VOx Surface Coverage Optimization of V2O5/WO3-TiO2 SCR Catalysts by Variation of the V Loading and by Aging
Abstract
:1. Introduction
2. Results and Discussion
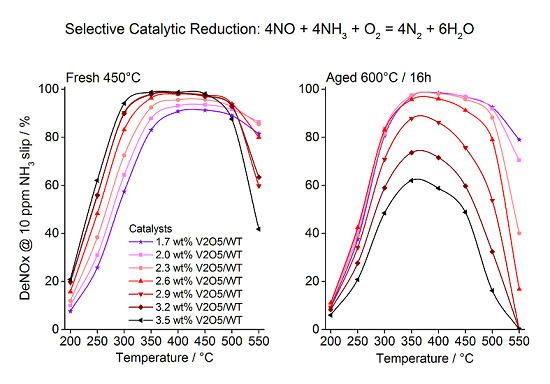
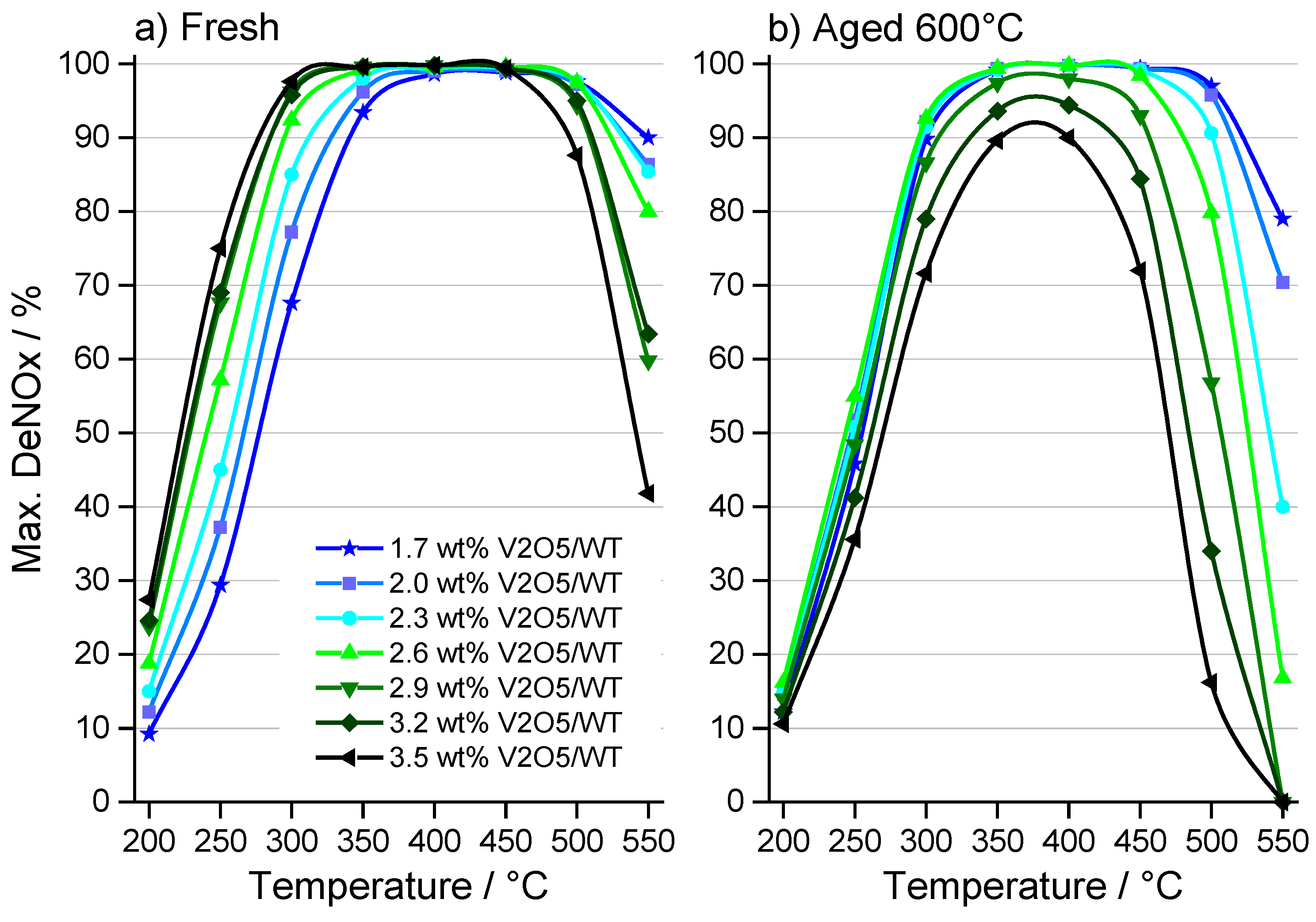
2.1. Catalytic Activity
| V2O5 (wt. %) | 1.7 | 2.0 | 2.3 | 2.6 | 2.9 | 3.2 | 3.5 |
|---|---|---|---|---|---|---|---|
| fresh | 29.4 | 37.2 | 45.0 | 57.2 | 67.6 | 69.0 | 75.0 |
| aged | 45.8 | 51.8 | 50.8 | 55.0 | 48.4 | 41.2 | 35.6 |
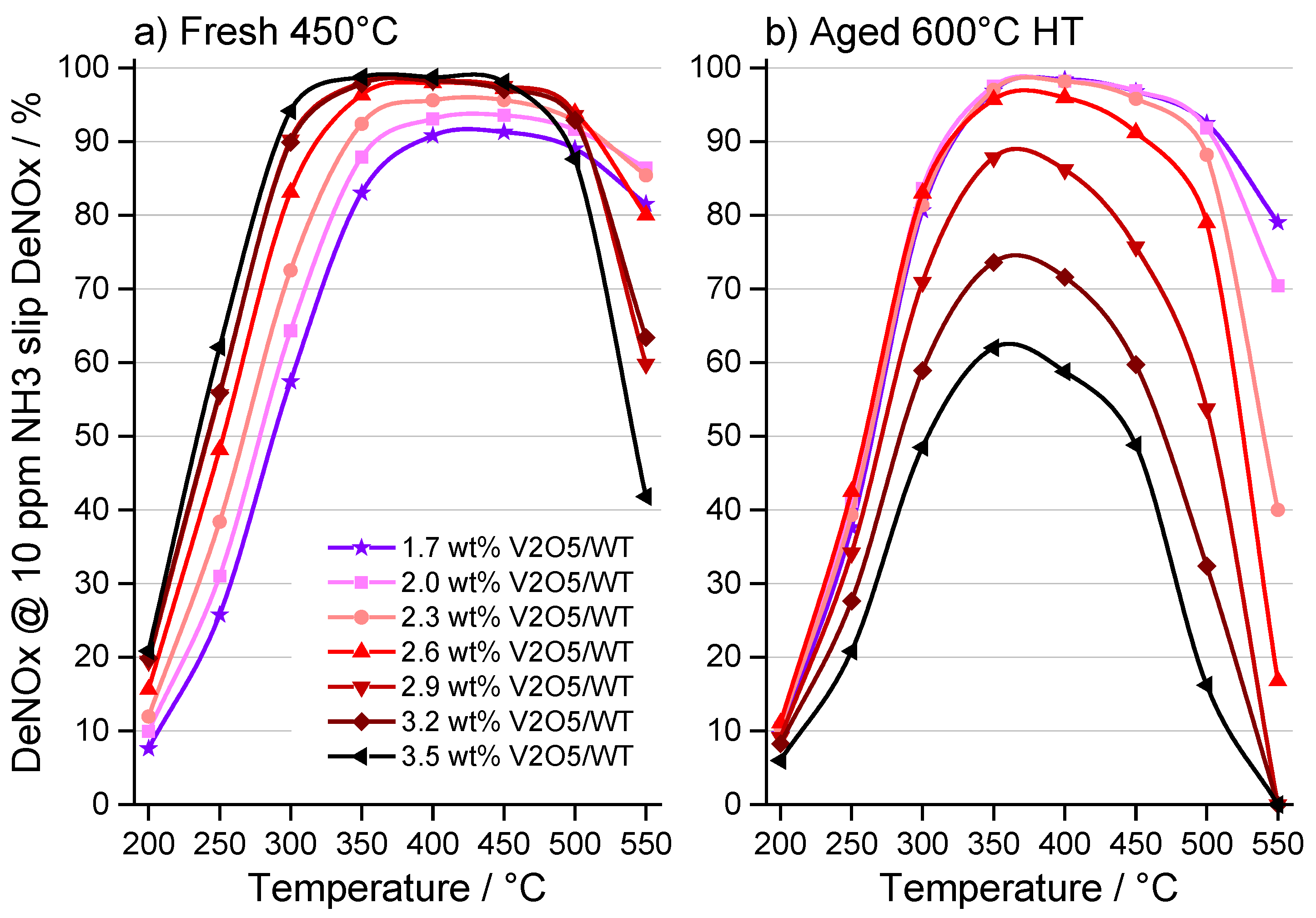
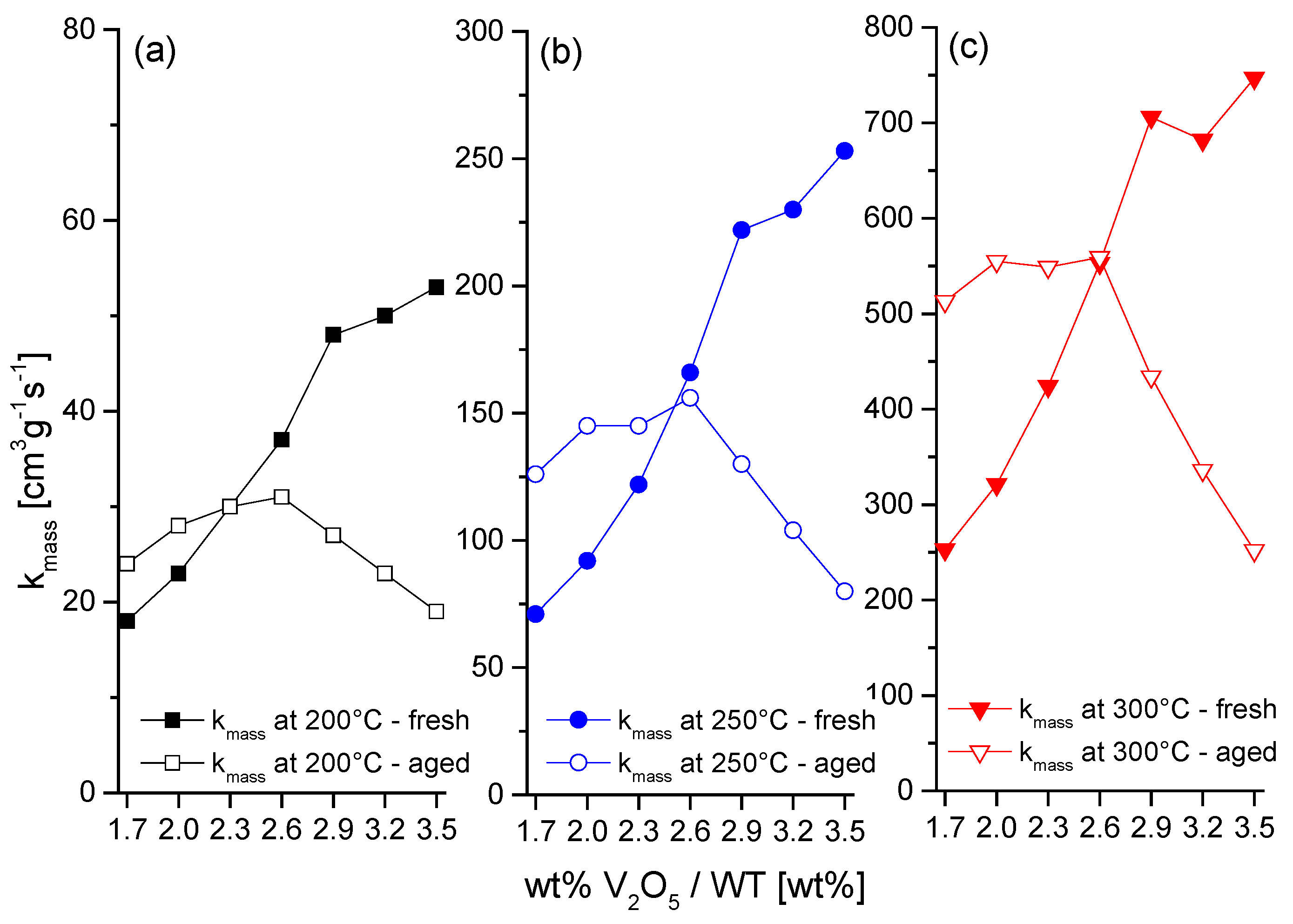
2.2. Characterization
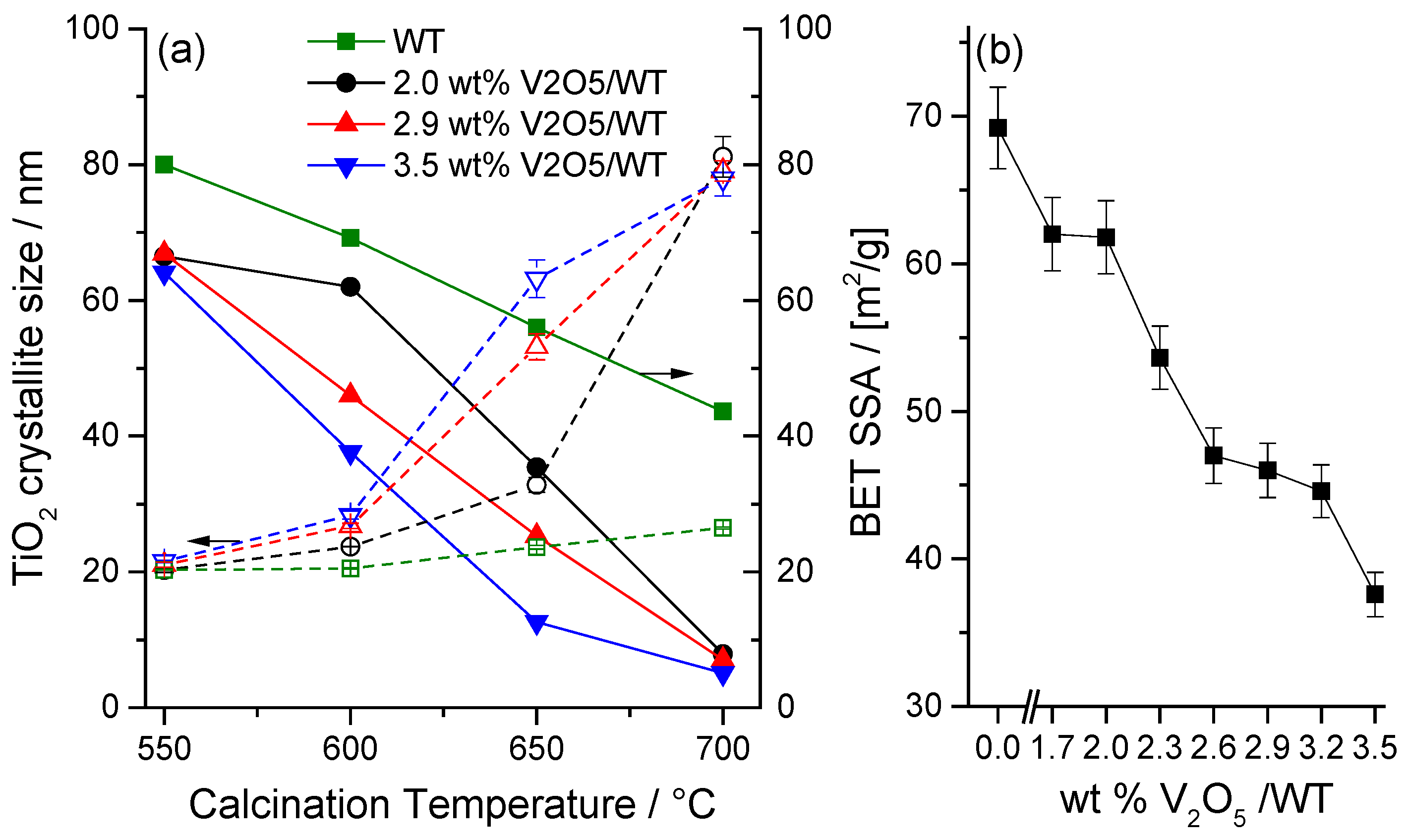
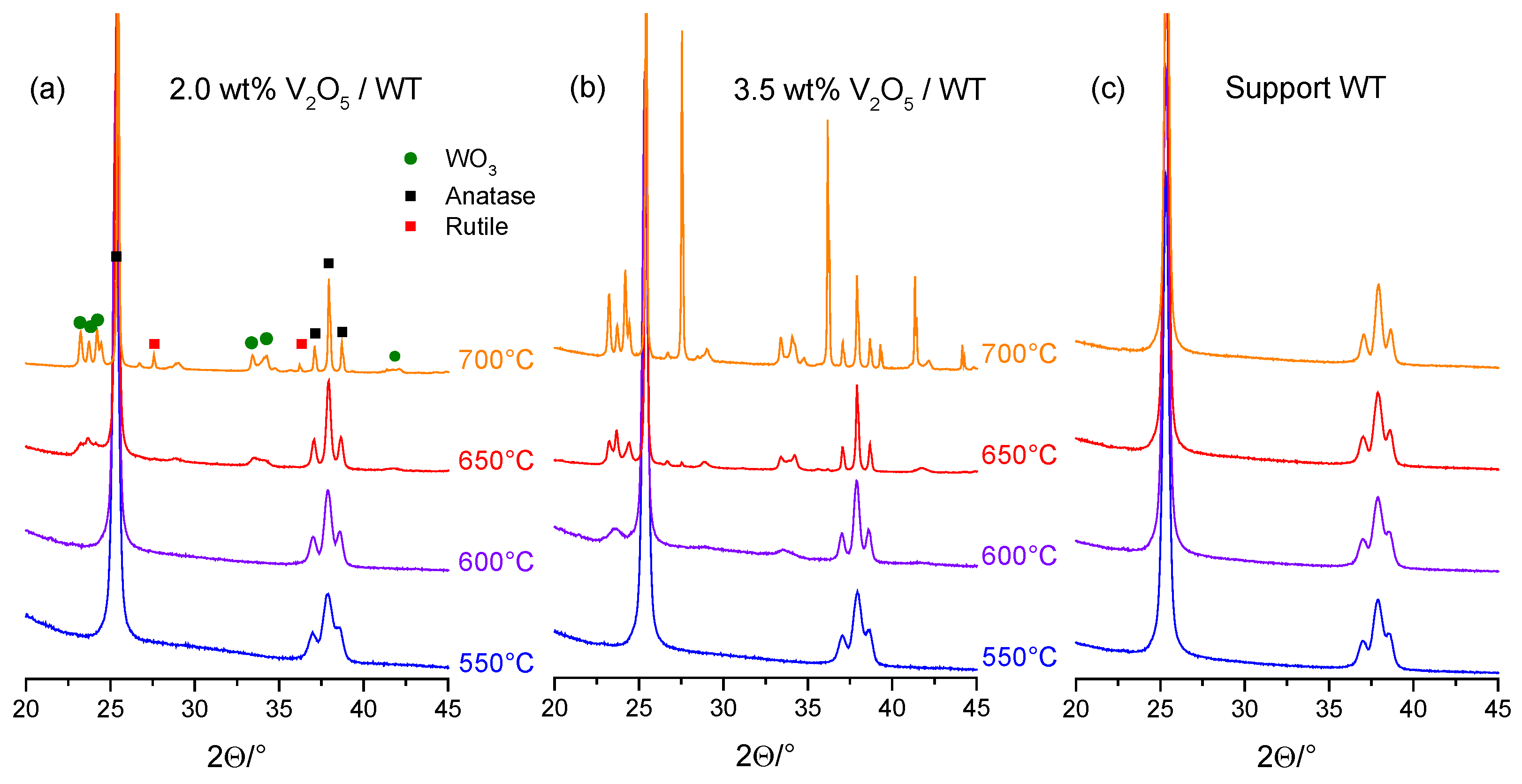
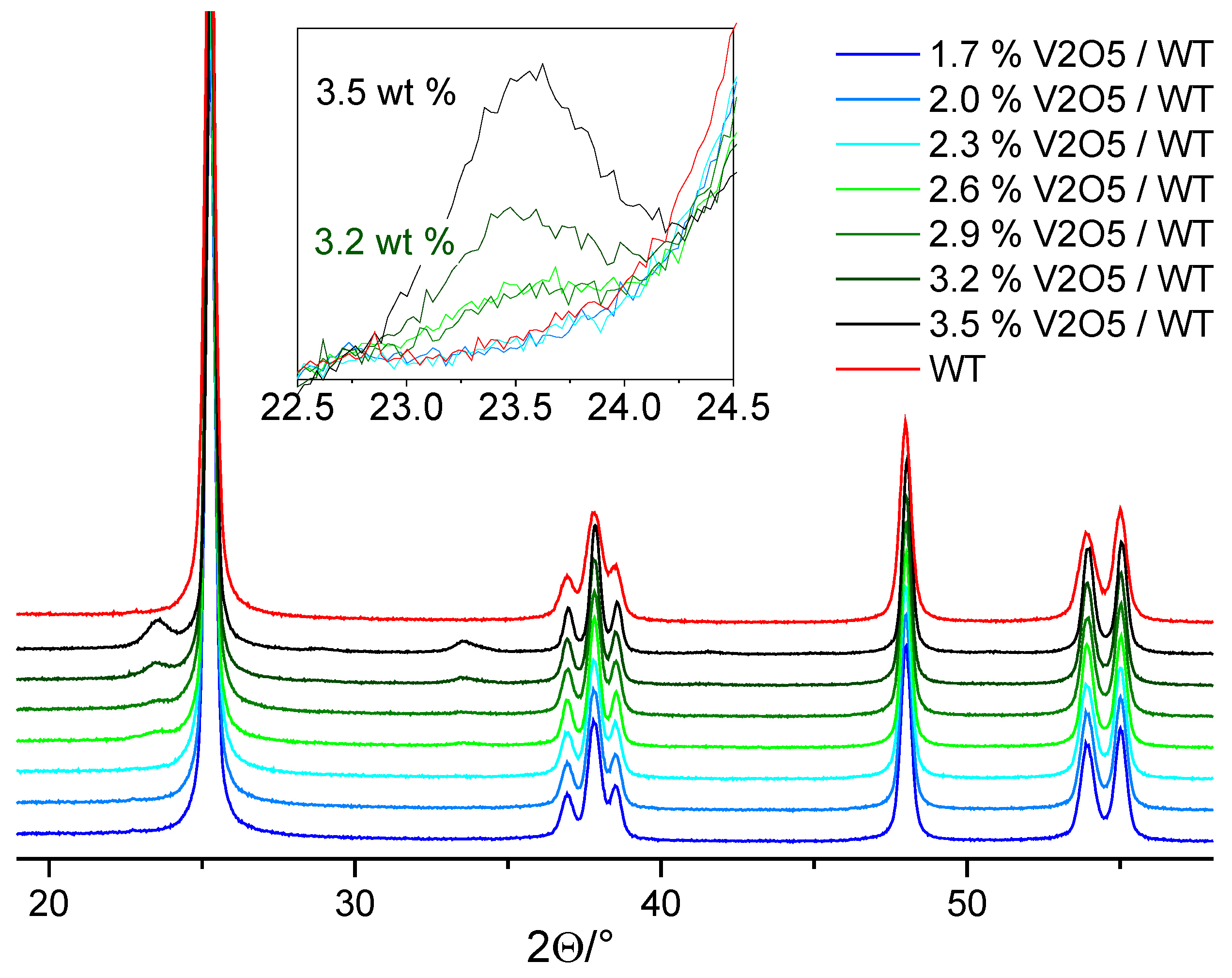

2.2.1. VOx Surface Coverage
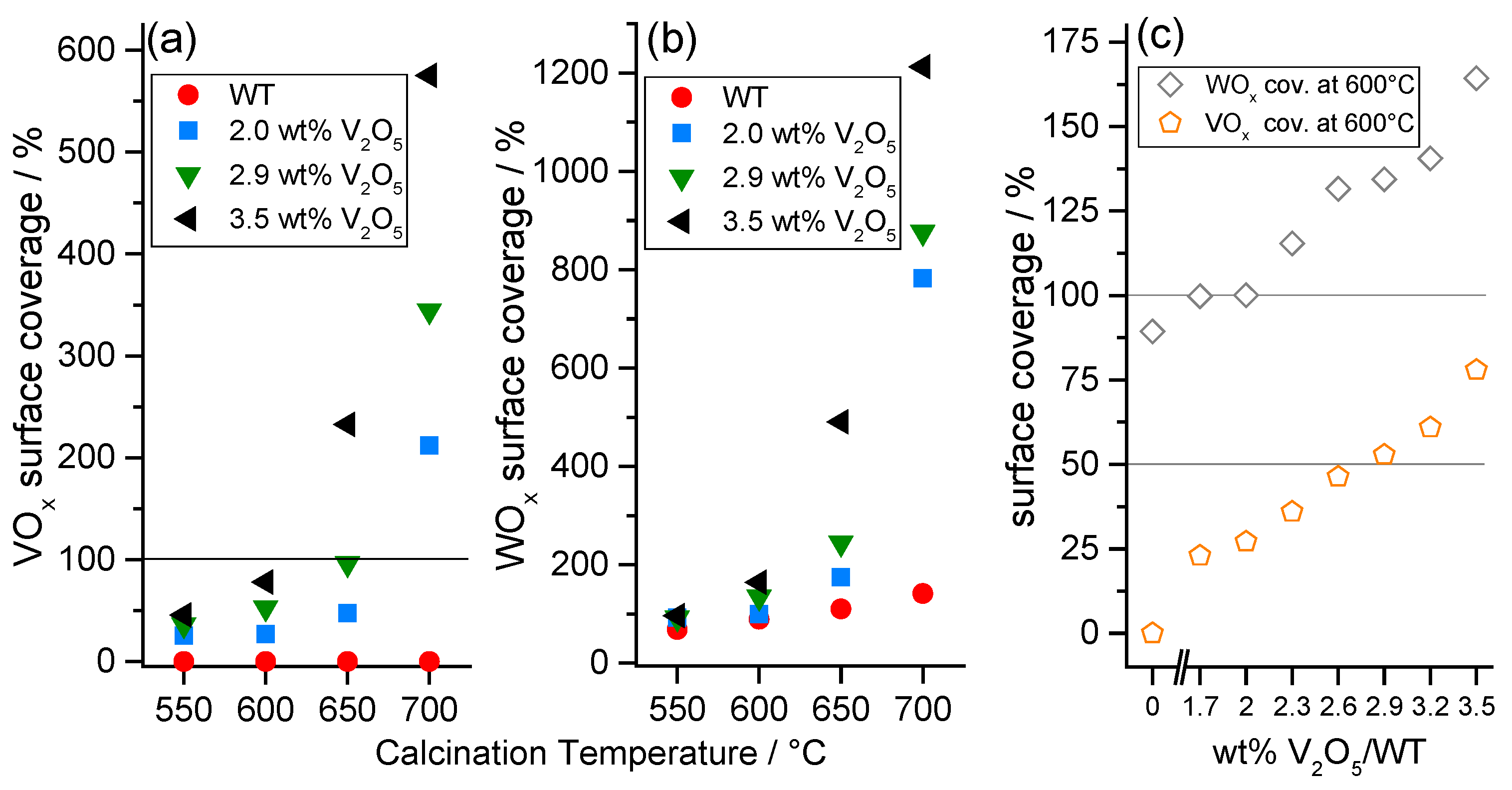
2.2.2. WOx Surface Coverage
3. Experimental Section
3.1. Materials
3.2. Catalytic Measurements
3.3. Characterization Methods
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Koebel, M.; Elsener, M.; Kleemann, M. Urea-SCR: A promising technique to reduce NOx emissions from automotive diesel engines. Catal. Today 2000, 59, 335–345. [Google Scholar] [CrossRef]
- Beale, A.M.; Lezcano-Gonzalez, I.; Maunula, T.; Palgrave, R.G. Development and characterization of thermally stable supported V–W–TiO2 catalysts for mobile NH3-SCR applications. Catal. Struct. React. 2015, 1, 25–34. [Google Scholar] [CrossRef]
