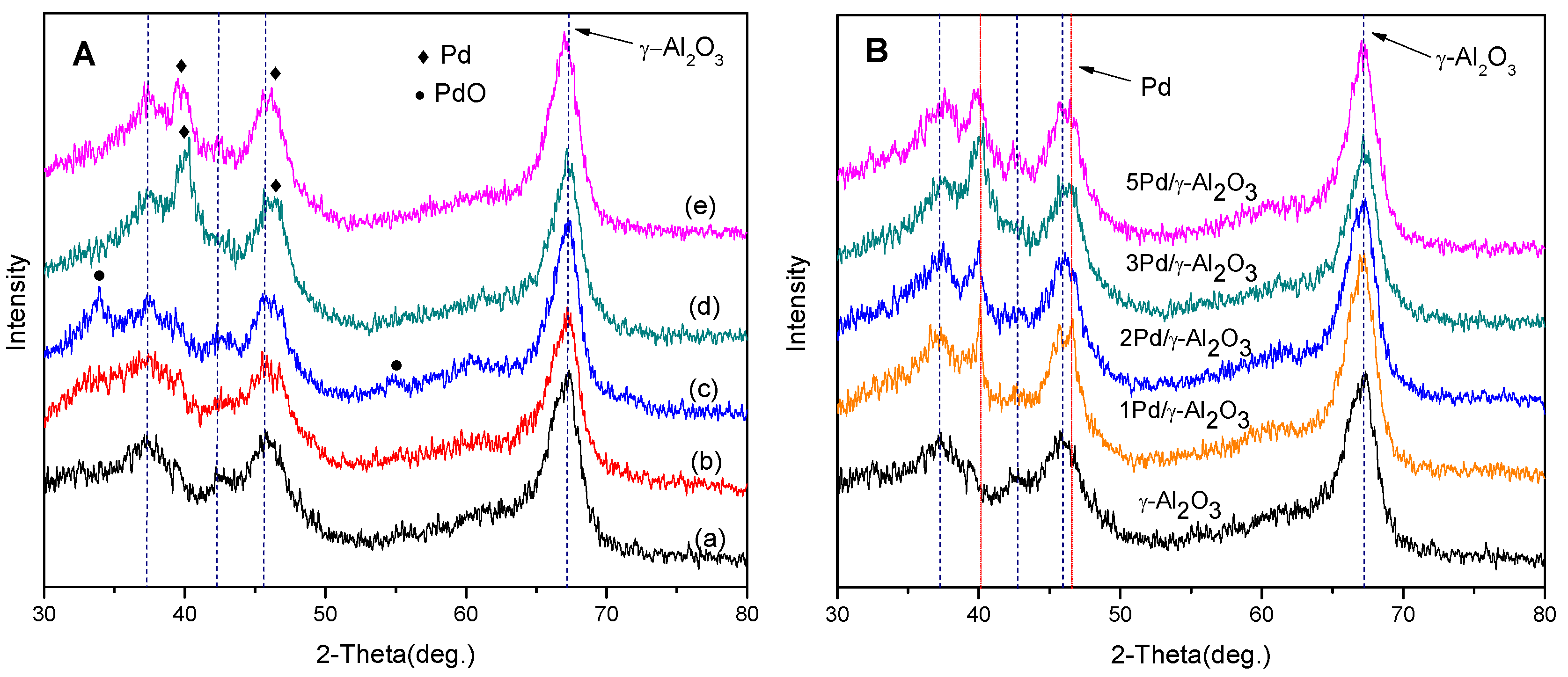
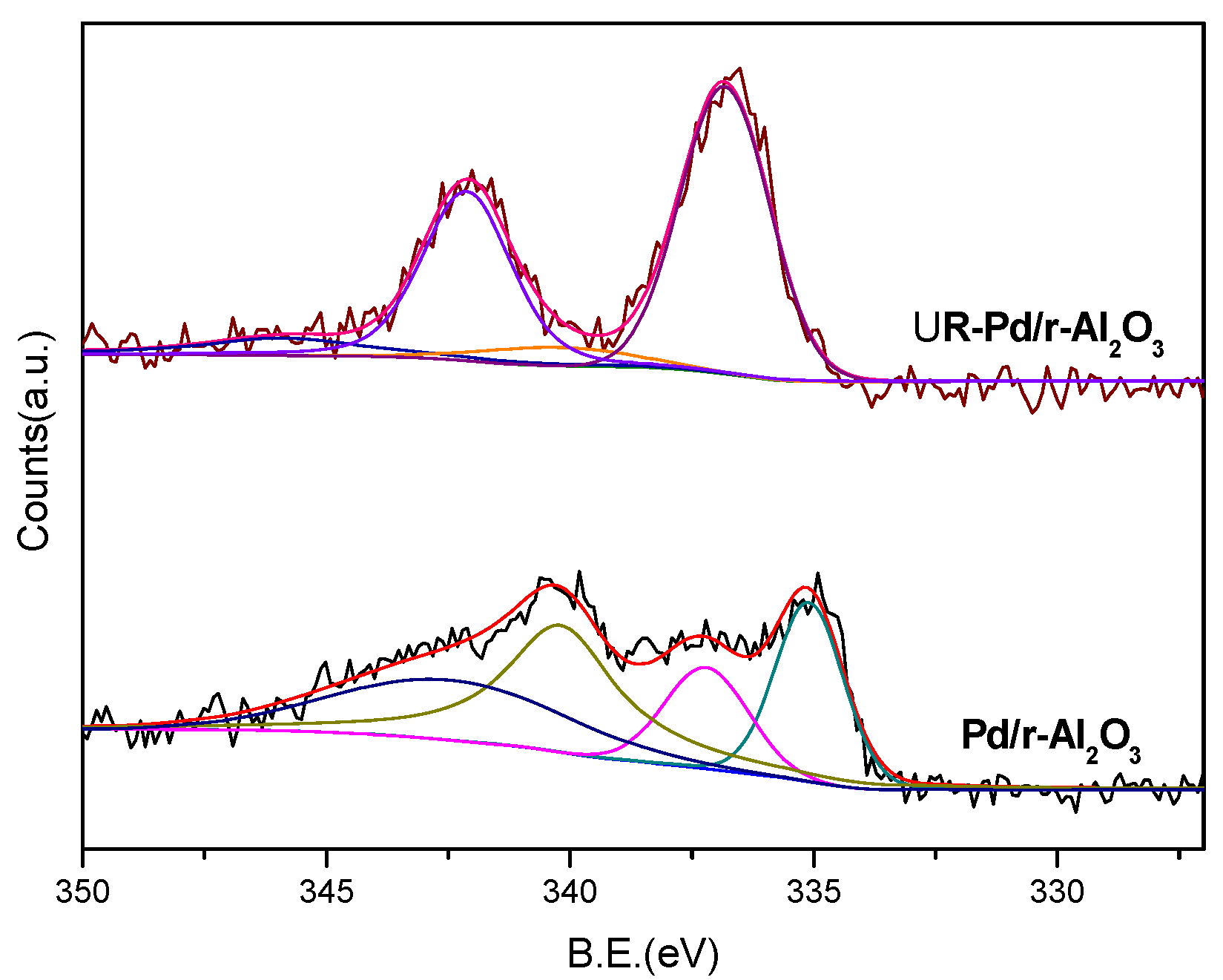
2.2. The Selective Hydrogenation of 4-Ethylphenol
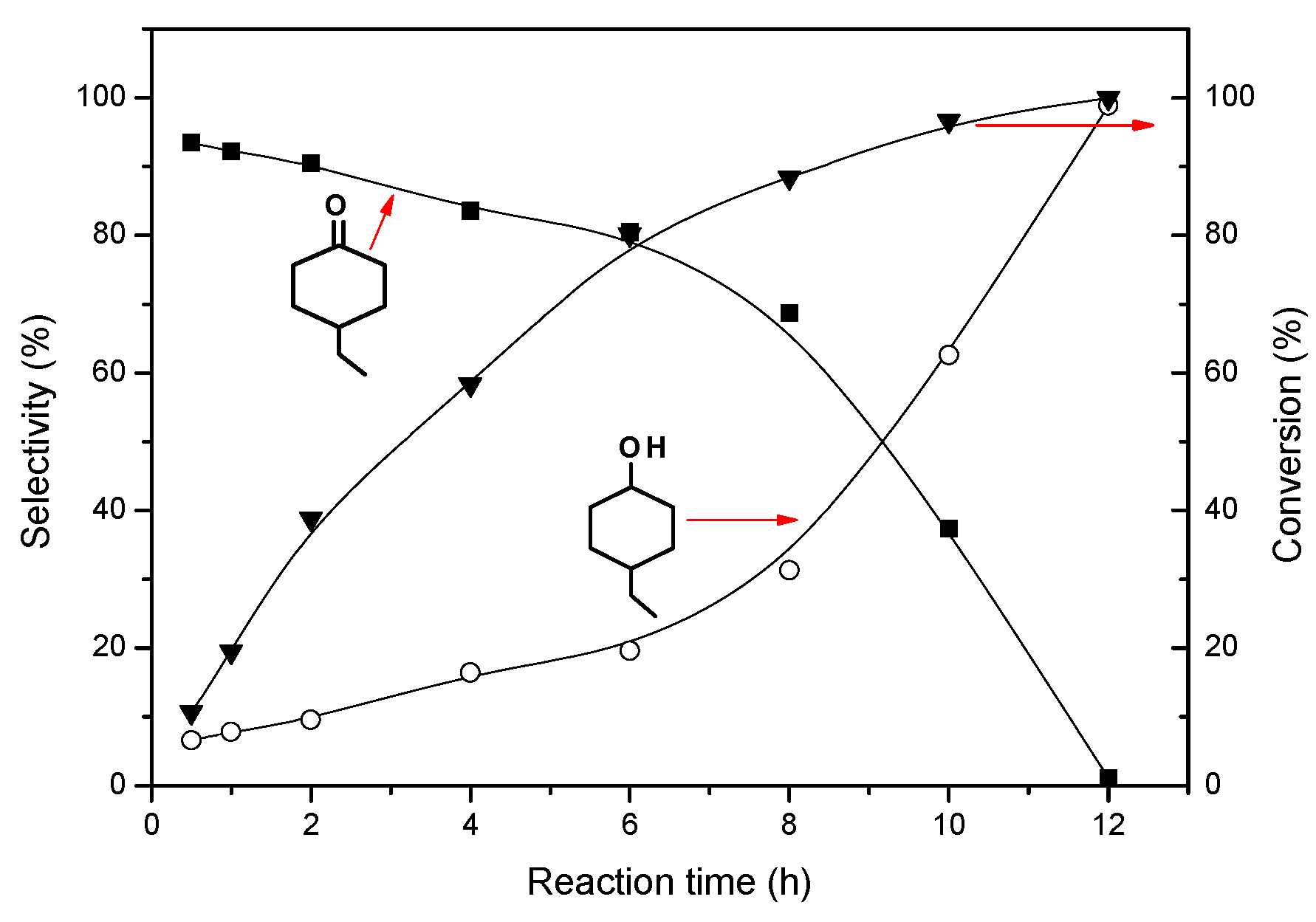
2.2.1. Effect of Reaction Temperature and Time on the Hydrogenation of 4-Ethylphenol

| T/°C a | 40 | 60 | 80 | 100 | 120 |
| Carbon balance/% | 79.6 | 100 | 86.8 | 70.9 | 68.9 |
| Pd loading/wt. % b | 0 | 1 | 2 | 3 | 5 |
| Carbon balance/% | - | 100 | 100 | 100 | 100 |
2.2.2. Effect of Pd Loading on the Hydrogenation of 4-Ethylphenol
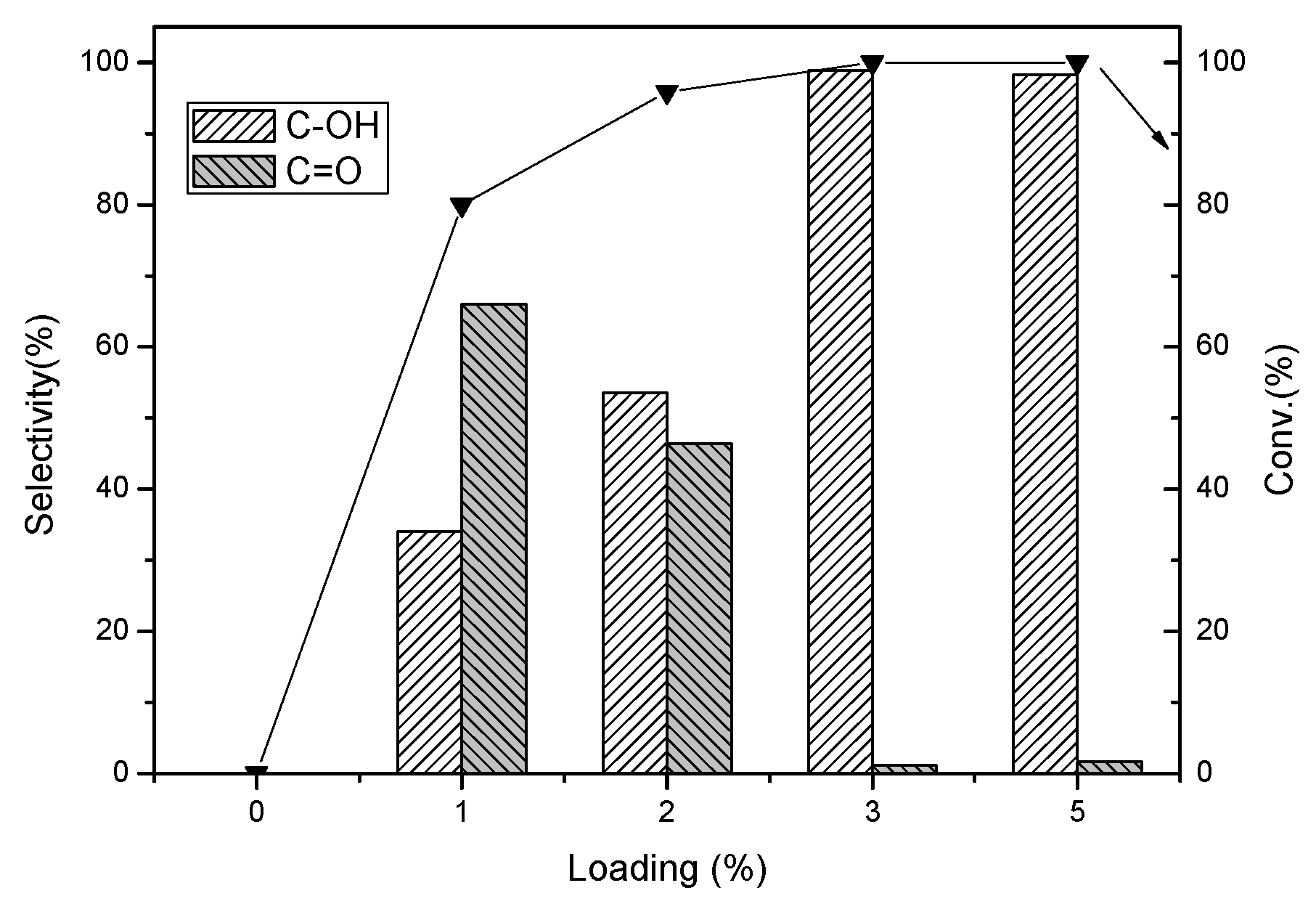
2.3. Hydrogenation of Other Substituted Phenols
| Entry | Reactant | Conversion (%) | Products | |||
|---|---|---|---|---|---|---|
| Selectivity (%) | Selectivity (%) | |||||
| 1 |  | 100 | - | - |  | 100 |
| 2 |  | 100 | - | - |  | 100 |
| 3 |  | 100 | - | - |  | 100 |
| 4 |  | 100 | - | - |  | 100 |
| 5 |  | 100 |  | 9.8 |  | 90.2 |
| 6 |  | 100 | - | - |  | 100 |
| 7 |  | 92.9 | - | - |  | 100 |
| 8 |  | 100 |  | 21.7 |  | 78.3 |
| 9 |  | 100 |  | 80.3 |  | 19.7 |
| 10 |  | 100 |  | 65.7 |  | 34.4 |
| 11 |  | 56.9 |  | 58.4 |  | 41.6 |
2.4. The Probable Reaction Pathway of 4-Ethylphenol Hydrogenation
2.5. The Hydrogenation of Phenols Directly from Raw Biomass on Pd/γ-Al2O3
| Entry | Monophenols | Yield (mg) | Hydrogenation Products (mg) | ||||
|---|---|---|---|---|---|---|---|
| Before a | After b | ||||||
| 1 |  | 3.4 | 1.5 |  | 1.2 |  | 0.7 |
| 2 |  | 3.8 | 2.7 |  | - c |  | - c |
| 3 |  | 4.4 | 3.7 |  | - c |  | - c |
| 4 |  | 25.9 | 3.4 | - c | - c | - c | - c |
| 5 |  | 27.9 | 49.2 |  | 2.5 |  | 12.1 |
