Advances in Ceramic Supports for Polymer Electrolyte Fuel Cells
Abstract
:1. Introduction
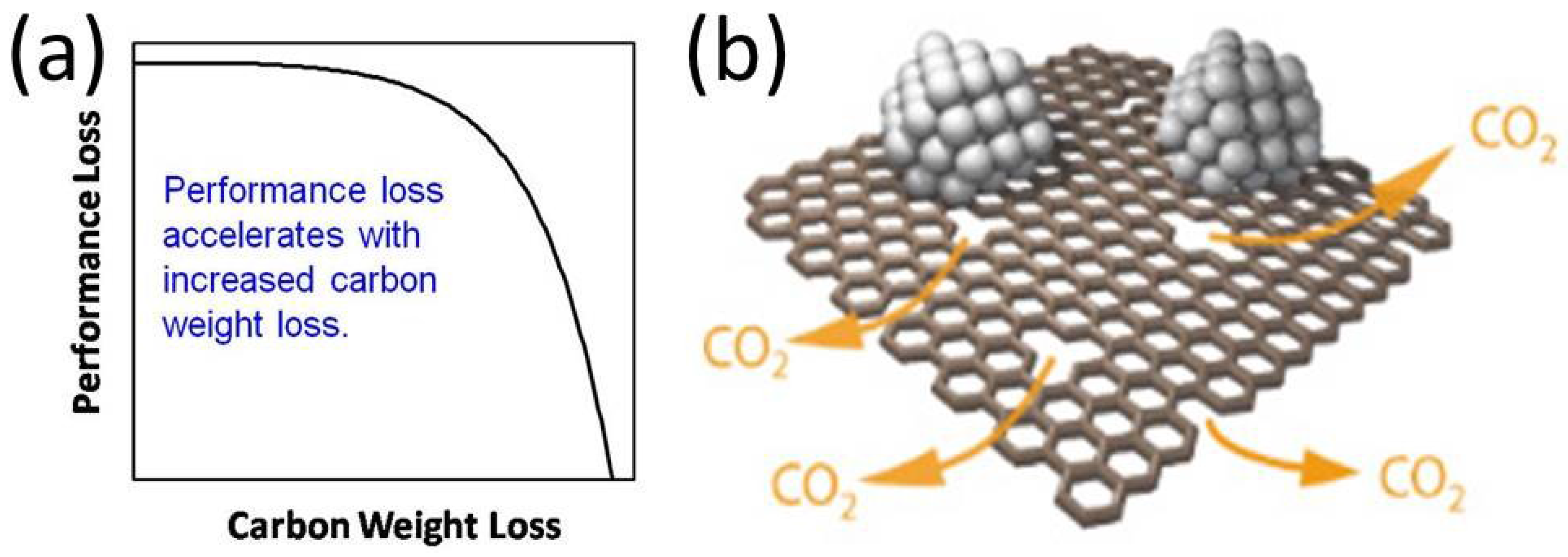
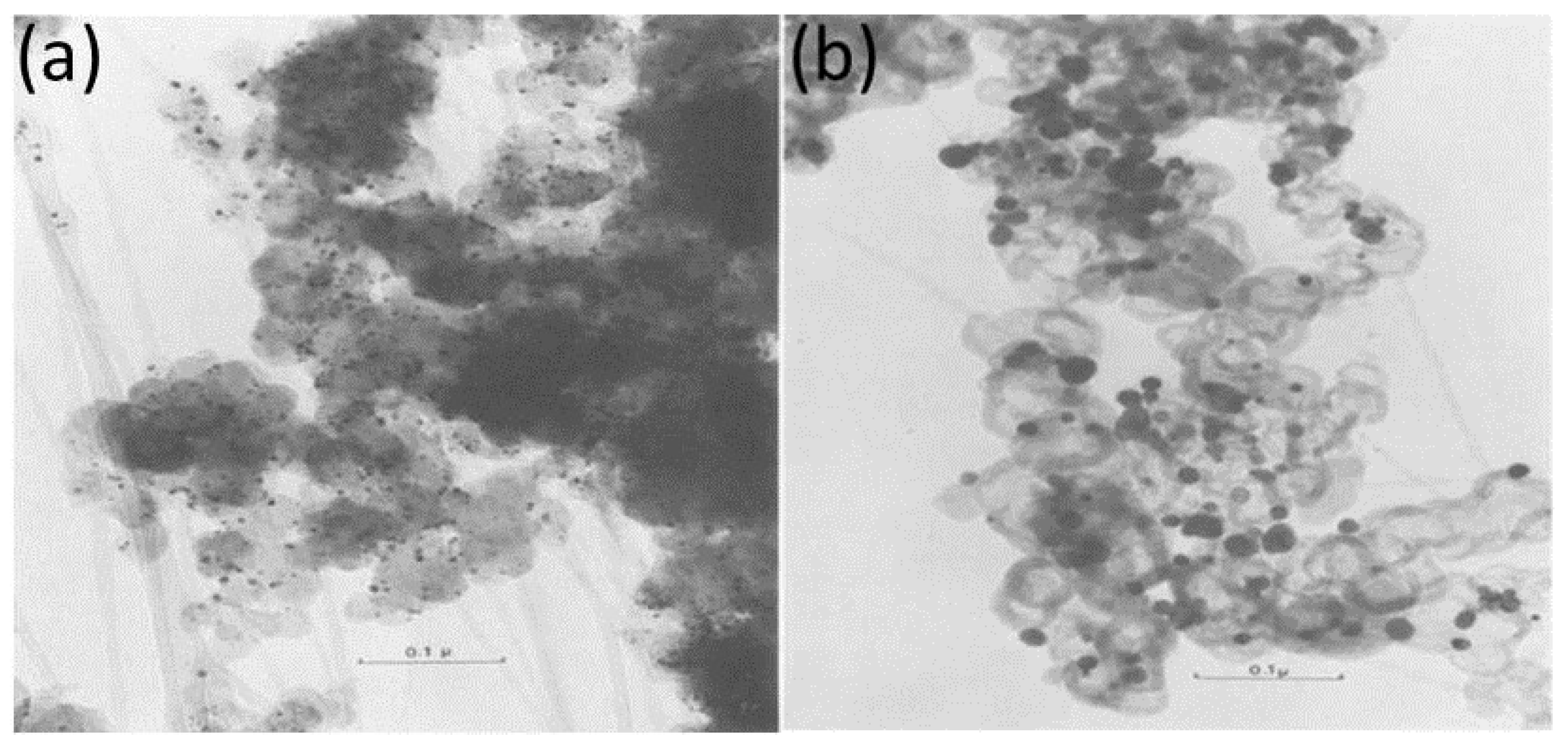
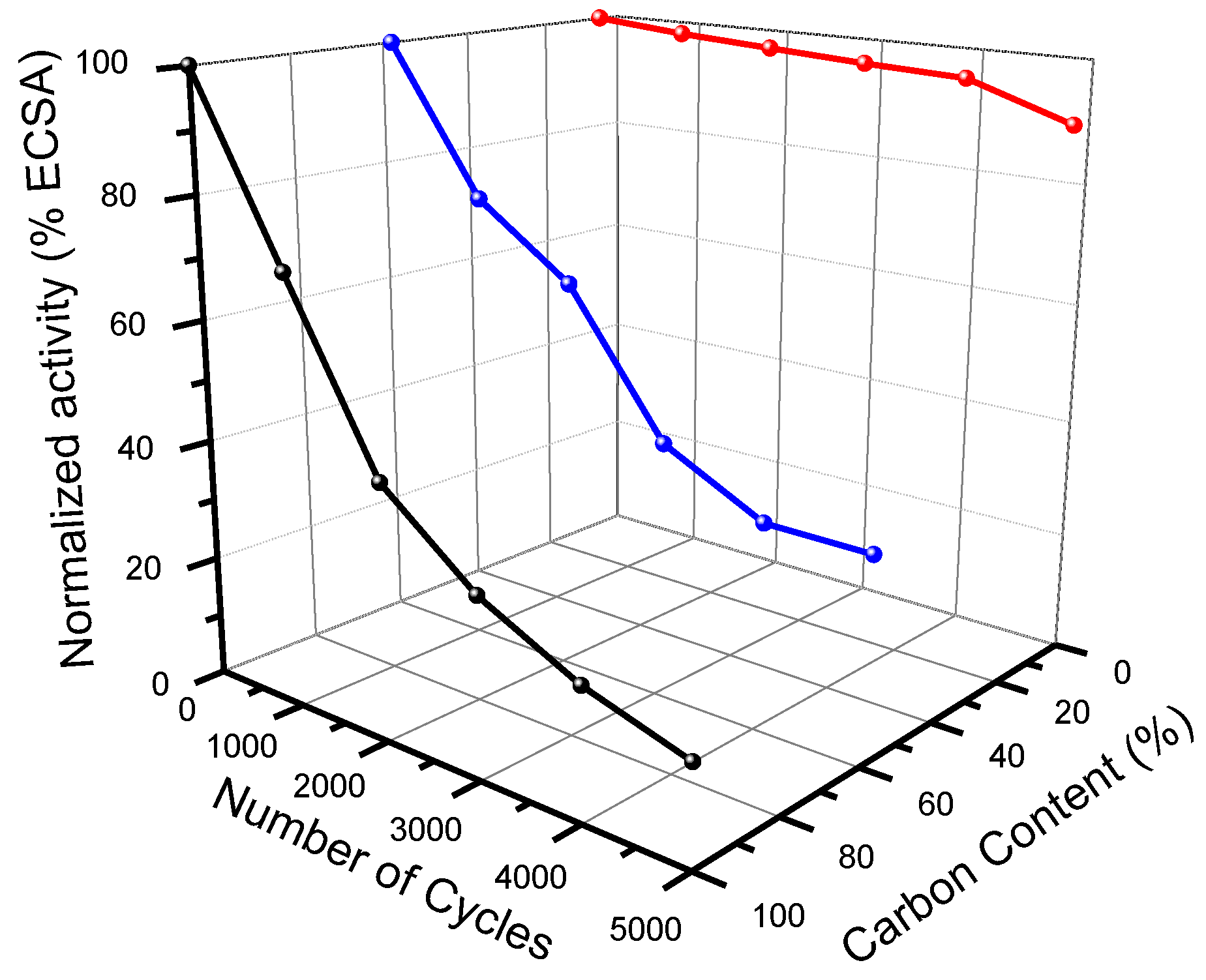
2. Carbides
3. Oxides
3.1. Titanium Oxide
3.2. Tungsten Oxide
3.3. Tin Oxide
4. Nitrides
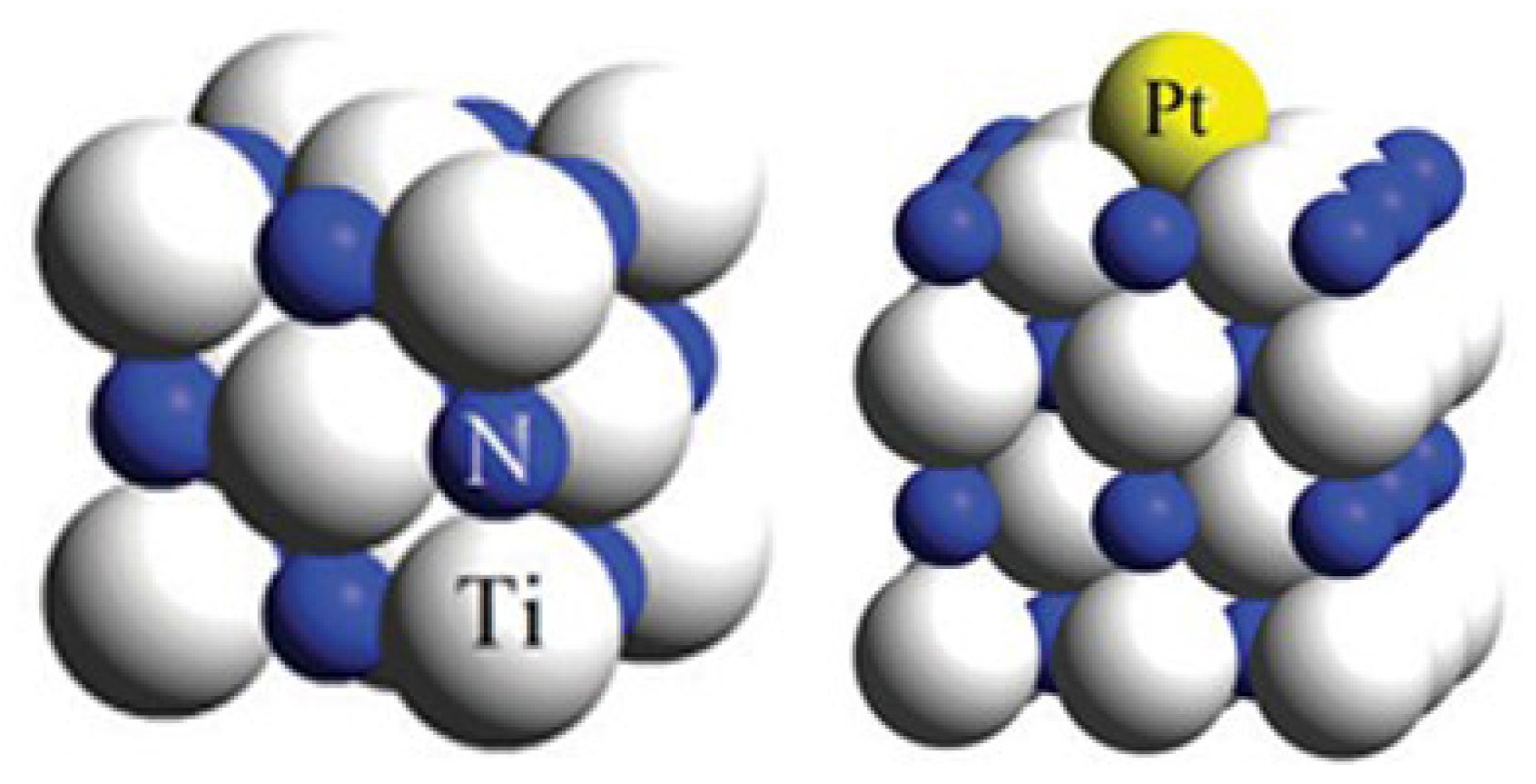
5. Composites/Hybrides
5.1. Carbon-Based Ceramic Composites
5.2. Other Ceramic Composites
6. Titanium Diboride
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Borup, R.; Meyers, J.; Pivovar, B.; Kim, Y.S.; Mukundan, R.; Garland, N.; Myers, D.; Wilson, M.; Garzon, F.; Wood, D. Scientific aspects of polymer electrolyte fuel cell durability and degradation. Chem. Rev. 2007, 107, 3904–3951. [Google Scholar] [CrossRef] [PubMed]
- Meier, J.C.; Galeano, C.; Katsounaros, I.; Witte, J.; Bongard, H.J.; Topalov, A.A.; Baldizzone, C.; Mezzavilla, S.; Schueth, F.; Mayrhofer, K.J.J. Design criteria for stable pt/c fuel cell catalysts. Beilstein J. Nanotechnol. 2014, 5, 44–67. [Google Scholar] [CrossRef] [PubMed]
- Pourbaix, M. Atlas of Electrochemical Equilibria in Aqueous Solutions; National Association of Corrosion: Oxford, UK, 1974. [Google Scholar]
- Barth, T.; Lunde, G. The lattice constants of metallic platinum, silver and gold. Z. Phys. Chem. 1926, 121, 78–102. [Google Scholar]
- Clougherty, E.V.; Lothrop, K.H.; Kafalas, J.A. New phase formed by high-pressure treatment. Nature 1961. [Google Scholar] [CrossRef]
- Rudy, E.; Windisch, S.; Stosick, A.J.; Hoffman, J.R. The constitution of binary molybdenum-carbon alloys. AIME 1967, 239, 1247–1267. [Google Scholar]
- Gruver, G.A. Corrosion of Carbon-black in Phosphoric-acid. J. Electrochem. Soc. 1978, 125, 1719–1720. [Google Scholar] [CrossRef]
- Meyers, J.P.; Darling, R.M. Model of carbon corrosion in PEM fuel cells. J. Electrochem. Soc. 2006, 153, A1432–A1442. [Google Scholar] [CrossRef]
- Li, W.; Wang, X.; Chen, Z.; Waje, M.; Yan, Y. Pt-Ru Supported on Double-Walled Carbon Nanotubes as High-Performance Anode Catalysts for Direct Methanol Fuel Cells. J. Phys. Chem. B 2006, 110, 15353–15358. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Xu, B.-Q. Carbon nanotube supported Pt electrodes for methanol oxidation: A comparison between multi- and single-walled carbon nanotubes. J. Power Sources 2007, 174, 148–158. [Google Scholar] [CrossRef]
- Zhou, X.; Qiao, J.; Yang, L.; Zhang, J. A Review of Graphene-Based Nanostructural Materials for Both Catalyst Supports and Metal-Free Catalysts in PEM Fuel Cell Oxygen Reduction Reactions. Adv. Energy Mater. 2014, 4, 1301523:1–1301523:25. [Google Scholar] [CrossRef]
- Wang, X.; Li, W.; Chen, Z.; Waje, M.; Yan, Y. Durability investigation of carbon nanotube as catalyst support for proton exchange membrane fuel cell. J. Power Sources 2006, 158, 154–159. [Google Scholar] [CrossRef]
- Armstrong, K.J.; Elbaz, L.; Bauer, E.; Burrell, A.K.; McCleskey, T.M.; Brosha, E.L. Nanoscale titania ceramic composite supports for PEM fuel cells. J. Mater. Res. 2012, 27, 2046–2054. [Google Scholar] [CrossRef]
- Shanmugam, S.; Gedanken, A. Synthesis and electrochemical oxygen reduction of platinum nanoparticles supported on mesoporous TiO2. J. Phys. Chem. C 2009, 113, 18707–18712. [Google Scholar] [CrossRef]
- Blackmore, K.J.; Elbaz, L.; Bauer, E.; Brosha, E.L.; More, K.; McCleskey, T.M.; Burrell, A.K. High Surface Area Molybdenum Nitride Support for Fuel Cell Electrodes. J. Electrochem. Soc. 2011, 158, B1255–B1259. [Google Scholar] [CrossRef]