- Djerad, S.; Tifouti, L.; Crocoll, M.; Weisweiler, W. Effect of vanadia and tungsten loadings on the physical and chemical characteristics of V2O5-WO3/TiO2 catalysts. J. Mol. Catal. A 2004, 208, 257–265. [Google Scholar] [CrossRef]
- Forzatti, P. Present status and perspectives in de-NOx SCR catalysis. Appl. Catal. A 2001, 222, 221–236. [Google Scholar] [CrossRef]
- Nova, I.; Tronconi, E. Urea-SCR Technology for DeNOx After Treatment of Diesel Exhausts; Springer: New York, NY, USA, 2014; pp. 6, 25 and 68. [Google Scholar]
- Fierro, J.L.G. Metal Oxides: Chemistry and Applications; CRC Press: Florida, FI, USA, 2005; pp. 8–20. [Google Scholar]
- Kompio, P.G.W.A.; Brückner, A.; Hipler, F.; Auer, G.; Löffler, E.; Grünert, W. A new view on the relations between tungsten and vanadium in V2O5WO3/TiO2 catalysts for the selective reduction of NO with NH3. J. Catal. 2012, 286, 237–247. [Google Scholar] [CrossRef]
- Wachs, I.E. Recent conceptual advances in the catalysis science of mixed metal oxide catalytic materials. Catal. Today 2005, 100, 79–94. [Google Scholar] [CrossRef]
- Amiridis, M.D.; Duevel, R.V.; Wachs, I.E. The effect of metal oxide additives on the activity of V2O5/TiO2 catalysts for the selective catalytic reduction of nitric oxide by ammonia. Appl. Catal. B 1999, 20, 111–122. [Google Scholar] [CrossRef]