2.6. Recyclability of the Catalyst

3. Experimental Section
3.1. Materials
3.2. Methods
3.2.1. Catalyst Preparation
3.2.2. Catalyst Characterization
3.2.3. Hydrogenation Reactions of Phenols
4. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhang, X.; Wang, T.; Ma, L.; Zhang, Q.; Huang, X.; Yu, Y. Production of cyclohexane from lignin degradation compounds over Ni/ZrO2-SiO2 catalysts. Appl. Energy 2013, 112, 533–538. [Google Scholar] [CrossRef]
- Hasegawa, I.; Inoue, Y.; Muranaka, Y.; Yasukawa, T.; Mae, K. Selective production of organic acids and depolymerization of lignin by hydrothermal oxidation with diluted hydrogen peroxide. Energy Fuels 2011, 25, 791–796. [Google Scholar] [CrossRef]
- Deepa, A.K.; Dhepe, P.L. Solid acid catalyzed depolymerization of lignin into value added aromatic monomers. RSC Adv. 2014, 4, 12625–12629. [Google Scholar] [CrossRef]
- Ye, Y.; Zhang, Y.; Fan, J.; Chang, J. Selective production of 4-ethylphenolics from lignin via mild hydrogenolysis. Bioresour. Technol. 2012, 118, 648–651. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Zhang, Q.; Wang, T.; Zhang, X.; Xu, Y.; Ma, L. An efficient and economical process for lignin depolymerization in biomass-derived solvent tetrahydrofuran. Bioresour. Technol. 2014, 154, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Zakzeski, J.; Jongerius, A.L.; Bruijnincx, P.C.; Weckhuysen, B.M. Catalytic lignin valorization process for the production of aromatic chemicals and hydrogen. ChemSusChem 2012, 5, 1602–1609. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Asakura, H.; van Rijn, J.; Yang, J.; Duchesne, P.; Zhang, B.; Chen, X.; Zhang, P.; Saeys, M.; Yan, N. Highly efficient NiAu-catalyzed hydrogenolysis of lignin into phenolic chemicals. Green Chem. 2014, 16, 2432–2437. [Google Scholar] [CrossRef]