- Cui, X.; Guo, L.; Cui, F.; He, Q.; Shi, J. Electrocatalytic activity and co tolerance properties of mesostructured pt/wo3 composite as an anode catalyst for pemfcs. J. Phys. Chem. C 2009, 113, 4134–4138. [Google Scholar] [CrossRef]
- Levy, R.B.; Boudart, M. Platinum-like behavior of tungsten carbide in surface catalysis. Science 1973, 181, 547–549. [Google Scholar] [CrossRef] [PubMed]
- York, A.P.E.; Claridge, J.B.; Brungs, A.J.; Brungs, A.J.; Tsang, S.C.; Green, M.L.H. Molybdenum and tungsten carbides as catalysts for the conversion of methane to synthesis gas using stoichiometric feedstocks. Chem. Commun. 1997, 39–40. [Google Scholar] [CrossRef]
- Burns, S.; Gallagher, J.G.; Hargreaves, J.S.J.; Harris, P.J.F. Direct observation of carbon nanotube formation in Pd/H-ZSM-5 and MoO3/H-ZSM-5 based methane activation catalysts. Catal. Lett. 2007, 116, 122–127. [Google Scholar] [CrossRef]
- Ribeiro, F.H.; Boudart, M.; Dalla Betta, R.A.; Iglesia, E. Catalytic reactions of n-Alkanes on β-W2C and WC: The effect of surface oxygen on reaction pathways. J. Catal. 1991, 130, 498–513. [Google Scholar] [CrossRef]
- Ledoux, M.J.; Huu, C.P.; Guille, J.; Dunlop, H. Compared activities of platinum and high specific surface area Mo2C and WC catalysts for reforming reactions: I. Catalyst activation and stabilization: Reaction of n-hexane. J. Catal. 1992, 134, 383–398. [Google Scholar] [CrossRef]
- Esposito, D.V.; Chen, J.G. Monolayer platinum supported on tungsten carbides as low-cost electrocatalysts: opportunities and limitations. Energy Environ. Sci. 2011, 4, 3900–3912. [Google Scholar] [CrossRef]
- Hunt, S.T.; Nimmanwudipong, T.; Román-Leshkov, Y. Engineering Non-sintered, Metal-Terminated Tungsten Carbide Nanoparticles for Catalysis. Angew. Chem. Int. Ed. 2014, 53, 5131–5136. [Google Scholar]
- Curry, K.E.; Thompson, L.T. Carbon-hydrogen bond activation over tungsten carbide catalysts. Catal. Today 1994, 21, 171–184. [Google Scholar] [CrossRef]
- Pantea, D.; Darmstadt, H.; Kaliaguine, S.; Sümmchen, L.; Roy, C. Electrical conductivity of thermal carbon blacks: Influence of surface chemistry. Carbon 2001, 39, 1147–1158. [Google Scholar] [CrossRef]
- Hassan, A.; Paganin, V.A.; Ticianelli, E.A. Pt modified tungsten carbide as anode electrocatalyst for hydrogen oxidation in proton exchange membrane fuel cell: CO tolerance and stability. Appl. Catal. B 2015, 165, 611–619. [Google Scholar] [CrossRef]
- Poh, C.K.; Lim, S.H.; Lin, J.; Feng, Y.P. Tungsten Carbide Supports for Single-Atom Platinum-Based Fuel-Cell Catalysts: First-Principles Study on the Metal–Support Interactions and O2 Dissociation on WxC Low-Index Surfaces. J. Phys. Chem. C 2014, 118, 13525–13538. [Google Scholar] [CrossRef]
- Nikolic, V.M.; Perovic, I.M.; Gavrilov, N.M.; Pašti, I.A.; Saponjic, A.B.; Vulic, P.J.; Karic, S.D.; Babic, B.M.; Marceta Kaninski, M.P. On the tungsten carbide synthesis for PEM fuel cell application—Problems, challenges and advantages. Int. J. Hydrogen Energy 2014, 39, 11175–11185. [Google Scholar] [CrossRef]
- Tang, H.; Qi, Z.; Ramani, M.; Elter, J.F. PEM fuel cell cathode carbon corrosion due to the formation of air/fuel boundary at the anode. J. Power Sources 2006, 158, 1306–1312. [Google Scholar] [CrossRef]
- Elbaz, L.; Kreller, C.R.; Henson, N.J.; Brosha, E.L. Electrocatalysis of oxygen reduction with platinum supported on molybdenum carbide-carbon composite. J. Electroanal. Chem. 2014, 720–721, 34–40. [Google Scholar] [CrossRef]
