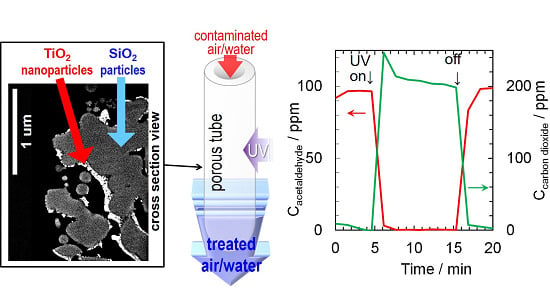
TiO2-Impregnated Porous Silica Tube and Its Application for Compact Air- and Water-Purification Units
Abstract
:1. Introduction
2. Results and Discussion
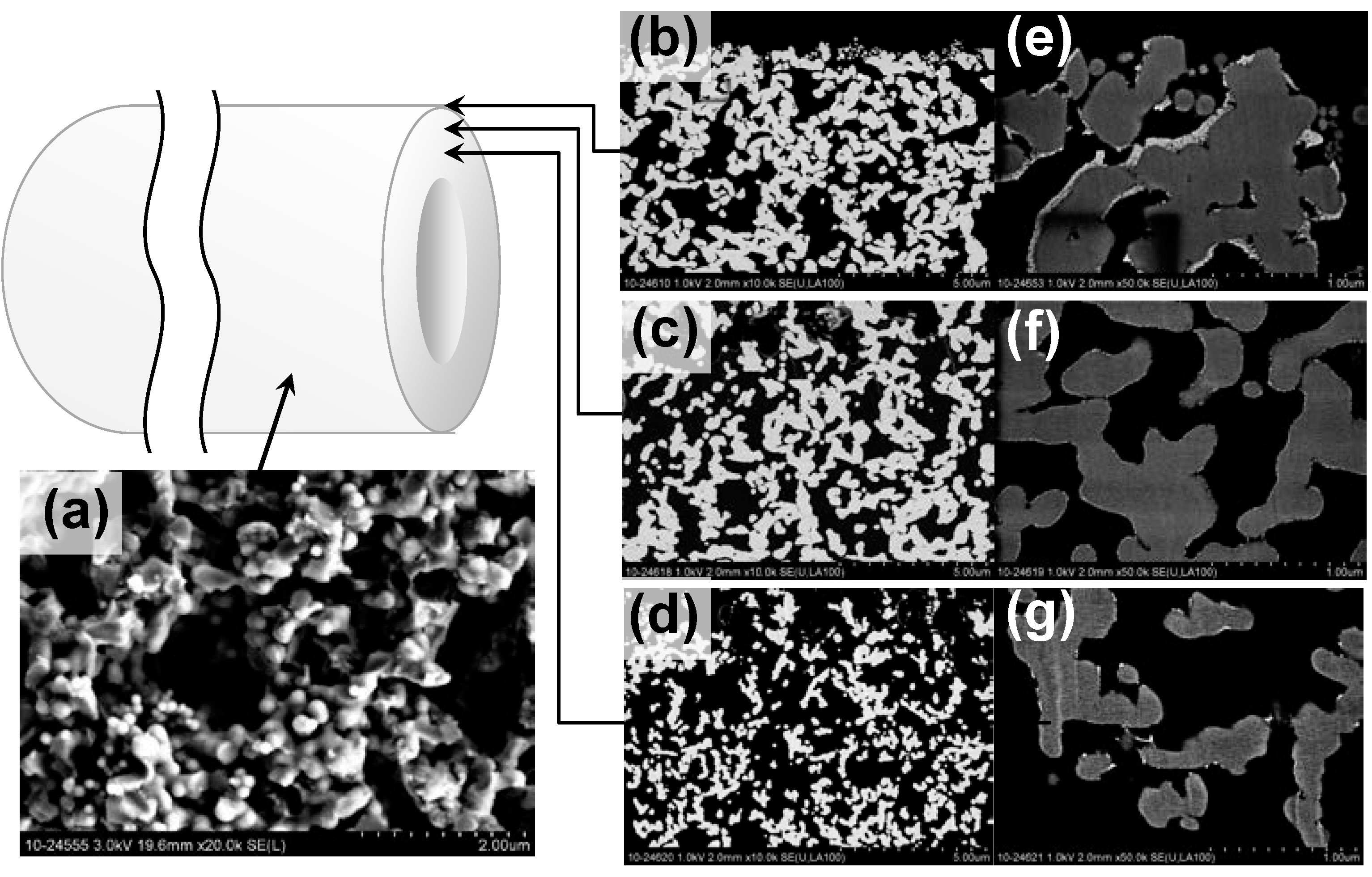
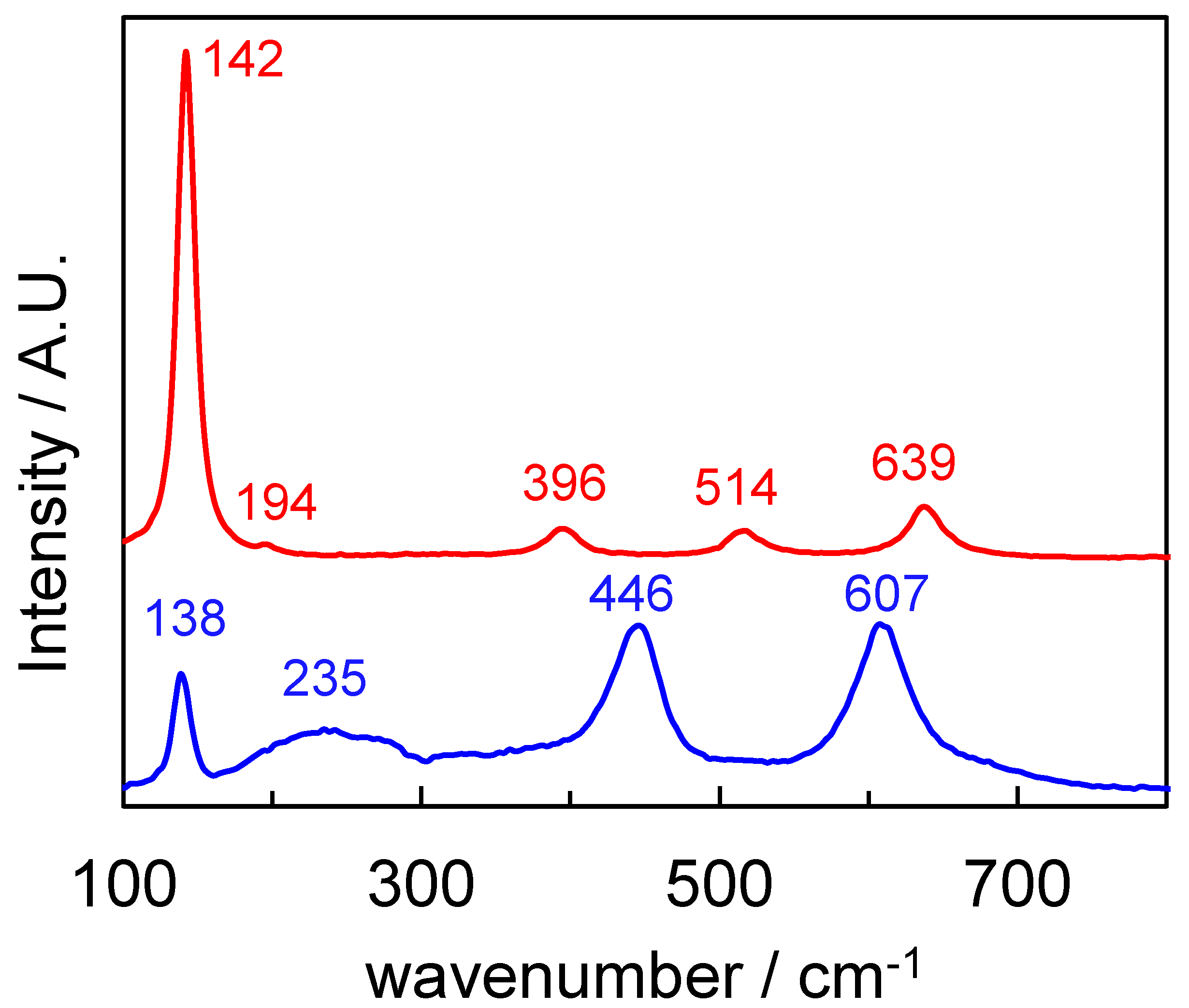
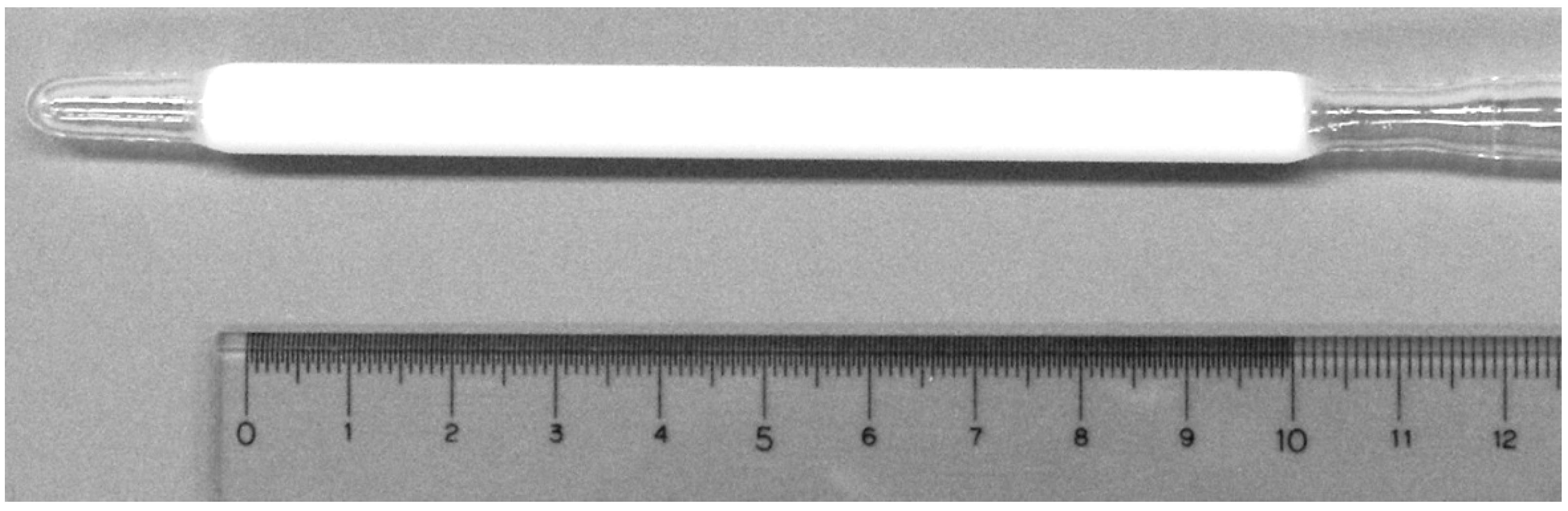
2.1. Characterization

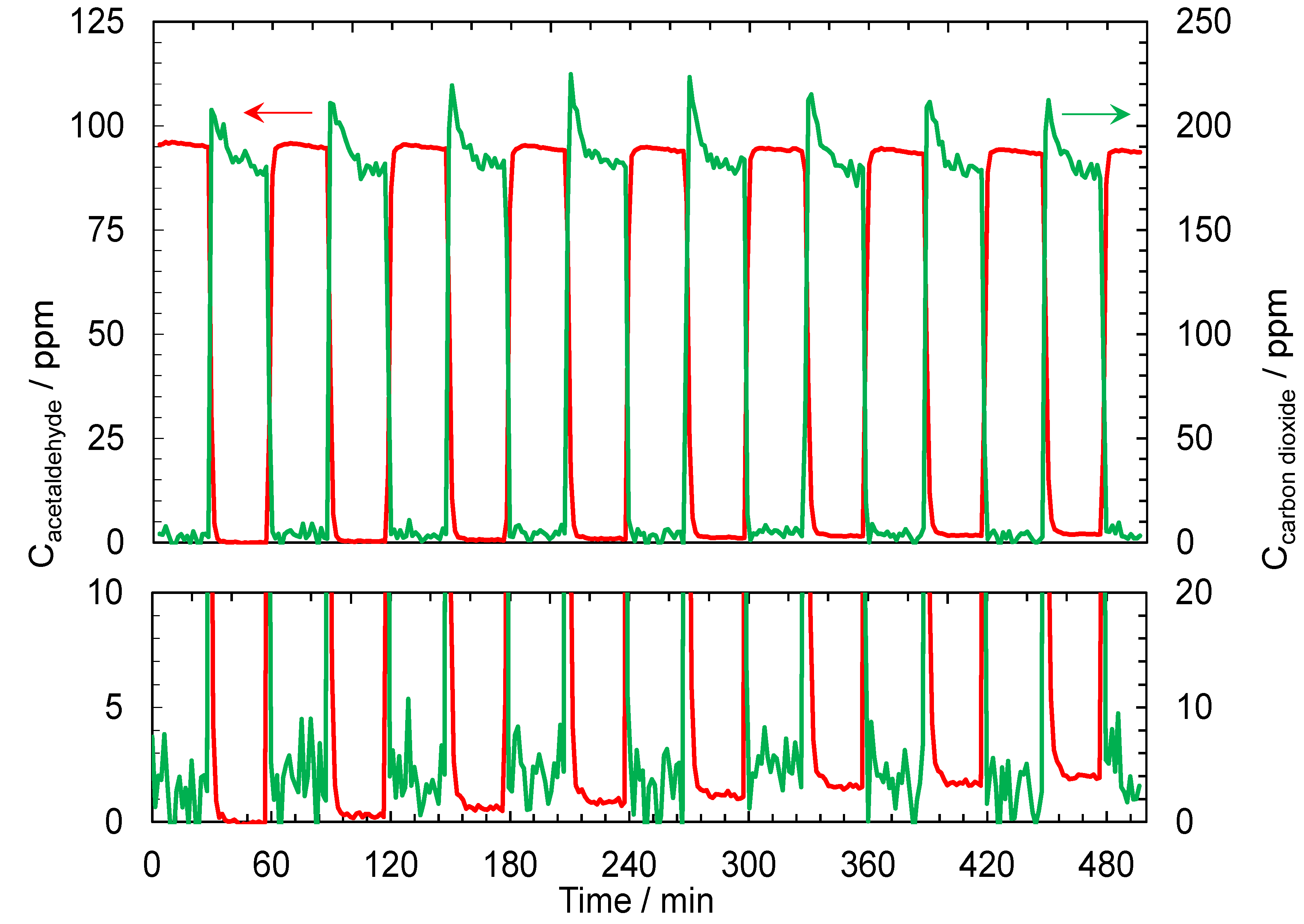
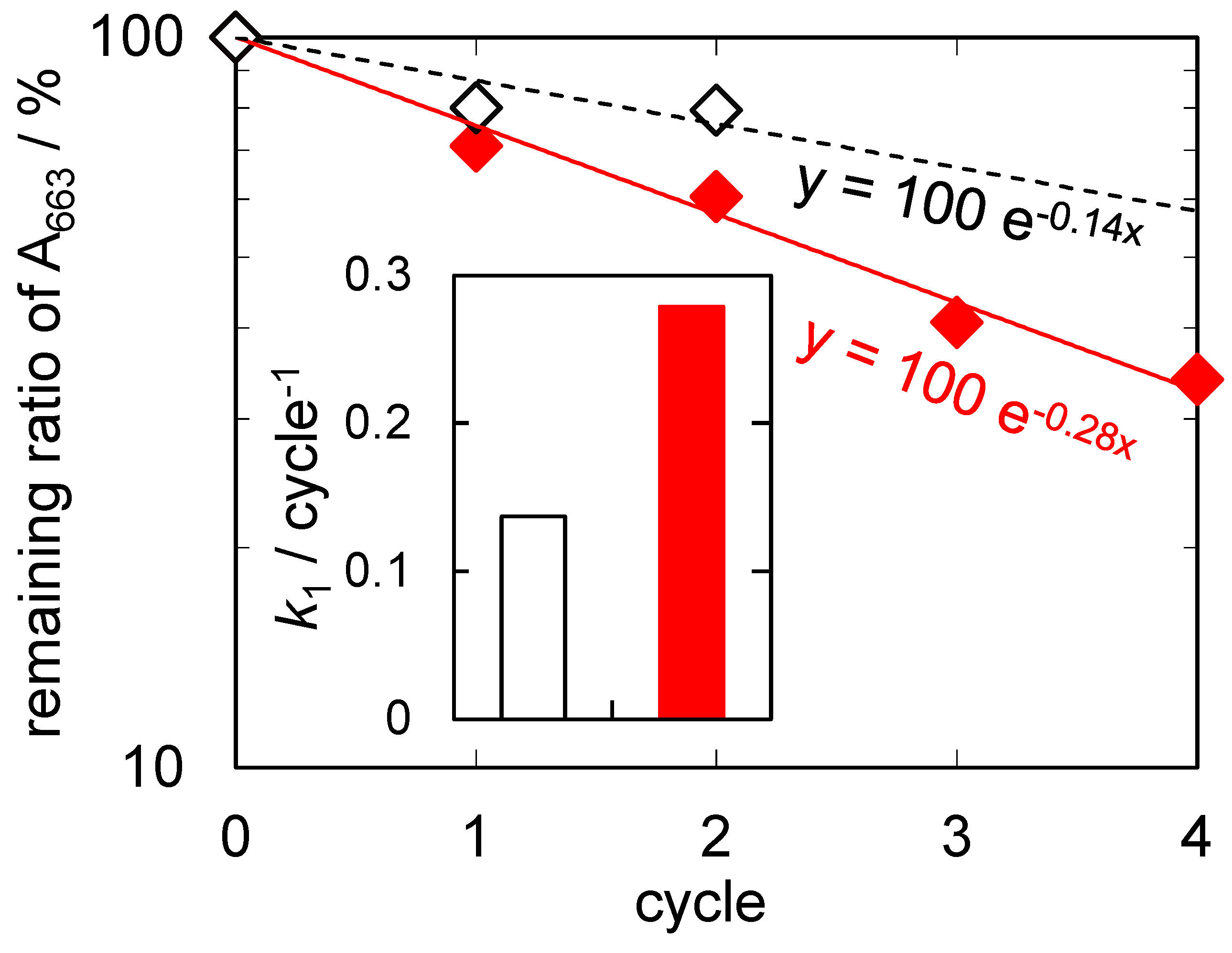
2.2. Results of Air- and Water-Purification Test

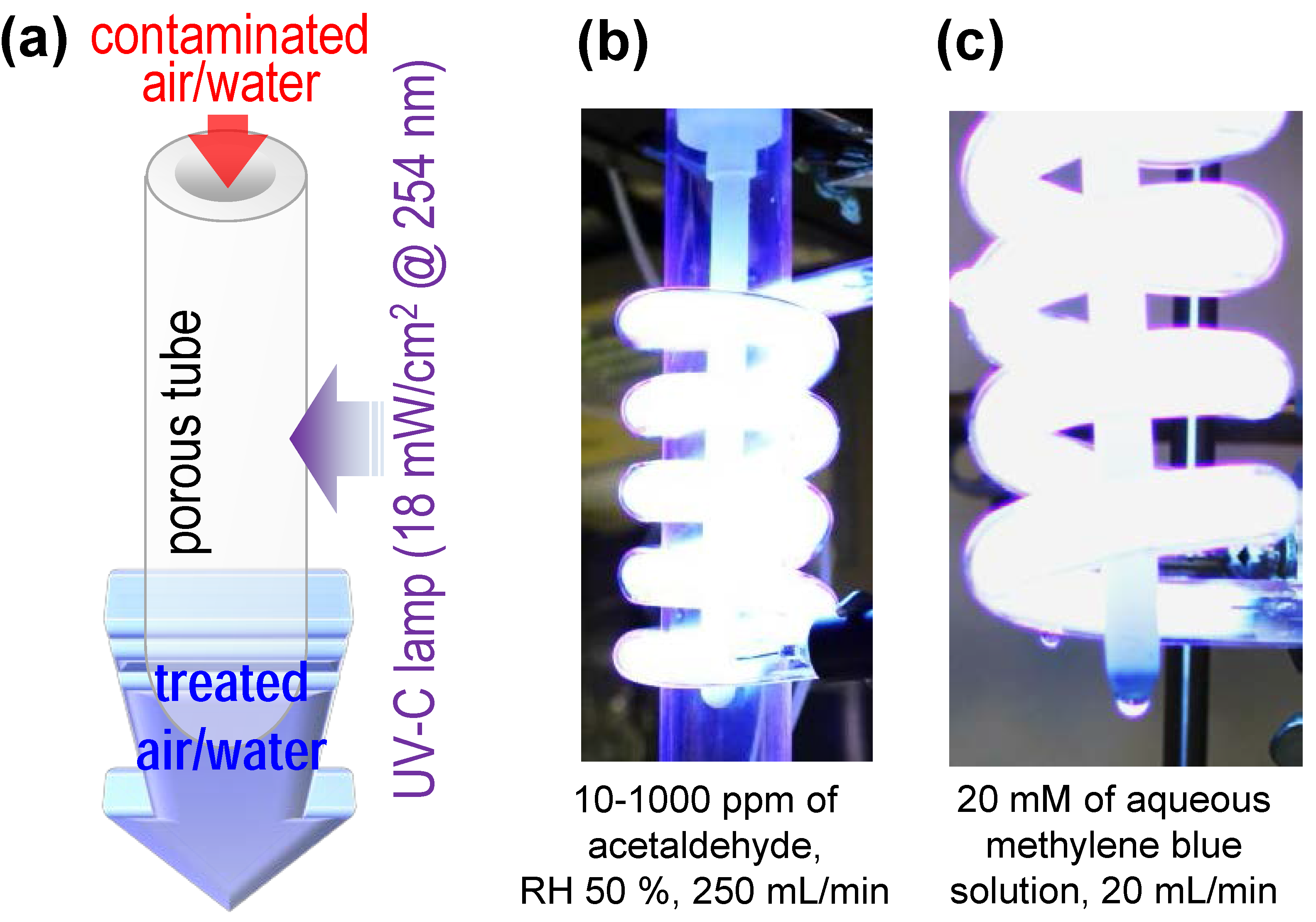
3. Experimental Section

4. Conclusions
Author Contributions
Conflicts of Interest
References