- Zhao, C.; Kou, Y.; Lemonidou, A.A.; Li, X.; Lercher, J.A. Highly selective catalytic conversion of phenolic bio-oil to alkanes. Angew. Chem. 2009, 48, 3987–3990. [Google Scholar] [CrossRef] [PubMed]
- Van den Bosch, S.; Schutyser, W.; Vanholme, R.; Driessen, T.; Koelewijn, S.; Renders, T.; de Meester, B.; Huijgen, W.; Dehaen, W.; Courtin, C.; et al. Reductive lignocellulose fractionation into soluble lignin-derived phenolic monomers and dimers an processable carbohydrate pulps. Energy Environ. Sci. 2015, 8, 1748–1763. [Google Scholar] [CrossRef]
- Jiang, Z.; He, T.; Li, J.; Hu, C. Selective conversion of lignin in corncob residue to monophenols with high yield and selectivity. Green Chem. 2014, 16, 4257–4265. [Google Scholar] [CrossRef]
- Claus, P.; Berndt, H.; Mohr, C.; Radnik, J.; Shin, E.-J.; Keane, M.A. Pd/MgO: Catalyst characterization and phenol hydrogenation activity. J. Catal. 2000, 192, 88–97. [Google Scholar] [CrossRef]
- Sikhwivhilu, L.M.; Coville, N.J.; Naresh, D.; Chary, K.V.R.; Vishwanathan, V. Nanotubulartitanate supported palladium catalysts: The influence of structure and morphology on phenol hydrogenation activity. Appl. Catal. A 2007, 324, 52–61. [Google Scholar] [CrossRef]
- Velu, S.; Kapoor, M.P.; Inagaki, S.; Suzuki, K. Vapor phase hydrogenation of phenol over palladium supported on mesoporous CeO2 and ZrO2. Appl. Catal. A 2003, 245, 317–331. [Google Scholar] [CrossRef]
