Sb Surface Modification of Pd by Mimetic Underpotential Deposition for Formic Acid Oxidation
Abstract
:1. Introduction
2. Results and Discussion
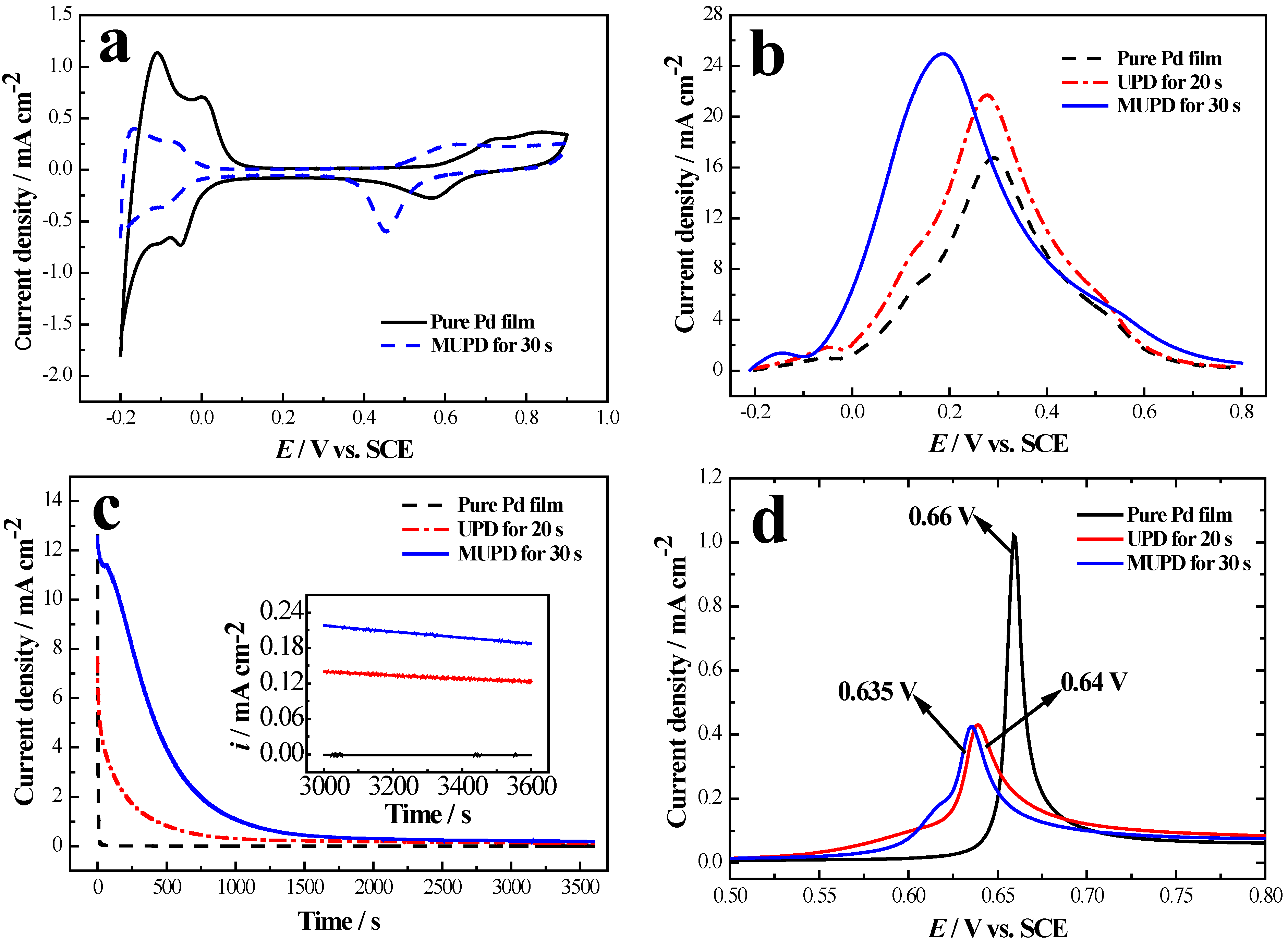
2.1. Sb UPD on Pd Film Electrodes

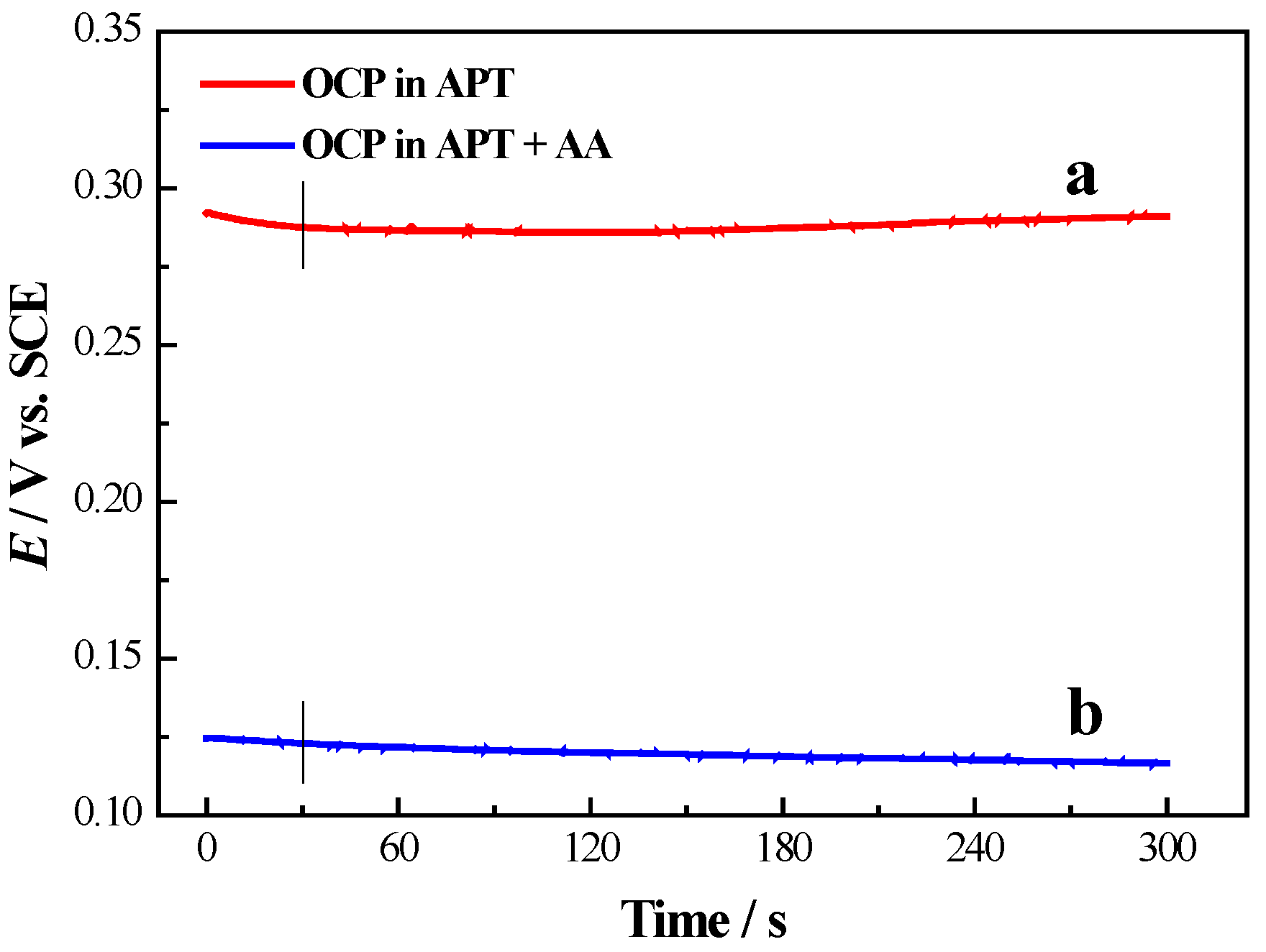
2.2. Sb MUPD on Pd Film Electrodes
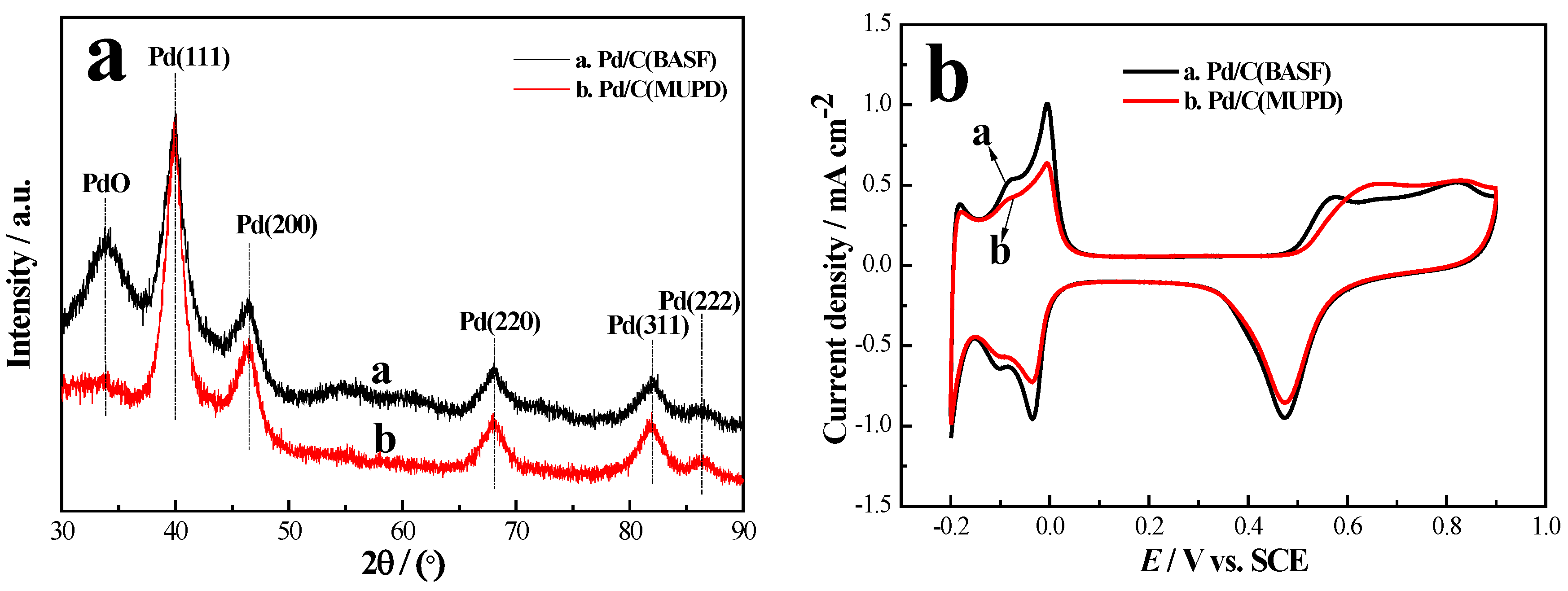
2.3. Sb MUPD on Pd/C Powder Catalyst

3. Experimental Section
3.1. Modification of Pd Surfaces
3.2. X-ray Diffraction
3.3. Electrochemical Measurements
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rice, C.; Ha, S.; Masel, R.I.; Waszczuk, P.; Wieckowski, A.; Barnard, T. Direct formic acid fuel cells. J. Power Sources 2002, 111, 83–89. [Google Scholar] [CrossRef]
- Yu, X.W.; Pickup, P.G. Recent advances in Direct Formic Acid Fuel Cells (DFAFC). J. Power Sources 2008, 182, 124–132. [Google Scholar] [CrossRef]
- Rice, C.; Ha, S.; Masel, R.I.; Wieckowski, A. Catalysts for direct formic acid fuel cells. J. Power Sources 2003, 115, 229–235. [Google Scholar] [CrossRef]
- Markovic, N.M.; Gasteiger, H.; Ross, P.N.; Jian, X.; Villegas, I.; Weaver, M. Electrooxidation mechanism of methanol and formic acid on Pt–Ru alloy surfaces. Electrochim. Acta 1995, 40, 91–98. [Google Scholar] [CrossRef]
- Markovic, N.M.; Ross, P.N. Surface science studies of model fuel cell electrocatalysts. Surf. Sci. Rep. 2002, 45, 117–229. [Google Scholar] [CrossRef]
- Waszczuk, P.; Barnard, T.M.; Rice, C.; Masel, R.I.; Wieckowski, A. A nanoparticle catalyst with superior activity for electrooxidation of formic acid. Electrochem. Commun. 2002, 4, 599–603. [Google Scholar] [CrossRef]