- Anstrom, M.; Topsøe, N.-Y.; Dumesic, J.A. Density functional theory studies of mechanistic aspects of the SCR reaction on vanadium oxide catalysts. J. Catal. 2003, 213, 115–125. [Google Scholar] [CrossRef]
- Gruber, M.; Hermann, K. Elementary steps of the catalytic NOx reduction with NH3: Cluster studies on reaction paths and energetics at vanadium oxide substrate. J. Chem. Phys. 2013, 139, 244701–244708. [Google Scholar] [CrossRef] [PubMed]
- Ramis, G.; Yi, L.; Busca, G. Ammonia activation over catalysts for the selective catalytic reduction of NOx and the selective catalytic oxidation of NH3. An FT-IR study. Catal. Today 1996, 28, 373–380. [Google Scholar] [CrossRef]
- Topsøe, N.-Y. Mechanism of the Selective Catalytic Reduction of Nitric Oxide by Ammonia Elucidated by in Situ On-Line Fourier Transform Infrared Spectroscopy. Science 1994, 265, 1217–1219. [Google Scholar] [CrossRef] [PubMed]
- Topsøe, N.Y.; Dumesic, J.A.; Topsøe, H. Vanadia-Titania Catalysts for Selective Catalytic Reduction of Nitric-Oxide by Ammonia: I.I. Studies of Active Sites and Formulation of Catalytic Cycles. J. Catal. 1995, 151, 241–252. [Google Scholar] [CrossRef]
- Tronconi, E.; Nova, I.; Ciardelli, C.; Chatterjee, D.; Weibel, M. Redox features in the catalytic mechanism of the “standard” and “fast” NH3-SCR of NOx over a V-based catalyst investigated by dynamic methods. J. Catal. 2007, 245, 1–10. [Google Scholar] [CrossRef]
