Adsorption and Diffusion of Xylene Isomers on Mesoporous Beta Zeolite
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization


| Sample | SBET (m2/g) | Smicb (m2/g) | Sextb (m2/g) | Vmicb (cm3/g) | Vmesoc (cm3/g) |
|---|---|---|---|---|---|
| Beta-0 | 743 | 681 | 62 | 0.27 | 0.06 |
| Beta-1 | 814 | 591 | 223 | 0.25 | 0.18 |
| Beta-2 | 879 | 539 | 340 | 0.23 | 0.29 |
| Beta-3 | 851 | 392 | 459 | 0.17 | 0.51 |
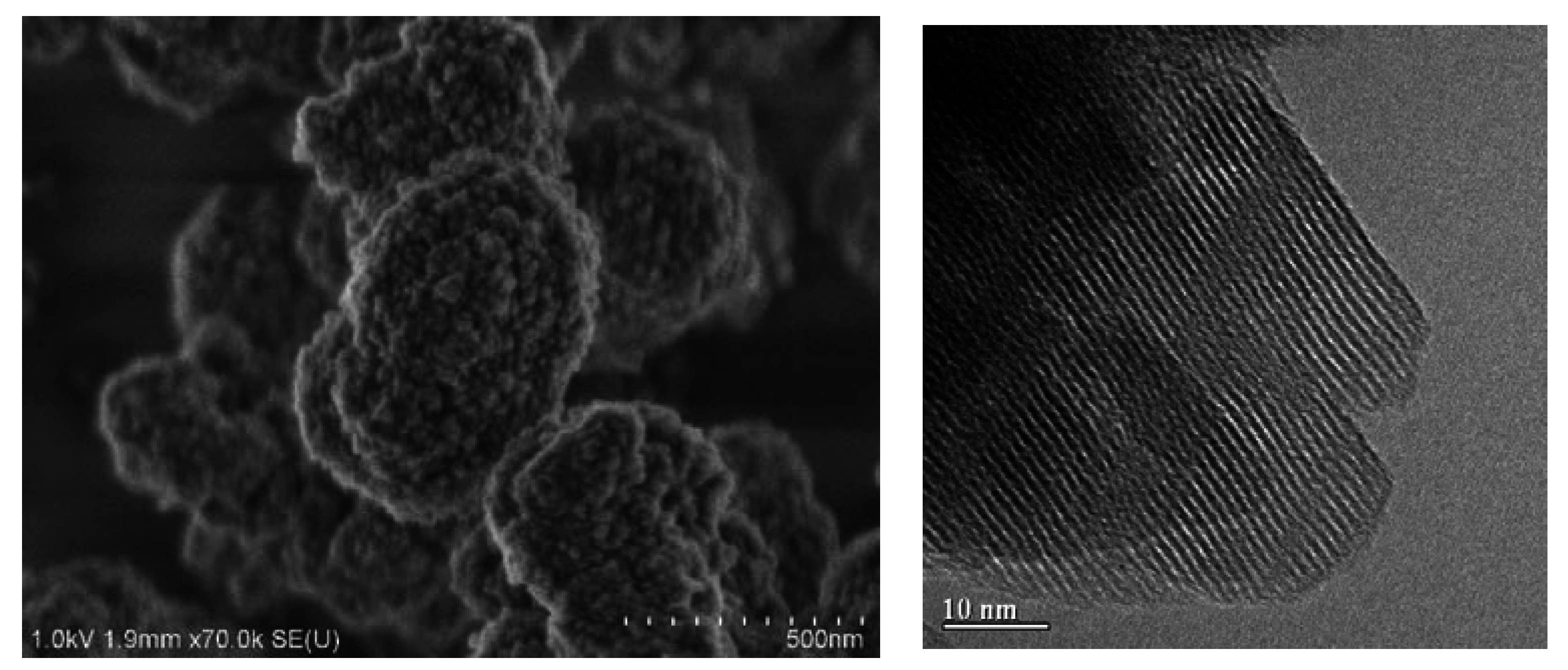
2.2. Adsorption Results
2.2.1. Adsorption Equilibrium Isotherms

2.2.2. Modeling of Equilibrium Isotherms
| T (K) | qm (mmol·g−1) | b (mbar−1·103) | n | R2 |
|---|---|---|---|---|
| m-xylene | ||||
| 308 | 2.74 | 4.93 | 0.25 | 0.9945 |
| 323 | 2.60 | 2.59 | 0.25 | 0.9815 |
| 338 | 2.40 | 1.20 | 0.27 | 0.9782 |
| p-xylene | ||||
| 308 | 2.79 | 2.20 | 0.26 | 0.9781 |
| 323 | 2.64 | 1.88 | 0.26 | 0.9913 |
| 338 | 2.44 | 0.99 | 0.27 | 0.9828 |
| o-xylene | ||||
| 308 | 2.90 | 6.83 | 0.24 | 0.9894 |
| 323 | 2.75 | 2.95 | 0.25 | 0.9880 |
| 338 | 2.59 | 1.94 | 0.25 | 0.9888 |
| Sample | Sorbate | T (K) | qm1 (mmol·g−1) | b1 (mbar−1·103) | n1 | qm2 (mmol·g−1) | b2 (mbar−1) | n2 | R2 |
|---|---|---|---|---|---|---|---|---|---|
| Beta-1 | m-xylene | 308 | 1.98 | 4.53 | 0.35 | 1.13 | 1.37 | 0.49 | 0.9865 |
| 323 | 1.80 | 2.24 | 0.31 | 1.01 | 0.92 | 0.53 | 0.9960 | ||
| 338 | 1.67 | 0.98 | 0.32 | 0.90 | 0.36 | 0.63 | 0.9949 | ||
| p-xylene | 308 | 2.02 | 1.98 | 0.30 | 1.21 | 0.91 | 0.58 | 0.9948 | |
| 323 | 1.87 | 1.65 | 0.32 | 1.10 | 0.42 | 0.55 | 0.9979 | ||
| 338 | 1.76 | 0.75 | 0.31 | 0.98 | 0.17 | 0.76 | 0.9976 | ||
| o-xylene | 308 | 2.13 | 6.52 | 0.35 | 1.34 | 1.68 | 0.41 | 0.9969 | |
| 323 | 1.91 | 2.49 | 0.30 | 1.25 | 1.32 | 0.44 | 0.9982 | ||
| 338 | 1.80 | 1.61 | 0.31 | 1.05 | 0.66 | 0.49 | 0.9969 | ||
| Beta-2 | m-xylene | 308 | 1.64 | 4.21 | 0.32 | 1.78 | 0.54 | 0.70 | 0.9979 |
| 323 | 1.49 | 1.97 | 0.38 | 1.66 | 0.22 | 0.82 | 0.9973 | ||
| 338 | 1.36 | 0.54 | 0.38 | 1.51 | 0.18 | 0.73 | 0.9964 | ||
| p-xylene | 308 | 1.69 | 1.67 | 0.44 | 1.85 | 0.50 | 0.67 | 0.9992 | |
| 323 | 1.55 | 1.18 | 0.39 | 1.67 | 0.19 | 0.86 | 0.9981 | ||