- Chary, K.V.R.; Naresh, D.; Vishwanathan, V.; Sadakane, M.; Ueda, W. Vapour phase hydrogenation of phenol over Pd/C catalysts: A relationship between dispersion, metal area and hydrogenation activity. Catal. Commun. 2007, 8, 471–477. [Google Scholar] [CrossRef]
- Mahata, N.; Vishwanathan, V. Gas phase hydrogenation of phenol over supported palladium catalysts. Catal. Today 1999, 49, 65–69. [Google Scholar] [CrossRef]
- Shin, E.-J.; Keane, M.A. Gas-phase hydrogenation/hydrogenolysis of phenol over supported nickel catalysts. Ind. Eng. Chem. Res. 2000, 39, 883–892. [Google Scholar] [CrossRef]
- Feng, G.; Chen, P.; Lou, H. Palladium catalysts supported on carbon-nitrogen composites for aqueous-phase hydrogenation of phenol. Catal. Sci. Technol. 2015, 5, 2300–2304. [Google Scholar] [CrossRef]
- Foster, A.J.; Do, P.T.M.; Lobo, R.F. The synergy of the support acid function and the metal function in the catalytic hydrodeoxygenation of m-cresol. Top. Catal. 2012, 55, 118–128. [Google Scholar] [CrossRef]
- Chatterjee, M.; Kawanami, H.; Sato, M.; Chatterjee, A.; Yokoyama, T.; Suzuki, T. Hydrogenation of phenol in supercritical carbon dioxide catalyzed by palladium supported on Al-MCM-41: A facile route for one-pot cyclohexanone formation. Adv. Synth. Catal. 2009, 351, 1912–1924. [Google Scholar] [CrossRef]
- Chase, Z.A.; Kasakov, S.; Shi, H.; Vjunov, A.; Fulton, J.L.; Camaioni, D.M.; Balasubramanian, M.; Zhao, C.; Wang, Y.; Lercher, J.A. State of Supported Nickel Nanoparticles during Catalysis in Aqueous Media. Chem. Eur. J. 2015, 21, 16541–16546. [Google Scholar] [CrossRef] [PubMed]