- Vittadini, A.; Casarin, M.; Selloni, A. First Principles Studies of Vanadia-Titania Monolayer Catalysts: Mechanisms of NO Selective Reduction. J. Phys. Chem. B 2005, 109, 1652–1655. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Jensen, A.D.; Johnsson, J.E.; Thøgersen, J.R. Deactivation of V2O5-WO3-TiO2 SCR catalyst at biomass fired power plants: Elucidation of mechanisms by lab- and pilot-scale experiments. Appl. Catal. B 2008, 83, 186–194. [Google Scholar] [CrossRef]
- Madia, G.; Elsener, M.; Koebel, M.; Raimondi, F.; Wokaun, A. Thermal stability of vanadia-tungsta-titania catalysts in the SCR process. Appl. Catal. B 2002, 39, 181–190. [Google Scholar] [CrossRef]
- Nicosia, D.; Elsener, M.; Kröcher, O.; Jansohn, P. Basic investigation of the chemical deactivation of V2O5/WO3-TiO2 SCR catalysts by potassium, calcium, and phosphate. Top. Catal. 2007, 42–43, 333–336. [Google Scholar] [CrossRef]
- Xie, X.; Lu, J.; Hums, E.; Huang, Q.; Lu, Z. Study on the Deactivation of V2O5-WO3/TiO2 Selective Catalytic Reduction Catalysts through Transient Kinetics. Energy Fuels 2015, 29, 3890–3896. [Google Scholar] [CrossRef]
- Kamata, H.; Takahashi, K.; Odenbrand, C.U.I. The role of K2O in the selective reduction of NO with NH3 over a V2O5(WO3)/TiO2 commercial selective catalytic reduction catalyst. J. Mol. Catal. A 1999, 139, 189–198. [Google Scholar] [CrossRef]