- Ochiai, T. Environmental and medical applications of TiO2 photocatalysts and boron-doped diamond electrodes. Electrochemistry 2014, 82, 720–725. [Google Scholar] [CrossRef]
- Nakata, K.; Kagawa, T.; Sakai, M.; Liu, S.; Ochiai, T.; Sakai, H.; Murakami, T.; Abe, M.; Fujishima, A. Preparation and photocatalytic activity of robust Titania monoliths for water remediation. ACS Appl. Mater. Interfaces 2013, 5, 500–504. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Nakata, K.; Sakai, M.; Saito, H.; Ochiai, T.; Murakami, T.; Takagi, K.; Fujishima, A. Hierarchical TiO2 spherical nanostructures with tunable pore size, pore volume, and specific surface area: Facile preparation and high-photocatalytic performance. Catal. Sci. Technol. 2012, 2, 1933–1939. [Google Scholar] [CrossRef]
- Reddy, K.R.; Nakata, K.; Ochiai, T.; Murakami, T.; Tryk, D.A.; Fujishima, A. Facile fabrication and photocatalytic application of Ag nanoparticles-TiO2 nanofiber composites. J. Nanosci. Nanotechnol. 2011, 11, 3692–3695. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, T.; Tago, S.; Tawarayama, H.; Hosoya, T.; Ishiguro, H.; Fujishima, A. Fabrication of a porous TiO2-coated silica glass tube and its application for a handy water purification unit. Int. J. Photoenergy 2014. [Google Scholar] [CrossRef]