- Zhou, W.P.; Lewera, A.; Larsen, R.; Masel, R.I.; Bagus, P.S.; Wieckowski, A. Size Effects in Electronic and Catalytic Properties of Unsupported Palladium Nanoparticles in Electrooxidation of Formic Acid. J. Phys. Chem. B 2006, 110, 13393–13398. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.J.; Lee, J.Y. Particle Size Effects in Pd-Catalyzed Electrooxidation of Formic Acid. J. Phys. Chem. C 2008, 112, 3789–3793. [Google Scholar] [CrossRef]
- Zhu, Y.; Kang, Y.Y.; Zou, Z.Q.; Zhou, Q.; Zheng, J.W.; Xia, B.J.; Yang, H. A facile preparation of carbon-supported Pd nanoparticles for electrocatalytic oxidation of formic acid. Electrochem. Commun. 2008, 10, 802–805. [Google Scholar] [CrossRef]
- Ge, J.J.; Xing, W.; Xue, X.Z.; Liu, C.P.; Lu, T.H.; Liao, J.H. Controllable Synthesis of Pd Nanocatalysts for Direct Formic Acid Fuel Cell (DFAFC) Application: From Pd Hollow Nanospheres to Pd Nanoparticles. J. Phys. Chem. C 2007, 111, 17305–17310. [Google Scholar] [CrossRef]
- Zhang, J.T.; Qiu, C.C.; Ma, H.Y.; Liu, X.Y. Facile Fabrication and Unexpected Electrocatalytic Activity of Palladium Thin Films with Hierarchical Architectures. J. Phys. Chem. C 2008, 112, 13970–13975. [Google Scholar] [CrossRef]
- Wang, J.Y.; Zhang, H.X.; Jiang, K.; Cai, W.B. From HCOOH to CO at Pd Electrodes: A Surface-Enhanced Infrared Spectroscopy Study. J. Am. Chem. Soc. 2011, 133, 14876–14879. [Google Scholar] [CrossRef] [PubMed]