- Zhang, X.; Li, X.; Wu, J.; Yang, R.; Zhang, Z. Selective Catalytic Reduction of NO by Ammonia on V2O5/TiO2 Catalyst Prepared by Sol-Gel Method. Catal. Lett. 2009, 130, 235–238. [Google Scholar]
- Georgiadou, I.; Papadopoulou, C.; Matralis, H.K.; Voyiatzis, G.A.; Lycourghiotis, A.; Kordulis, C. Preparation, Characterization, and Catalytic Properties for the SCR of NO by NH3 of V2O5/TiO2 Catalysts Prepared by Equilibrium Deposition Filtration. J. Phys. Chem. B 1998, 102, 8459–8468. [Google Scholar] [CrossRef]
- Alemany, L.J.; Lietti, L.; Ferlazzo, N.; Forzatti, P.; Busca, G.; Giamello, E.; Bregani, F. Reactivity and Physicochemical Characterization of V2O5-WO3/TiO2 De-NOx Catalysts. J. Catal. 1995, 155, 117–130. [Google Scholar] [CrossRef]
- Marberger, A.; Elsener, M.; Ferri, D.; Sagar, A.; Schermanz, K.; Kröcher, O. Generation of NH3 Selective Catalytic Reduction Active Catalysts from Decomposition of Supported FeVO4. ACS Catal. 2015, 4180–4188. [Google Scholar] [CrossRef]
- Went, G.T.; Leu, L.-j.; Bell, A.T. Quantitative structural analysis of dispersed vanadia species in TiO2(anatase)-supported V2O5. J. Catal. 1992, 134, 479–491. [Google Scholar] [CrossRef]