- Oh, S.-M.; Ishigaki, T. Preparation of pure rutile and anatase TiO2 nanopowders using RF thermal plasma. Thin Solid Films 2004, 457, 186–191. [Google Scholar] [CrossRef]
- Balachandran, U.; Eror, N.G. Raman spectra of Titanium dioxide. J. Solid State Chem. 1982, 42, 276–282. [Google Scholar] [CrossRef]
- Liu, M.; Piao, L.; Zhao, L.; Ju, S.; Yan, Z.; He, T.; Zhou, C.; Wang, W. Anatase TiO2 single crystals with exposed {001} and {110} facets: Facile synthesis and enhanced photocatalysis. Chem. Commun. 2010, 46, 1664–1666. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Wang, S.; Bian, Z.; Xie, S.; Cai, C.; Wang, J.; Yang, H.; Li, H. Solvothermally controllable synthesis of anatase TiO2 nanocrystals with dominant {001} facets and enhanced photocatalytic activity. CrystEngComm 2010, 12, 2219–2224. [Google Scholar] [CrossRef]
- Ochiai, T.; Hoshi, T.; Slimen, H.; Nakata, K.; Murakami, T.; Tatejima, H.; Koide, Y.; Houas, A.; Horie, T.; Morito, Y.; et al. Fabrication of TiO2 nanoparticles impregnated Titanium mesh filter and its application for environmental purification unit. Catal. Sci. Technol. 2011, 1, 1324–1327. [Google Scholar] [CrossRef]