| 338 | 1.44 | 0.38 | 0.38 | 1.52 | 0.12 | 0.75 | 0.9973 | ||
| o-xylene | 308 | 1.74 | 4.70 | 0.38 | 1.97 | 0.88 | 0.59 | 0.9951 | |
| 323 | 1.63 | 2.14 | 0.32 | 1.83 | 0.42 | 0.64 | 0.9971 | ||
| 338 | 1.51 | 1.21 | 0.35 | 1.73 | 0.27 | 0.59 | 0.9974 | ||
| Beta-3 | m-xylene | 308 | 1.35 | 3.64 | 0.35 | 2.53 | 0.45 | 0.78 | 0.9980 |
| 323 | 1.20 | 1.36 | 0.50 | 2.40 | 0.19 | 0.99 | 0.9983 | ||
| 338 | 1.05 | 0.27 | 0.53 | 2.25 | 0.12 | 0.99 | 0.9992 | ||
| p-xylene | 308 | 1.41 | 0.77 | 0.49 | 2.63 | 0.40 | 0.73 | 0.9985 | |
| 323 | 1.26 | 0.46 | 0.51 | 2.50 | 0.16 | 0.81 | 0.9982 | ||
| 338 | 1.13 | 0.19 | 0.58 | 2.36 | 0.09 | 0.91 | 0.9984 | ||
| o-xylene | 308 | 1.51 | 3.92 | 0.34 | 2.95 | 0.68 | 0.59 | 0.9989 | |
| 323 | 1.40 | 1.76 | 0.37 | 2.77 | 0.25 | 0.72 | 0.9992 | ||
| 338 | 1.27 | 0.63 | 0.40 | 2.63 | 0.16 | 0.68 | 0.9990 |
2.2.3. Henry’s Constants and Initial Heats of Adsorption
| Sample | Sorbates | T (K) | KH (mmol/g mbar·103) | Qst (kJ/moL) |
|---|---|---|---|---|
| Beta-0 | p-xylene | 308 | 10.9 | 55.5 |
| 323 | 4.35 | |||
| 338 | 1.53 | |||
| m-xylene | 308 | 13.4 | 66.1 | |
| 323 | 4.38 | |||
| 338 | 1.68 | |||
| o-xylene | 308 | 17.8 | 69.5 | |
| 323 | 4.94 | |||
| 338 | 1.77 | |||
| Beta-1 | p-xylene | 308 | 0.84 | 43.5 |
| 323 | 0.41 | |||
| 338 | 0.19 | |||
| m-xylene | 308 | 1.28 | 55.0 | |
| 323 | 0.47 | |||
| 338 | 0.21 | |||
| o-xylene | 308 | 1.37 | 56.1 | |
| 323 | 0.51 | |||
| 338 | 0.22 | |||
| Beta-2 | p-xylene | 308 | 0.11 | 35.1 |
| 323 | 0.06 | |||
| 338 | 0.04 | |||
| m-xylene | 308 | 0.19 | 42.8 | |
| 323 | 0.09 | |||
| 338 | 0.05 | |||
| o-xylene | 308 | 0.31 | 43.6 | |
| 323 | 0.15 | |||
| 338 | 0.07 | |||
| Beta-3 | p-xylene | 308 | 0.04 | 25.5 |
| 323 | 0.03 | |||
| 338 | 0.02 | |||
| m-xylene | 308 | 0.08 | 31.0 | |
| 323 | 0.05 | |||
| 338 | 0.03 | |||
| o-xylene | 308 | 0.15 | 33.2 | |
| 323 | 0.07 | |||
| 338 | 0.05 |
2.3. Diffusion Results
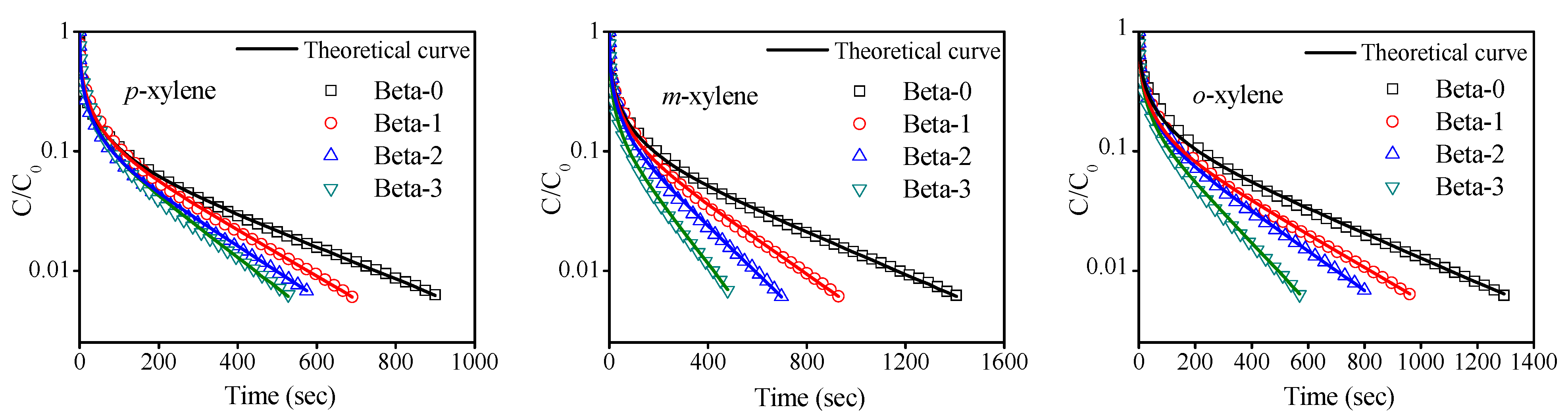
| Sample | L | β | Deff/R2(s−1·10−4) | ||
|---|---|---|---|---|---|
| p-xylene | |||||
| Beta-0 | 17.7 | 2.97 | 3.74 | ||
| Beta-1 | 15.6 | 2.94 | 5.18 | ||
| Beta-2 | 15.9 | 2.95 | 6.06 | ||