- Jia, Q.X.; McCleskey, T.M.; Burrell, A.K.; Lin, Y.; Collis, G.E.; Wang, H.; Li, A.D.Q.; Foltyn, S.R. Polymer-assisted deposition of metal-oxide films. Nat. Mater. 2004, 3, 529–532. [Google Scholar] [CrossRef] [PubMed]
- Elbaz, L.; Phillips, J.; Artyushkova, K.; More, K.; Brosha, E.L. Evidence of High Electrocatalytic Activity of Molybdenum Carbide Supported Platinum Nanorafts. J. Electrochem. Soc. 2015, 162, H681–H685. [Google Scholar] [CrossRef]
- Ma, L.; Sui, S.; Zhai, Y. Preparation and characterization of Ir/TiC catalyst for oxygen evolution. J. Power Sources 2008, 177, 470–477. [Google Scholar] [CrossRef]
- Ou, Y.; Cui, X.; Zhang, X.; Jiang, Z. Titanium carbide nanoparticles supported Pt catalysts for methanol electrooxidation in acidic media. J. Power Sources 2010, 195, 1365–1369. [Google Scholar] [CrossRef]
- Chiwata, M.; Kakinuma, K.; Wakisaka, M.; Uchida, M.; Deki, S.; Watanabe, M.; Uchida, H. Oxygen Reduction Reaction Activity and Durability of Pt Catalysts Supported on Titanium Carbide. Catalysts 2015, 5, 966–980. [Google Scholar] [CrossRef]
- Ignaszak, A.; Song, C.; Zhu, W.; Zhang, J.; Bauer, A.; Baker, R.; Neburchilov, V.; Ye, S.; Campbell, S. Titanium carbide and its core-shelled derivative TiC@TiO2 as catalyst supports for proton exchange membrane fuel cells. Electrochim. Acta 2012, 69, 397–405. [Google Scholar] [CrossRef]
- Toyoda, M.; Yano, T.; Tryba, B.; Mozia, S.; Tsumura, T.; Inagaki, M. Preparation of carbon-coated Magneli phases TinO2n−1 and their photocatalytic activity under visible light. Appl. Catal. B 2009, 88, 160–164. [Google Scholar] [CrossRef]
- Toyoda, M.; Yano, T.; Mozia, S.; Tsumura, T.; Itoh, E.; Amao, Y.; Inagaki, M. Development of visible-light sensitive reduced phases of titania, TinO2n−1, through carbon coating. Tanso 2005, 220, 265–269. [Google Scholar] [CrossRef]
- Ni, M.; Leung, M.K.; Leung, D.Y.; Sumathy, K. A review and recent developments in photocatalytic water-splitting using TiO2 for hydrogen production. Renewable Sustainable Energy Rev. 2007, 11, 401–425. [Google Scholar] [CrossRef]
- Zhu, Y.; Shi, J.; Zhang, Z.; Zhang, C.; Zhang, X. Development of a gas sensor utilizing chemiluminescence on nanosized titanium dioxide. Anal. Chem. 2002, 74, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Bartholomew, R.F.; Frankl, D. Electrical properties of some titanium oxides. Phys. Rev. 1969, 187. [Google Scholar] [CrossRef]
- Wu, Q.; Ruan, J.; Zhou, Z.; Sang, S. Magneli phase titanium sub-oxide conductive ceramic TinO2n−1 as support for electrocatalyst toward oxygen reduction reaction with high activity and stability. J. Cent. South Univ. 2015, 22, 1212–1219. [Google Scholar] [CrossRef]
- Ioroi, T.; Akita, T.; Yamazaki, S.-i.; Siroma, Z.; Fujiwara, N.; Yasuda, K. Corrosion-Resistant PEMFC Cathode Catalysts Based on a Magnéli-Phase Titanium Oxide Support Synthesized by Pulsed UV Laser Irradiation. J. Electrochem. Soc. 2011, 158, C329–C334. [Google Scholar] [CrossRef]
- Ioroi, T.; Senoh, H.; Siroma, Z.; Yamazaki, S.-i.; Fujiwara, N.; Yasuda, K. Stability of Corrosion-Resistant Magnéli-Phase Ti4O7-Supported PEMFC Catalysts. ECS Trans. 2007, 11, 1041–1048. [Google Scholar]
- Ioroi, T.; Siroma, Z.; Fujiwara, N.; Yamazaki, S.-i.; Yasuda, K. Sub-stoichiometric titanium oxide-supported platinum electrocatalyst for polymer electrolyte fuel cells. Electrochem. Commun. 2005, 7, 183–188. [Google Scholar] [CrossRef]