- Mikolajczuk, A.; Borodzinski, A.; Kedzierzawski, P.; Stobinski, L.; Mierzwa, B.; Dziura, R. Deactivation of Carbon Supported Palladium Catalyst in Direct Formic Acid Fuel Cell. Appl. Surf. Sci. 2011, 257, 8211–8214. [Google Scholar] [CrossRef]
- Nitze, F.; Sandstrom, R.; Barzegar, H.R.; Hu, G.; Mazurkiewicz, M.; Malolepszy, A.; Stobinski, L.; Wagberg, T. Direct Support Mixture Painting, Using Pd(0) Organo-Metallic Compounds—An Easy and Environmentally Sound Approach to Combine Decoration and Electrode Preparation for Fuel Cells. J. Mater. Chem. A 2014, 2, 20973–20979. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, J.G.; Ye, J.L.; Zou, Z.G.; Ye, J.H.; Gu, J.; Yu, T.; Yang, A.D. Poisoning and Regeneration of Pd Catalyst in Direct Formic Acid Fuel Cell. Electrochim. Acta 2010, 55, 5024–5027. [Google Scholar] [CrossRef]
- Yu, X.W.; Pickup, P.G. Mechanistic Study of the Deactivation of Carbon Supported Pd during Formic Acid Oxidation. Electrochem. Commun. 2009, 11, 2012–2014. [Google Scholar] [CrossRef]
- Miyake, H.; Okada, T.; Osawa, G.S.M. Formic acid electrooxidation on Pd in acidic solutions studied by surface enhanced infrared absorption spectroscopy. Phys. Chem. Chem. Phys. 2008, 10, 3662–3669. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.W.; Pickup, P.G. Deactivation resistant PdSb/C catalysts for direct formic acid fuel cells. Electrochem. Commun. 2010, 12, 800–803. [Google Scholar] [CrossRef]
- Haan, J.L.; Stafford, K.M.; Morgan, R.D.; Masel, R.I. Performance of the direct formic acid fuel cell with electrochemically modified palladium-antimony anode catalyst. Electrochim. Acta 2010, 55, 2477–2481. [Google Scholar] [CrossRef]