- Hellinger, M.; Baier, S.; Mortensen, P.; Kleist, W.; Jensen, A.; Grunwaldt, J.-D. Continuouscatalytic hydrodeoxygenation of guaiacol over Pt/SiO2 and Pt/H-MFI-90. Catalysts 2015, 5, 1152–1166. [Google Scholar] [CrossRef] [Green Version]
- Santillan-Jimenez, E.; Perdu, M.; Pace, R.; Morgan, T.; Crocker, M. Activated carbon, carbon nanofiber and carbon nanotube supported molybdenum carbide catalysts for the hydrodeoxygenation of guaiacol. Catalysts 2015, 5, 424–441. [Google Scholar] [CrossRef]
- Ishikawa, M.; Tamura, M.; Nakagawa, Y.; Tomishige, K. Demethoxylation of guaiacol and methoxybenzenes over carbon-supported Ru-Mn catalyst. Appl. Catal. B 2016, 182, 193–203. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Ishikawa, M.; Tamura, M.; Tomishige, K. Selective production of cyclohexanol and methanol from guaiacol over Ru catalyst combined with MgO. Green Chem. 2014, 16, 2197–2203. [Google Scholar] [CrossRef]
- Schutyser, W.; van den Bosch, S.; Dijkmans, J.; Turner, S.; Meledina, M.; van Tendeloo, G.; Debecker, D.P.; Sels, B.F. Selective nickel-catalyzed conversion of model and lignin-derived phenolic compounds to cyclohexanone-based polymer building blocks. ChemSusChem 2015, 8, 1805–1818. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Yang, X.; Yang, H.; Huang, P.; Song, H.; Liao, S. High-performance PdRu bimetallic catalyst supported on mesoporous silica nanoparticles for phenol hydrogenation. Appl. Surf. Sci. 2014, 315, 138–143. [Google Scholar] [CrossRef]