- Vračar, L.M.; Krstajić, N.V.; Radmilović, V.R.; Jakšić, M.M. Electrocatalysis by nanoparticles- oxygen reduction on Ebonex/Pt electrode. J. Electroanal. Chem. 2006, 587, 99–107. [Google Scholar] [CrossRef]
- Geng, P.; Su, J.Y.; Miles, C.; Comninellis, C.; Chen, G.H. Highly-Ordered Magneli Ti4O7 Nanotube Arrays as Effective Anodic Material for Electro-oxidation. Electrochim. Acta 2015, 153, 316–324. [Google Scholar] [CrossRef]
- Du, Q.; Wu, J.; Yang, H. Pt@Nb-TiO2 catalyst membranes fabricated by electrospinning and atomic layer deposition. ACS Catal. 2013, 4, 144–151. [Google Scholar] [CrossRef]
- Chevallier, L.; Bauer, A.; Cavaliere, S.; Hui, R.; Rozière, J.; Jones, D.J. Mesoporous nanostructured Nb-doped titanium dioxide microsphere catalyst supports for PEM fuel cell electrodes. ACS Appl. Mater. Interfaces 2012, 4, 1752–1759. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.; Chevallier, L.; Hui, R.; Cavaliere, S.; Zhang, J.; Jones, D.; Rozière, J. Synthesis and characterization of Nb-TiO2 mesoporous microsphere and nanofiber supported Pt catalysts for high temperature PEM fuel cells. Electrochim. Acta 2012, 77, 1–7. [Google Scholar] [CrossRef]
- Elezović, N.; Babić, B.; Gajić-Krstajić, L.; Radmilović, V.; Krstajić, N.; Vračar, L. Synthesis, characterization and electrocatalytical behavior of Nb-TiO2/Pt nanocatalyst for oxygen reduction reaction. J. Power Sources 2010, 195, 3961–3968. [Google Scholar] [CrossRef]
- Siracusano, S.; Stassi, A.; Modica, E.; Baglio, V.; Aricò, A.S. Preparation and characterisation of Ti oxide based catalyst supports for low temperature fuel cells. Int. J. Hydrogen Energy 2013, 38, 11600–11608. [Google Scholar] [CrossRef]
- Huang, S.-Y.; Ganesan, P.; Popov, B.N. Titania supported platinum catalyst with high electrocatalytic activity and stability for polymer electrolyte membrane fuel cell. Appl. Catal. B 2011, 102, 71–77. [Google Scholar] [CrossRef]
- Ioroi, T.; Akita, T.; Asahi, M.; Yamazaki, S.; Siroma, Z.; Fujiwara, N.; Yasuda, K. Platinum-titanium alloy catalysts on a Magnéli-phase titanium oxide support for improved durability in Polymer Electrolyte Fuel Cells. J. Power Sources 2013, 223, 183–189. [Google Scholar] [CrossRef]
- Huang, D.; Zhang, B.; Bai, J.; Zhang, Y.; Wittstock, G.; Wang, M.; Shen, Y. Pt Catalyst Supported within TiO2 Mesoporous Films for Oxygen Reduction Reaction. Electrochim. Acta 2014, 130, 97–103. [Google Scholar] [CrossRef]
- Micoud, F.; Maillard, F.; Gourgaud, A.; Chatenet, M. Unique CO-tolerance of Pt-WOx materials. Electrochem. Commun. 2009, 11, 651–654. [Google Scholar] [CrossRef]
- Rajeswari, J.; Viswanathan, B.; Varadarajan, T.K. Tungsten trioxide nanorods as supports for platinum in methanol oxidation. Mater. Chem. Phys. 2007, 106, 168–174. [Google Scholar] [CrossRef]
- Cui, X.; Shi, J.; Chen, H.; Zhang, L.; Guo, L.; Gao, J.; Li, J. Platinum/mesoporous WO3 as a carbon-free electrocatalyst with enhanced electrochemical activity for methanol oxidation. J. Phys. Chem. B 2008, 112, 12024–12031. [Google Scholar] [CrossRef] [PubMed]
- Park, K.W.; Ahn, K.S.; Choi, J.H.; Nah, Y.C.; Kim, Y.M.; Sung, Y.E. Pt-WOx electrode structure for thin-film fuel cells. Appl. Phys. Lett. 2002, 81, 907–909. [Google Scholar] [CrossRef]
- Chhina, H.; Campbell, S.; Kesler, O. Ex situ Evaluation of Tungsten Oxide as a Catalyst Support for PEMFCs. J. Electrochem. Soc. 2007, 154, B533–B539. [Google Scholar] [CrossRef]