- Petit, V.; le Rouge, A.; Béclin, F.; Hamzaoui, H.E.; Bigot, L. Experimental study of SiO2 soot deposition using the outside vapor deposition method. Aerosol Sci. Technol. 2010, 44, 388–394. [Google Scholar] [CrossRef]
- Ochiai, T.; Fukuda, T.; Nakata, K.; Murakami, T.; Tryk, D.; Koide, Y.; Fujishima, A. Photocatalytic inactivation and removal of algae with TiO2-coated materials. J. Appl. Electrochem. 2010, 40, 1737–1742. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ochiai, T.; Tago, S.; Hayashi, M.; Tawarayama, H.; Hosoya, T.; Fujishima, A. TiO2-Impregnated Porous Silica Tube and Its Application for Compact Air- and Water-Purification Units. Catalysts 2015, 5, 1498-1506. https://doi.org/10.3390/catal5031498
Ochiai T, Tago S, Hayashi M, Tawarayama H, Hosoya T, Fujishima A. TiO2-Impregnated Porous Silica Tube and Its Application for Compact Air- and Water-Purification Units. Catalysts. 2015; 5(3):1498-1506. https://doi.org/10.3390/catal5031498
Chicago/Turabian StyleOchiai, Tsuyoshi, Shoko Tago, Mio Hayashi, Hiromasa Tawarayama, Toshifumi Hosoya, and Akira Fujishima. 2015. "TiO2-Impregnated Porous Silica Tube and Its Application for Compact Air- and Water-Purification Units" Catalysts 5, no. 3: 1498-1506. https://doi.org/10.3390/catal5031498