- Rode, C.V.; Joshi, U.D.; Sato, O.; Shirai, M. Catalytic ring hydrogenation of phenol under supercritical carbon dioxide. Chem. Commun. 2003, 1960–1961. [Google Scholar] [CrossRef]
- Li, H.; Liu, J.; Li, H. Liquid-phase selective hydrogenation of phenol to cyclohexanone over the Ce-doped Pd-B amorphous alloy catalyst. Mater. Lett. 2008, 62, 297–300. [Google Scholar] [CrossRef]
- Díaz, E.; Mohedano, A.F.; Calvo, L.; Gilarranz, M.A.; Casas, J.A.; Rodríguez, J.J. Hydrogenation of phenol in aqueous phase with palladium on activated carbon catalysts. Chem. Eng. J. 2007, 131, 65–71. [Google Scholar] [CrossRef]
- Li, Y.; Xu, X.; Zhang, P.; Gong, Y.; Li, H.; Wang, Y. Highly selective Pd@mpg-C3N4 catalyst for phenol hydrogenation in aqueous phase. RSC Adv. 2013, 3, 10973–10982. [Google Scholar] [CrossRef]
- Pérez, Y.; Fajardo, M.; Corma, A. Highly selective palladium supported catalyst for hydrogenation of phenol in aqueous phase. Catal. Commun. 2011, 12, 1071–1074. [Google Scholar] [CrossRef]
- Yang, X.; Du, L.; Liao, S.; Li, Y.; Song, H. High-performance gold-promoted palladium catalyst towards the hydrogenation of phenol with mesoporous hollow spheres as support. Catal. Commun. 2012, 17, 29–33. [Google Scholar] [CrossRef]
- Yin, F.; Ji, S.; Wu, P.; Zhao, F.; Li, C. Deactivation behavior of Pd-based SBA-15 mesoporous silica catalysts for the catalytic combustion of methane. J. Catal. 2008, 257, 108–116. [Google Scholar] [CrossRef]
- Mortensen, P.; Grunwaldt, J.; Jensen, P.; Jensen, D. Influence on nickel particle size on the hydrodeoxygenation of phenolover Ni/SiO2. Catal. Today 2016, 259, 277–284. [Google Scholar] [CrossRef]