- Liu, Y.; Shrestha, S.; Mustain, W.E. Synthesis of Nanosize Tungsten Oxide and Its Evaluation as an Electrocatalyst Support for Oxygen Reduction in Acid Media. ACS Catal. 2012, 2, 456–463. [Google Scholar] [CrossRef]
- Jaksic, J.M.; Labou, D.; Papakonstantinou, G.D.; Siokou, A.; Jaksic, M.M. Novel spillover interrelating reversible electrocatalysts for oxygen and hydrogen electrode reactions. J. Phys. Chem. C 2010, 114, 18298–18312. [Google Scholar] [CrossRef]
- Lu, Y.; Jiang, Y.; Gao, X.; Wang, X.; Chen, W. Strongly Coupled Pd Nanotetrahedron/Tungsten Oxide Nanosheet Hybrids with Enhanced Catalytic Activity and Stability as Oxygen Reduction Electrocatalysts. J. Am. Chem. Soc. 2014, 136, 11687–11697. [Google Scholar] [CrossRef] [PubMed]
- Lima, F.H.B.; Zhang, J.; Shao, M.H.; Sasaki, K.; Vukmirovic, M.B.; Ticianelli, E.A.; Adzic, R.R. Catalytic Activity-d-Band Center Correlation for the O2 Reduction Reaction on Platinum in Alkaline Solutions. J. Phys. Chem. C 2007, 111, 404–410. [Google Scholar] [CrossRef]
- Fagan, J.G.; Amarakoon, R. Reliability and reproducibility of ceramic sensors. III: Humidity sensors. Am. Ceram. Soc. Bull. 1993, 72, 119–130. [Google Scholar]
- Pianaro, S.; Bueno, P.; Longo, E.; Varela, J.A. A new SnO2-based varistor system. J. Mater. Sci. Lett. 1995, 14, 692–694. [Google Scholar] [CrossRef]
- Sekizawa, K.; Widjaja, H.; Maeda, S.; Ozawa, Y.; Eguchi, K. Low temperature oxidation of methane over Pd catalyst supported on metal oxides. Catal. Today 2000, 59, 69–74. [Google Scholar] [CrossRef]
- Amalric-Popescu, D.; Bozon-Verduraz, F. SnO2-supported palladium catalysts: activity in deNOx at low temperature. Catal. Lett. 2000, 64, 125–128. [Google Scholar] [CrossRef]
- Du, W.; Yang, G.; Wong, E.; Deskins, N.A.; Frenkel, A.I.; Su, D.; Teng, X. Platinum-Tin Oxide Core-Shell Catalysts for Efficient Electro-Oxidation of Ethanol. J. Am. Chem. Soc. 2014, 136, 10862–10865. [Google Scholar] [CrossRef] [PubMed]
- Katayama, A. Electrooxidation of methanol on a platinum-tin oxide catalyst. J. Phys. Chem. 1980, 84, 376–381. [Google Scholar] [CrossRef]
- Dou, M.; Hou, M.; Wang, F.; Liang, D.; Zhao, Q.; Shao, Z.; Yi, B. Sb-Doped SnO2 Supported Platinum Catalyst with High Stability for Proton Exchange Membrane Fuel Cells. J. Electrochem. Soc. 2014, 161, F1231–F1236. [Google Scholar] [CrossRef]
- Kakinuma, K.; Chino, Y.; Senoo, Y.; Uchida, M.; Kamino, T.; Uchida, H.; Deki, S.; Watanabe, M. Characterization of Pt catalysts on Nb-doped and Sb-doped SnO2−δ support materials with aggregated structure by rotating disk electrode and fuel cell measurements. Electrochim. Acta 2013, 110, 316–324. [Google Scholar] [CrossRef]
- Senoo, Y.; Kakinuma, K.; Uchida, M.; Uchida, H.; Deki, S.; Watanabe, M. Improvements in electrical and electrochemical properties of Nb-doped SnO2−δ supports for fuel cell cathodes due to aggregation and Pt loading. RSC Adv. 2014, 4, 32180–32188. [Google Scholar] [CrossRef]
- Yin, M.; Xu, J.; Li, Q.; Jensen, J.O.; Huang, Y.; Cleemann, L.N.; Bjerrum, N.J.; Xing, W. Highly active and stable Pt electrocatalysts promoted by antimony-doped SnO2 supports for oxygen reduction reactions. Appl. Catal. B 2014, 144, 112–120. [Google Scholar] [CrossRef]
- Kakinuma, K.; Uchida, M.; Kamino, T.; Uchida, H.; Watanabe, M. Synthesis and electrochemical characterization of Pt catalyst supported on Sn0.96Sb0.04O2−δ with a network structure. Electrochim. Acta 2011, 56, 2881–2887. [Google Scholar] [CrossRef]