| Beta-3 | 13.1 | 2.91 | 7.48 | ||
| m-xylene | |||||
| Beta-0 | 13.2 | 2.91 | 2.84 | ||
| Beta-1 | 12.9 | 2.90 | 4.26 | ||
| Beta-2 | 15.8 | 2.95 | 5.01 | ||
| Beta-3 | 16.9 | 2.96 | 6.85 | ||
| o-xylene | |||||
| Beta-0 | 15.4 | 2.94 | 2.73 | ||
| Beta-1 | 13.4 | 2.91 | 3.97 | ||
| Beta-2 | 13.4 | 2.91 | 4.64 | ||
| Beta-3 | 14.3 | 2.93 | 6.35 | ||
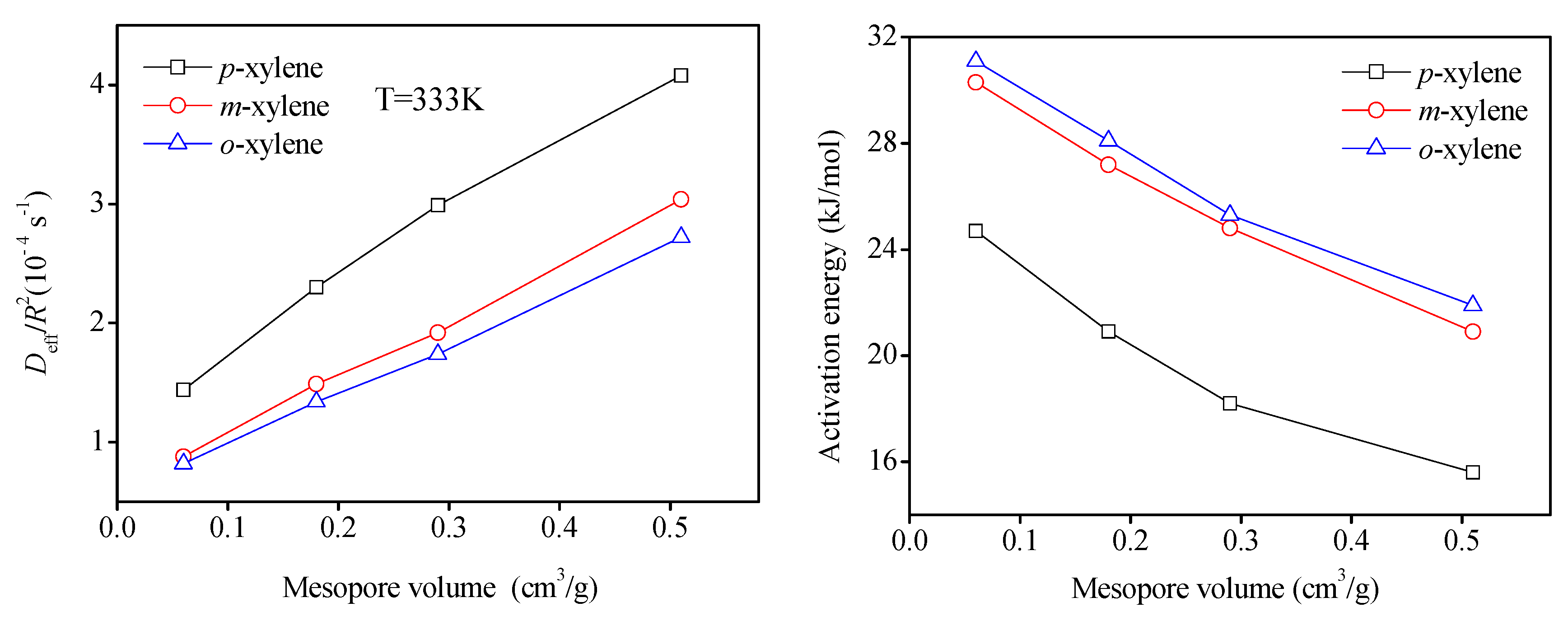
3. Experimental Section
3.1. Materials and Characterization
3.2. Adsorption Isotherms Measurements
3.3. ZLC Measurement and Theory
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Baerlocher, C.; McCusker, L.B.; Olson, D.H. Atlas of Zeolite Framework Types; Elsevier Science: Amsterdam, The Netherlands, 2007; p. 73. [Google Scholar]
- Ackley, M.W.; Rege, S.U.; Saxena, H. Application of natural zeolites in the purification and separation of gases. Microporous Mesoporous Mater. 2003, 61, 25–42. [Google Scholar] [CrossRef]
- Degnan, T.F., Jr. Applications of zeolites in petroleum refining. Top. Catal. 2000, 13, 349–356. [Google Scholar] [CrossRef]
- Christensen, C.H.; Johannsen, K.; Törnqvist, E.; Schmidt, I.; Topsøe, H. Mesoporous zeolite single crystal catalysts: Diffusion and catalysis in hierarchical zeolites. Catal. Today 2007, 128, 117–122. [Google Scholar] [CrossRef]
- Verboekend, D.; Pérez-Ramírez, J. Design of hierarchical zeolite catalysts by desilication. Catal. Sci. Technol. 2011, 1, 879–890. [Google Scholar] [CrossRef]
- Choi, M.; Cho, H.S.; Srivastava, R.; Venkatesan, C.; Choi, D.H.; Ryoo, R. Amphiphilic organosilane-directed synthesis of crystalline zeolite with tunable mesoporosity. Nat. Mater. 2006, 5, 718–723. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Kanoh, H.; Abrams, L.; Kaneko, K. Mesopore-modified zeolites: Preparation, characterization, and applications. Chem. Rev. 2006, 106, 896–910. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Ma, J.; Zhang, Q.; Liu, Z.; Li, R. Adsorption and diffusion of n-heptane and toluene over mesoporous ZSM-5 zeolites. Ind. Eng. Chem. Res. 2014, 53, 13810–13819. [Google Scholar] [CrossRef]