- Watanabe, M.; Horiuchi, M.; Motoo, S. Electrocatalysis by ad-atoms: Part XXIII. Design of platinum ad-electrodes for formic acid fuel cells with ad-atoms of the IVth and the Vth groups. J. Electroanal. Chem. Interfacial Electrochem. 1988, 250, 117–125. [Google Scholar] [CrossRef]
- Fernandez-Vega, A.; Feliu, J.M.; Aldaz, A.; Claviller, J. Heterogeneous Electrocatalysis on Well Defined Platinum Surfaces Modified by Controlled Amounts of Irreversibly Adsorbed Adatoms: Part II. Formic Acid Oxidation on the Pt(100)–Sb System. J. Electroanal. Chem. 1989, 258, 101–113. [Google Scholar] [CrossRef]
- Yang, Y.Y.; Sun, S.G.; Gu, Y.J.; Zhou, Z.Y.; Zhen, C.H. Surface modification and electrocatalytic properties of Pt(100), Pt(110), Pt(320) and Pt(331) electrodes with Sb towards HCOOH oxidation. Electrochim. Acta 2001, 46, 4339–4348. [Google Scholar] [CrossRef]
- Peng, B.; Wang, J.Y.; Zhang, H.X.; Lin, Y.H.; Cai, W.B. A versatile electroless approach to controlled modification of Sb on Pt surfaces towards efficient electrocatalysis of formic acid. Electrochem. Commun. 2009, 11, 831–833. [Google Scholar] [CrossRef]
- Wang, S.H.; Zhang, H.X.; Cai, W.B. Mimetic underpotential deposition technique extended for surface nanoengineering of electrocatalysts. J. Power Sources 2012, 212, 100–104. [Google Scholar] [CrossRef]
- Lee, J.K.; Jeon, H.; Uhm, S.Y.; Lee, J.Y. Influence of underpotentially deposited Sb onto Pt anode surface on the performance of direct formic acid fuel cells. Electrochim. Acta 2008, 53, 6089–6092. [Google Scholar] [CrossRef]