- Savych, J.; Subianto, S.; Nabil, Y.; Cavaliere, S.; Jones, D.; Rozière, J. Negligible degradation on in situ voltage cycling of a PEMFC with electrospun niobium-doped tin oxide supported Pt cathode. Phys. Chem. Chem. Phys. 2015, 17, 16970–16976. [Google Scholar] [CrossRef] [PubMed]
- Chino, Y.; Taniguchi, K.; Senoo, Y.; Kakinuma, K.; Hara, M.; Watanabe, M.; Uchida, M. Effect of Added Graphitized CB on Both Performance and Durability of Pt/Nb-SnO2 Cathodes for PEFCs. J. Electrochem. Soc. 2015, 162, F736–F743. [Google Scholar] [CrossRef]
- Senoo, Y.; Taniguchi, K.; Kakinuma, K.; Uchida, M.; Uchida, H.; Deki, S.; Watanabe, M. Cathodic performance and high potential durability of Ta-SnO2−δ-supported Pt catalysts for PEFC cathodes. Electrochem. Commun. 2015, 51, 37–40. [Google Scholar] [CrossRef]
- Saha, M.S.; Li, R.; Cai, M.; Sun, X. High electrocatalytic activity of platinum nanoparticles on SnO2 nanowire-based electrodes. Electrochem. Solid State Lett. 2007, 10, B130–B133. [Google Scholar] [CrossRef]
- Zhang, P.; Huang, S.-Y.; Popov, B.N. Mesoporous Tin Oxide as an Oxidation-Resistant Catalyst Support for Proton Exchange Membrane Fuel Cells. J. Electrochem. Soc. 2010, 157, B1163–B1172. [Google Scholar] [CrossRef]
- Wang, X.; Yang, Z.; Zhang, Y.; Jing, L.; Zhao, Y.; Yan, Y.; Sun, K. MnO2 Supported Pt Nanoparticels with High Electrocatalytic Activity for Oxygen Reduction Reaction. Fuel Cells 2014, 14, 35–41. [Google Scholar] [CrossRef]
- Dong, H.-Q.; Chen, Y.-Y.; Han, M.; Li, S.-L.; Zhang, J.; Li, J.-S.; Lan, Y.-Q.; Dai, Z.-H.; Bao, J.-C. Synergistic effect of mesoporous Mn2O3-supported Pd nanoparticle catalysts for electrocatalytic oxygen reduction reaction with enhanced performance in alkaline medium. J. Mater. Chem. A 2014, 2, 1272–1276. [Google Scholar] [CrossRef]
- Seger, B.; Kongkanand, A.; Vinodgopal, K.; Kamat, P.V. Platinum dispersed on silica nanoparticle as electrocatalyst for PEM fuel cell. J. Electroanal. Chem. 2008, 621, 198–204. [Google Scholar] [CrossRef]
- Sasaki, K.; Zhang, L.; Adzic, R.R. Niobium oxide-supported platinum ultra-low amount electrocatalysts for oxygen reduction. Phys. Chem. Chem. Phys. 2008, 10, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.X.; Zhang, H.M.; Liu, G.; Liang, Y.M.; Hu, J.W.; Yi, B.L. A novel non-noble electrocatalyst for PEM fuel cell based on molybdenum nitride. Electrochem. Commun. 2006, 8, 707–712. [Google Scholar] [CrossRef]
- Huang, T.; Mao, S.; Zhou, G.; Wen, Z.; Huang, X.; Ci, S.; Chen, J. Hydrothermal synthesis of vanadium nitride and modulation of its catalytic performance for oxygen reduction reaction. Nanoscale 2014, 6, 9608–9613. [Google Scholar] [CrossRef] [PubMed]
- Oyama, S.T. Introduction to the chemistry of transition metal carbides and nitrides. In The Chemistry of Transition Metal Carbides and Nitrides; Oyama, S.T., Ed.; Springers: Heidelberg, Germany, 1996; pp. 1–27. [Google Scholar]
- Giner, J.; Swette, L. Oxygen Reduction on Titanium Nitride in Alkaline Electrolyte. Nature 1966, 211, 1291–1292. [Google Scholar] [CrossRef]
- Avasarala, B.; Haldar, P. On the stability of TiN-based electrocatalysts for fuel cell applications. Int. J. Hydrogen Energy 2011, 36, 3965–3974. [Google Scholar] [CrossRef]
- Seifitokaldani, A.; Savadogo, O. Electrochemically Stable Titanium Oxy-Nitride Support for Platinum Electro-Catalyst for PEM Fuel Cell Applications. Electrochim. Acta 2015, 167, 237–245. [Google Scholar] [CrossRef]