- Lee, S.; Kim, H.; Choi, M. Controlled decationization of X zeolite: Mesopore generation within zeolite crystallites for bulky molecular adsorption and transformation. J. Mater. Chem. A 2013, 1, 12096–12102. [Google Scholar] [CrossRef]
- Gu, F.N.; Wei, F.; Yang, J.Y.; Lin, N.; Lin, W.G.; Wang, Y.; Zhu, J.H. New strategy to synthesis of hierarchical mesoporous zeolites. Chem. Mater. 2010, 22, 2442–2450. [Google Scholar] [CrossRef]
- Bonilla, M.R.; Valiullin, R.; Kärger, J.; Bhatia, S.K. Understanding adsorption and transport of light gases in hierarchical materials using molecular simulation and effective medium theory. J. Phys. Chem. C 2014, 118, 14355–14370. [Google Scholar] [CrossRef]
- Groen, J.C.; Zhu, W.; Brouwer, S.; Huynink, S.J.; Kapteijn, F.; Moulijn, J.A.; Pérez-Ramírez, J. Direct demonstration of enhanced diffusion in mesoporous ZSM-5 zeolite obtained via controlled desilication. J. Am. Chem. Soc. 2007, 129, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Shen, B.; Gao, J.; Xu, C. Investigation on the mechanism of diffusion in mesopore structured ZSM-5 and improved heavy oil conversion. J. Catal. 2008, 258, 228–234. [Google Scholar] [CrossRef]
- Newsam, J.M.; Treacy, M.M.; Koetsier, W.T.; de Gruyter, C.B. Structural characterization of zeolite beta. Proc. R. Soc. Lond. A 1988, 420, 375–405. [Google Scholar] [CrossRef]
- Brito, A.; Borges, M.E.; Otero, N. Zeolite Y as a heterogeneous catalyst in biodiesel fuel production from used vegetable oil. Energy Fuels 2007, 21, 3280–3283. [Google Scholar] [CrossRef]
- Perez-Pariente, J.; Sastre, E.; Fornes, V.; Martens, J.A.; Jacobs, P.A.; Corma, A. Isomerization and disproportionation of m-xylene over zeolite β. Appl. Catal. 1991, 69, 125–137. [Google Scholar] [CrossRef]
- Ratnasamy, P.; Bhat, R.N.; Pokhriyal, S.K.; Hegde, S.G.; Kumar, R. Reactions of aromatic hydrocarbons over zeolite β. J. Catal. 1989, 119, 65–70. [Google Scholar] [CrossRef]
- Llopis, F.J.; Sastre, G.; Corma, A. Xylene isomerization and aromatic alkylation in zeolites NU-87, SSZ-33, β, and ZSM-5: Molecular dynamics and catalytic studies. J. Catal. 2004, 227, 227–241. [Google Scholar] [CrossRef]