- Burkardt, A.; Weisweiler, W.; van den Tillaart, J.A.A.; Schäfer-Sindlinger, A.; Lox, E.S. Influence of the V2O5 Loading on the Structure and Activity of V2O5/TiO2 SCR Catalysts for Vehicle Application. Top. Catal. 2001, 16–17, 369–375. [Google Scholar] [CrossRef]
- Briand, L.E.; Tkachenko, O.P.; Guraya, M.; Gao, X.; Wachs, I.E.; Grünert, W. Surface-Analytical Studies of Supported Vanadium Oxide Monolayer Catalysts. J. Phys. Chem. B 2004, 108, 4823–4830. [Google Scholar] [CrossRef]
- Lee, B.W.; Hyun, C.; Shin, D.W. Characterization and De-NOx activity of binary V2O5/TiO2 and WO3/TiO2, and ternary V2O5-WO3/TiO2 SCR catalysts. J. Ceram. Process. Res. 2007, 8, 203–207. [Google Scholar]
- Putluru, S.; Schill, L.; Gardini, D.; Mossin, S.; Wagner, J.; Jensen, A.; Fehrmann, R. Superior DeNOx activity of V2O5-WO3/TiO2 catalysts prepared by deposition–precipitation method. J. Mater. Sci. 2014, 49, 2705–2713. [Google Scholar] [CrossRef]
- Amiridis, M.D.; Solar, J.P. Selective Catalytic Reduction of Nitric Oxide by Ammonia over V2O5/TiO2, V2O5/TiO2/SiO2, and V2O5-WO3/TiO2 Catalysts: Effect of Vanadia Content on the Activation Energy. Ind. Eng. Chem. Res. 1996, 35, 978–981. [Google Scholar] [CrossRef]
- Kwon, D.W.; Park, K.H.; Hong, S.C. Influence of VOx surface density and vanadyl species on the selective catalytic reduction of NO by NH3 over VOx/TiO2 for superior catalytic activity. Appl. Catal. A 2015, 499, 1–12. [Google Scholar]