- Wang, W.; Savadogo, O.; Ma, Z.-F. The oxygen reduction reaction on Pt/TiOxNy-based electrocatalyst for PEM fuel cell applications. J. Appl. Electrochem. 2012, 42, 857–866. [Google Scholar] [CrossRef]
- Pan, Z.; Xiao, Y.; Fu, Z.; Zhan, G.; Wu, S.; Xiao, C.; Hu, G.; Wei, Z. Hollow and porous titanium nitride nanotubes as high-performance catalyst supports for oxygen reduction reaction. J. Mater. Chem. A 2014, 2, 13966–13975. [Google Scholar] [CrossRef]
- Zhang, R.-Q.; Lee, T.-H.; Yu, B.-D.; Stampfl, C.; Soon, A. The role of titanium nitride supports for single-atom platinum-based catalysts in fuel cell technology. Phys. Chem. Chem. Phys. 2012, 14, 16552–16557. [Google Scholar] [CrossRef] [PubMed]
- Avasarala, B.; Haldar, P. Durability and degradation mechanism of titanium nitride based electrocatalysts for PEM (proton exchange membrane) fuel cell applications. Energy 2013, 57, 545–553. [Google Scholar] [CrossRef]
- Yang, M.; Cui, Z.; DiSalvo, F.J. Mesoporous vanadium nitride as a high performance catalyst support for formic acid electrooxidation. Chem. Commun. 2012, 48, 10502–10504. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Chen, H.; Shu, D.; He, C.; Nan, J. Study on the electrochemical behavior of vanadium nitride as a promising supercapacitor material. J. Phys. Chem. Solids 2009, 70, 495–500. [Google Scholar] [CrossRef]
- Cao, B.; Neuefeind, J.C.; Adzic, R.R.; Khalifah, P.G. Molybdenum Nitrides as Oxygen Reduction Reaction Catalysts: Structural and Electrochemical Studies. Inorg. Chem. 2015, 54, 2128–2136. [Google Scholar] [CrossRef] [PubMed]
- Di Noto, V.; Negro, E. Development of nano-electrocatalysts based on carbon nitride supports for the ORR processes in PEM fuel cells. Electrochim. Acta 2010, 55, 7564–7574. [Google Scholar] [CrossRef]
- Di Noto, V.; Negro, E.; Giffin, G.A. (Keynote Lecture) Multi-Metal Nano-Electrocatalysts Based on Carbon Nitride Supports for the ORR and FOR in PEM Fuel Cells. ECS Trans. 2012, 40, 3–10. [Google Scholar]
- Xu, L.; Li, H.; Xia, J.; Wang, L.; Xu, H.; Ji, H.; Li, H.; Sun, K. Graphitic carbon nitride nanosheet supported high loading silver nanoparticle catalysts for the oxygen reduction reaction. Mater. Lett. 2014, 128, 349–353. [Google Scholar] [CrossRef]
- Yan, Z.; Zhang, M.; Xie, J.; Zhu, J.; Shen, P.K. A bimetallic carbide Fe2MoC promoted Pd electrocatalyst with performance superior to Pt/C towards the oxygen reduction reaction in acidic media. Appl. Catal. B 2015, 165, 636–641. [Google Scholar] [CrossRef]
- Li, Z.; Ji, S.; Pollet, B.G.; Shen, P.K. A Co3W3C promoted Pd catalyst exhibiting competitive performance over Pt/C catalysts towards the oxygen reduction reaction. Chem. Commun. 2014, 50, 566–568. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Meng, H.; Cai, M.; Shen, P.K. Bimetallic Carbide Nanocomposite Enhanced Pt Catalyst with High Activity and Stability for the Oxygen Reduction Reaction. J. Am. Chem. Soc. 2012, 134, 1954–1957. [Google Scholar] [CrossRef] [PubMed]
- Chhina, H.; Campbell, S.; Kesler, O. Thermal and electrochemical stability of tungsten carbide catalyst supports. J. Power Sources 2007, 164, 431–440. [Google Scholar] [CrossRef]
- Liang, C.; Ding, L.; Li, C.; Pang, M.; Su, D.; Li, W.; Wang, Y. Nanostructured WCx/CNTs as highly efficient support of electrocatalysts with low Pt loading for oxygen reduction reaction. Energy Environ. Sci. 2010, 3, 1121–1127. [Google Scholar] [CrossRef]