- Byun, Y.; Jo, D.; Shin, D.N.; Hong, S.B. Theoretical investigation of the isomerization and disproportionation of m-Xylene over medium-pore zeolites with different framework topologies. ACS Catal. 2014, 4, 1764–1776. [Google Scholar] [CrossRef]
- Fernandez, C.; Stan, I.; Gilson, J.P.; Thomas, K.; Vicente, A.; Bonilla, A.; Pérez-Ramírez, J. hierarchical ZSM-5 zeolites in shape-selective xylene isomerization: Role of mesoporosity and acid site speciation. Chem. A Eur. J. 2010, 16, 6224–6233. [Google Scholar] [CrossRef] [PubMed]
- Dehkordi, A.M.; Khademi, M. Adsorption of xylene isomers on Na-BETA zeolite: Equilibrium in batch adsorber. Microporous Mesoporous Mater. 2013, 172, 136–140. [Google Scholar] [CrossRef]
- Rasouli, M.; Yaghobi, N.; Allahgholipour, F.; Atashi, H. Para-xylene adsorption separation process using nano-zeolite Ba-X. Chem. Eng. Res. Des. 2014, 92, 1192–1199. [Google Scholar] [CrossRef]
- Sing, K.S. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Do, D.D. Adsorption Analysis: Equilibria and Kinetics; Imperial College Press: London, UK, 1998; p. 64. [Google Scholar]
- Cavalcante, C.L., Jr.; Azevêdo, D.C.; Souza, I.G.; Silva, A.C.M.; Alsina, O.L.; Lima, V.E.; Araujo, A.S. Sorption and diffusion of p-xylene and o-xylene in aluminophosphate molecular sieve AlPO4–11. Adsorption 2000, 6, 53–59. [Google Scholar] [CrossRef]
- Chiang, A.S.; Lee, C.K.; Chang, Z.H. Adsorption and diffusion of aromatics in AIPO4–5. Zeolites 1991, 11, 380–386. [Google Scholar] [CrossRef]
- Peralta, D.; Barthelet, K.; Pérez-Pellitero, J.; Chizallet, C.; Chaplais, G.; Simon-Masseron, A.; Pirngruber, G.D. Adsorption and separation of xylene isomers: CPO-27-Ni vs HKUST-1 vs NaY. J. Phys. Chem. C 2012, 116, 21844–21855. [Google Scholar] [CrossRef]
- Ruthven, D.M.; Kaul, B.K. Adsorption of n-hexane and intermediate molecular weight aromatic hydrocarbons on LaY zeolite. Ind. Eng. Chem. Res. 1996, 35, 2060–2064. [Google Scholar] [CrossRef]
- Silva, J.A.; Rodrigues, A.E. Sorption and diffusion of n-pentane in pellets of 5A zeolite. Ind. Eng. Chem. Res. 1997, 36, 493–500. [Google Scholar] [CrossRef]