- Yuan, G. Role of base addition in the liquid-phase hydrodechlorination of 2,4-dichlorophenol over Pd/Al2O3 and Pd/C. J. Catal. 2004, 225, 510–522. [Google Scholar] [CrossRef]
- Chen, H.; Xu, Z.; Wan, H.; Zheng, J.; Yin, D.; Zheng, S. Aqueous bromate reduction by catalytic hydrogenation over Pd/Al2O3 catalysts. Appl. Catal. B 2010, 96, 307–313. [Google Scholar] [CrossRef]
- Cheng, L.; Dai, Q.; Li, H.; Wang, X. Highly selective hydrogenation of phenol and derivatives over Pd catalysts supported on SiO2 and γ-Al2O3 in aqueous media. Catal. Commun. 2014, 57, 23–28. [Google Scholar] [CrossRef]
- Hu, L.; Luo, Y.; Cai, B.; Li, J.; Tong, D.; Hu, C. The degradation of the lignin in Phyllostachysheterocycla cv. Pubescens in an ethanol solvothermal system. Green Chem. 2014, 16, 3107–3116. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yi, J.; Luo, Y.; He, T.; Jiang, Z.; Li, J.; Hu, C. High Efficient Hydrogenation of Lignin-Derived Monophenols to Cyclohexanols over Pd/γ-Al2O3 under Mild Conditions. Catalysts 2016, 6, 12. https://doi.org/10.3390/catal6010012
Yi J, Luo Y, He T, Jiang Z, Li J, Hu C. High Efficient Hydrogenation of Lignin-Derived Monophenols to Cyclohexanols over Pd/γ-Al2O3 under Mild Conditions. Catalysts. 2016; 6(1):12. https://doi.org/10.3390/catal6010012
Chicago/Turabian StyleYi, Jian, Yiping Luo, Ting He, Zhicheng Jiang, Jianmei Li, and Changwei Hu. 2016. "High Efficient Hydrogenation of Lignin-Derived Monophenols to Cyclohexanols over Pd/γ-Al2O3 under Mild Conditions" Catalysts 6, no. 1: 12. https://doi.org/10.3390/catal6010012