- Garcia, A.C.; Ticianelli, E.A. Investigation of the oxygen reduction reaction on Pt-WC/C electrocatalysts in alkaline media. Electrochim. Acta 2013, 106, 453–459. [Google Scholar] [CrossRef]
- Farsi, H.; Barzgari, Z. Chemical Synthesis of Nanostructured SrWO4 for Electrochemical Energy Storage and Conversion Applications. Int. J. Nanosci. 2014, 13, 1450013:1–1450013:9. [Google Scholar] [CrossRef]
- Farsi, H.; Barzgari, Z. Synthesis, characterization and electrochemical studies of nanostructured CaWO4 as platinum support for oxygen reduction reaction. Mater. Res. Bull. 2014, 59, 261–266. [Google Scholar] [CrossRef]
- Kukino, T.; Kikuchi, R.; Takeguchi, T.; Matsui, T.; Eguchi, K. Proton conductivity and stability of Cs2HPW12O40 electrolyte at intermediate temperatures. Solid State Ionics 2005, 176, 1845–1848. [Google Scholar] [CrossRef]
- Dsoke, S.; Kolary-Zurowska, A.; Zurowski, A.; Mignini, P.; Kulesza, P.J.; Marassi, R. Rotating disk electrode study of Cs2.5H0.5PW12O40 as mesoporous support for Pt nanoparticles for PEM fuel cells electrodes. J. Power Sources 2011, 196, 10591–10600. [Google Scholar] [CrossRef]
- Yu, H.Y.; Feng, X.D.; Grozea, D.; Lu, Z.H.; Sodhi, R.N.S.; Hor, A.-M.; Aziz, H. Surface electronic structure of plasma-treated indium tin oxides. Appl. Phys. Lett. 2001, 78, 2595–2597. [Google Scholar] [CrossRef]
- Chhina, H.; Campbell, S.; Kesler, O. An oxidation-resistant indium tin oxide catalyst support for proton exchange membrane fuel cells. J. Power Sources 2006, 161, 893–900. [Google Scholar] [CrossRef]
- Liu, Y.; Mustain, W.E. High Stability, High Activity Pt/ITO Oxygen Reduction Electrocatalysts. J. Am. Chem. Soc. 2013, 135, 530–533. [Google Scholar] [CrossRef] [PubMed]
- Munro, R.G. Material properties of titanium diboride. J. Res. Nat. Inst. Stand. Technol. 2000, 105, 709–720. [Google Scholar] [CrossRef]
- Yin, S.; Mu, S.; Lv, H.; Cheng, N.; Pan, M.; Fu, Z. A highly stable catalyst for PEM fuel cell based on durable titanium diboride support and polymer stabilization. Appl. Catal. B 2010, 93, 233–240. [Google Scholar] [CrossRef]
- Yin, S.; Mu, S.; Pan, M.; Fu, Z. A highly stable TiB2-supported Pt catalyst for polymer electrolyte membrane fuel cells. J. Power Sources 2011, 196, 7931–7936. [Google Scholar] [CrossRef]
- Huang, Z.; Lin, R.; Fan, R.; Fan, Q.; Ma, J. Effect of TiB2 Pretreatment on Pt/TiB2 Catalyst Performance. Electrochim. Acta 2014, 139, 48–53. [Google Scholar] [CrossRef]
- Roth, C.; Bleith, P.; Schwöbel, C.A.; Kaserer, S.; Eichler, J. Importance of Fuel Cell Tests for Stability Assessment—Suitability of Titanium Diboride as an Alternative Support Material. Energies 2014, 7, 3642–3652. [Google Scholar] [CrossRef]
- Bača, L.; Stelzer, N. Adapting of sol-gel process for preparation of TiB2 powder from low-cost precursors. J. Eur. Ceram. Soc. 2008, 28, 907–911. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lori, O.; Elbaz , L. Advances in Ceramic Supports for Polymer Electrolyte Fuel Cells. Catalysts 2015, 5, 1445-1464. https://doi.org/10.3390/catal5031445
Lori O, Elbaz L. Advances in Ceramic Supports for Polymer Electrolyte Fuel Cells. Catalysts. 2015; 5(3):1445-1464. https://doi.org/10.3390/catal5031445
Chicago/Turabian StyleLori, Oran, and Lior Elbaz . 2015. "Advances in Ceramic Supports for Polymer Electrolyte Fuel Cells" Catalysts 5, no. 3: 1445-1464. https://doi.org/10.3390/catal5031445