- Vavlitis, A.P.; Ruthven, D.M.; Loughlin, K.F. Sorption of n-pentane, n-octane, and n-decane in 5A zeolite crystals. J. Colloid Interface Sci. 1981, 84, 526–531. [Google Scholar] [CrossRef]
- Saha, D.; Deng, S. Adsorption equilibrium, kinetics, and enthalpy of N2O on zeolite 4A and 13X. J. Chem. Eng. Data 2010, 55, 3312–3317. [Google Scholar] [CrossRef]
- Bárcia, P.S.; Silva, J.A.; Rodrigues, A.E. Adsorption equilibrium and kinetics of branched hexane isomers in pellets of Beta zeolite. Microporous Mesoporous Mater. 2005, 79, 145–163. [Google Scholar] [CrossRef]
- Liu, Z.; Fan, W.; Xue, Z.; Ma, J.; Li, R. Diffusion of n-alkanes in mesoporous 5A zeolites by ZLC method. Adsorption 2013, 19, 201–208. [Google Scholar] [CrossRef]
- Brandani, S. Effects of nonlinear equilibrium on zero length column experiments. Chem. Eng. Sci. 1998, 53, 2791–2798. [Google Scholar] [CrossRef]
- Brandani, S.; Jama, M.A.; Ruthven, D.M. ZLC measurements under non-linear conditions. Chem. Eng. Sci. 2000, 55, 1205–1212. [Google Scholar] [CrossRef]
- Ruthven, D.M.; Eic, M.; Richard, E. Diffusion of C8 aromatic hydrocarbons in silicalite. Zeolites 1991, 11, 647–653. [Google Scholar] [CrossRef]
- Brandani, S.; Jama, M.; Ruthven, D. Diffusion, self-diffusion and counter-diffusion of benzene and p-xylene in silicalite. Microporous Mesoporous Mater. 2000, 35, 283–300. [Google Scholar] [CrossRef]
- Zhang, Q.; Ming, W.; Ma, J.; Zhang, J.; Wang, P.; Li, R. De novo assembly of mesoporous beta zeolite with intracrystalline channels and its catalytic performance for biodiesel production. J. Mater. Chem. A 2014, 2, 8712–8718. [Google Scholar] [CrossRef]
- Rouquerol, F.; Rouquerol, J.; Sing, K. Adsorption by Powders and Porous Solids; Academic Press: San Diego, CA, USA, 1999; p. 267. [Google Scholar]
- Landers, J.; Gor, G.Y.; Neimark, A.V. Density functional theory methods for characterization of porous materials. Colloids Surf. A 2013, 437, 3–32. [Google Scholar] [CrossRef]
- Eic, M.; Ruthven, D.M. A new experimental technique for measurement of intracrystalline diffusivity. Zeolites 1988, 8, 40–45. [Google Scholar] [CrossRef]