- Brandt, K.; Steinhausen, M.; Wandelt, K. Catalytic and electrocatalytic oxidation of formic acid on the pure and Cu-modified Pd(111)-surface. J. Electroanal. Chem. 2008, 616, 27–37. [Google Scholar] [CrossRef]
- Peng, B.; Wang, H.F.; Liu, Z.P.; Cai, W.B. Combined Surface-Enhanced Infrared Spectroscopy and First-Principles Study on Electro-Oxidation of Formic Acid at Sb-Modified Pt Electrodes. J. Phys. Chem. C 2010, 114, 3102–3107. [Google Scholar] [CrossRef]
- Sadiki, A.; Vo, P.; Hu, S.Z.; Copenhaver, T.S.; Scudiero, L.; Ha, S.; Haan, J.L. Increased Electrochemical Oxidation Rate of Alcohols in Alkaline Media on Palladium Surfaces Electrochemically Modified by Antimony, Lead, and Tin. Electrochim. Acta 2014, 139, 302–307. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.-L.; Cao, X.-L.; Wang, Y.-J.; Li, Q.-X. Sb Surface Modification of Pd by Mimetic Underpotential Deposition for Formic Acid Oxidation. Catalysts 2015, 5, 1388-1398. https://doi.org/10.3390/catal5031388
Wang L-L, Cao X-L, Wang Y-J, Li Q-X. Sb Surface Modification of Pd by Mimetic Underpotential Deposition for Formic Acid Oxidation. Catalysts. 2015; 5(3):1388-1398. https://doi.org/10.3390/catal5031388
Chicago/Turabian StyleWang, Long-Long, Xiao-Lu Cao, Ya-Jun Wang, and Qiao-Xia Li. 2015. "Sb Surface Modification of Pd by Mimetic Underpotential Deposition for Formic Acid Oxidation" Catalysts 5, no. 3: 1388-1398. https://doi.org/10.3390/catal5031388