- Wachs, I.E. Raman and IR studies of surface metal oxide species on oxide supports: Supported metal oxide catalysts. Catal. Today 1996, 27, 437–455. [Google Scholar] [CrossRef]
- Kleemann, M.; Elsener, M.; Koebel, M.; Wokaun, A. Investigation of the ammonia adsorption on monolithic SCR catalysts by transient response analysis. Appl. Catal. B 2000, 27, 231–242. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, J.; Hua, L.; Shuai, S.; Wang, J. Ammonia Storage and Slip in a Urea Selective Catalytic Reduction Catalyst under Steady and Transient Conditions. Ind. Eng. Chem. Res. 2011, 50, 11863–11871. [Google Scholar] [CrossRef]
- Metkar, P.S.; Balakotaiah, V.; Harold, M.P. Experimental and kinetic modeling study of NO oxidation: Comparison of Fe and Cu-zeolite catalysts. Catal. Today 2012, 184, 115–128. [Google Scholar] [CrossRef]
- Koebel, M.; Madia, G.; Elsener, M. Selective catalytic reduction of NO and NO2 at low temperatures. Catal. Today 2002, 73, 239–247. [Google Scholar]
- Carreon, M.A.; Guliants, V.V. Ordered Meso- and Macroporous Binary and Mixed Metal Oxides. Eur. J. Inorg. Chem. 2005, 2005, 27–43. [Google Scholar] [CrossRef]
- Kröcher, O.; Devadas, M.; Elsener, M.; Wokaun, A.; Söger, N.; Pfeifer, M.; Demel, Y.; Mussmann, L. Investigation of the selective catalytic reduction of NO by NH3 on Fe-ZSM5 monolith catalysts. Appl. Catal. B 2006, 66, 208–216. [Google Scholar] [CrossRef]