- Gunadi, A.; Brandani, S. Diffusion of linear paraffins in NaCaA studied by the ZLC method. Microporous Mesoporous Mater. 2006, 90, 278–283. [Google Scholar] [CrossRef]
- Ruthven, D.M. Diffusion of aromatic hydrocarbons in silicalite/HZSM-5. Adsorption 2007, 13, 225–230. [Google Scholar] [CrossRef]
- Thang, H.V.; Malekian, A.; Eić, M.; On, D.T.; Kaliaguine, S. Diffusive characterization of large pore mesoporous materials with semi-crystalline zeolitic framework. Stud. Surf. Sci. Catal. 2003, 146, 145–148. [Google Scholar]
- Malekian, A.; Vinh-Thang, H.; Huang, Q.; Eic, M.; Kaliaguine, S. Evaluation of the main diffusion path in novel micro-mesoporous zeolitic materials with the zero length column method. Ind. Eng. Chem. Res. 2007, 46, 5067–5073. [Google Scholar] [CrossRef]
- Hufton, J.R.; Ruthven, D.M. Diffusion of light alkanes in silicalite studied by the zero length column method. Ind. Eng. Chem. Res. 1993, 32, 2379–2386. [Google Scholar] [CrossRef]
- Han, M.; Yin, X.; In, Y.; Chen, S. Diffusion of aromatic hydrocarbon in ZSM-5 studied by the improved zero length column method. Ind. Eng. Chem. Res. 1999, 38, 3172–3175. [Google Scholar] [CrossRef]
- Cavalcante, C.L., Jr.; Silva, N.M.; Souza-Aguiar, E.F.; Sobrinho, E.V. Diffusion of paraffins in dealuminated Y mesoporous molecular sieve. Adsorption 2003, 9, 205–212. [Google Scholar] [CrossRef]
- Gobin, O.C.; Huang, Q.; Vinh-Thang, H.; Kleitz, F.; Eic, M.; Kaliaguine, S. Mesostructured silica SBA-16 with tailored intrawall porosity part 2: Diffusion. J. Phys. Chem. C 2007, 111, 3059–3065. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, A.; Ma, J.; Xu, D.; Li, R. Adsorption and Diffusion of Xylene Isomers on Mesoporous Beta Zeolite. Catalysts 2015, 5, 2098-2114. https://doi.org/10.3390/catal5042098
Song A, Ma J, Xu D, Li R. Adsorption and Diffusion of Xylene Isomers on Mesoporous Beta Zeolite. Catalysts. 2015; 5(4):2098-2114. https://doi.org/10.3390/catal5042098
Chicago/Turabian StyleSong, Aixia, Jinghong Ma, Duo Xu, and Ruifeng Li. 2015. "Adsorption and Diffusion of Xylene Isomers on Mesoporous Beta Zeolite" Catalysts 5, no. 4: 2098-2114. https://doi.org/10.3390/catal5042098