- Koebel, M.; Elsener, M. Selective catalytic reduction of NO over commercial DeNOx-catalysts: experimental determination of kinetic and thermodynamic parameters. Chem. Eng. Sci. 1998, 53, 657–669. [Google Scholar] [CrossRef]
- Casapu, M.; Kröcher, O.; Elsener, M. Screening of doped MnOx-CeO2 catalysts for low-temperature NO-SCR. Appl. Catal. B 2009, 88, 413–419. [Google Scholar] [CrossRef]
- Ravel, B.; Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 2005, 12, 537–541. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marberger, A.; Elsener, M.; Ferri, D.; Kröcher, O. VOx Surface Coverage Optimization of V2O5/WO3-TiO2 SCR Catalysts by Variation of the V Loading and by Aging. Catalysts 2015, 5, 1704-1720. https://doi.org/10.3390/catal5041704
Marberger A, Elsener M, Ferri D, Kröcher O. VOx Surface Coverage Optimization of V2O5/WO3-TiO2 SCR Catalysts by Variation of the V Loading and by Aging. Catalysts. 2015; 5(4):1704-1720. https://doi.org/10.3390/catal5041704
Chicago/Turabian StyleMarberger, Adrian, Martin Elsener, Davide Ferri, and Oliver Kröcher. 2015. "VOx Surface Coverage Optimization of V2O5/WO3-TiO2 SCR Catalysts by Variation of the V Loading and by Aging" Catalysts 5, no. 4: 1704-1720. https://doi.org/10.3390/catal5041704