Positive Effect of Heat Treatment on Carbon-Supported CoS Nanocatalysts for Oxygen Reduction Reaction
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structure and Morphology
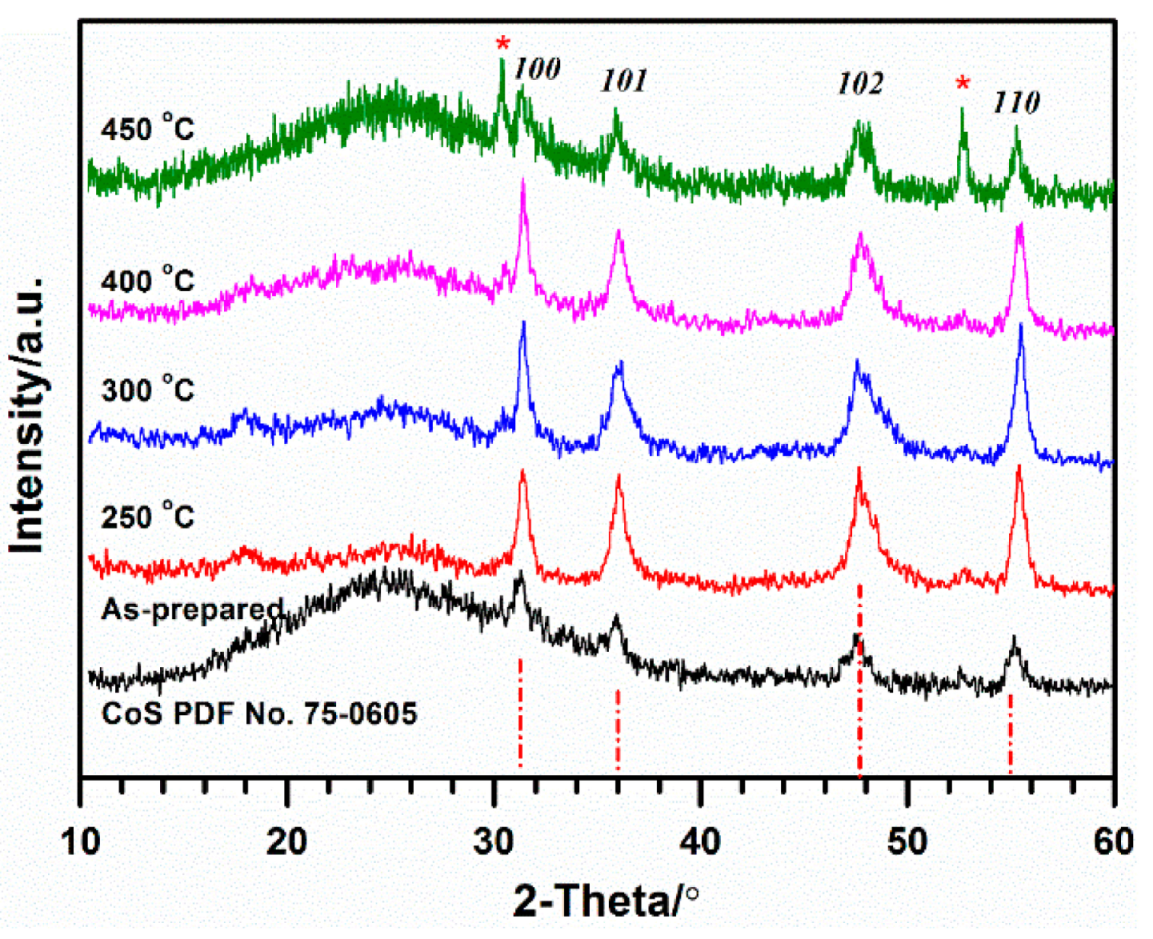
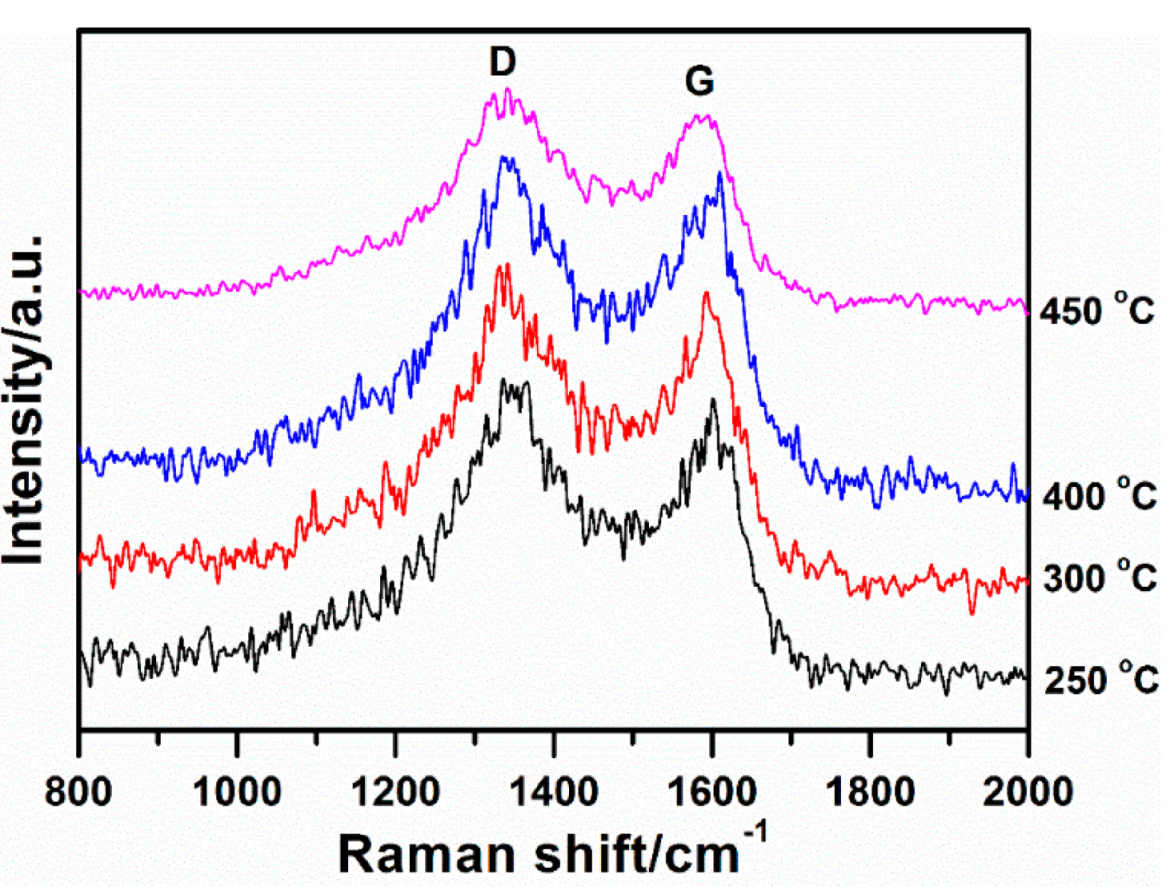
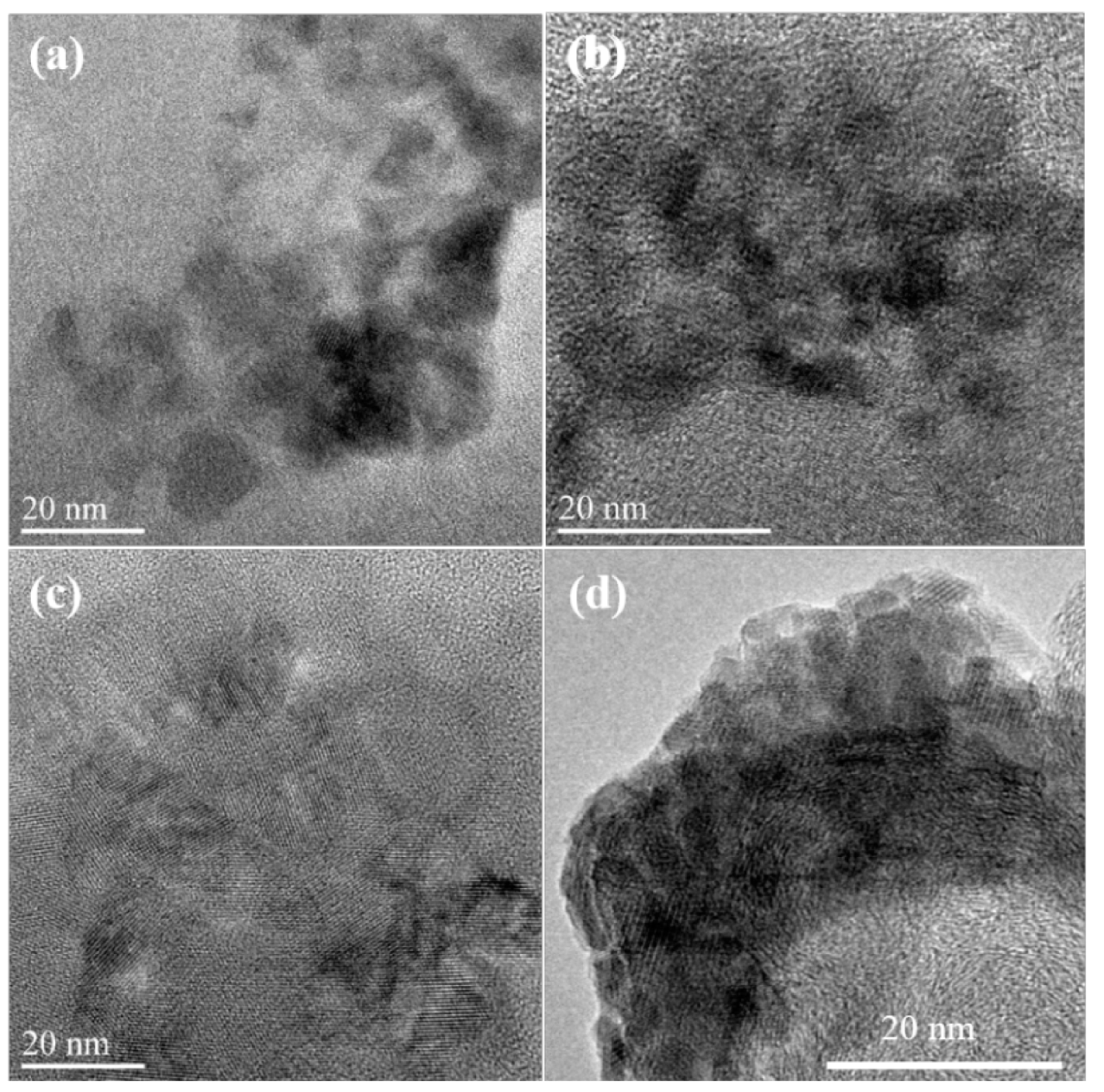
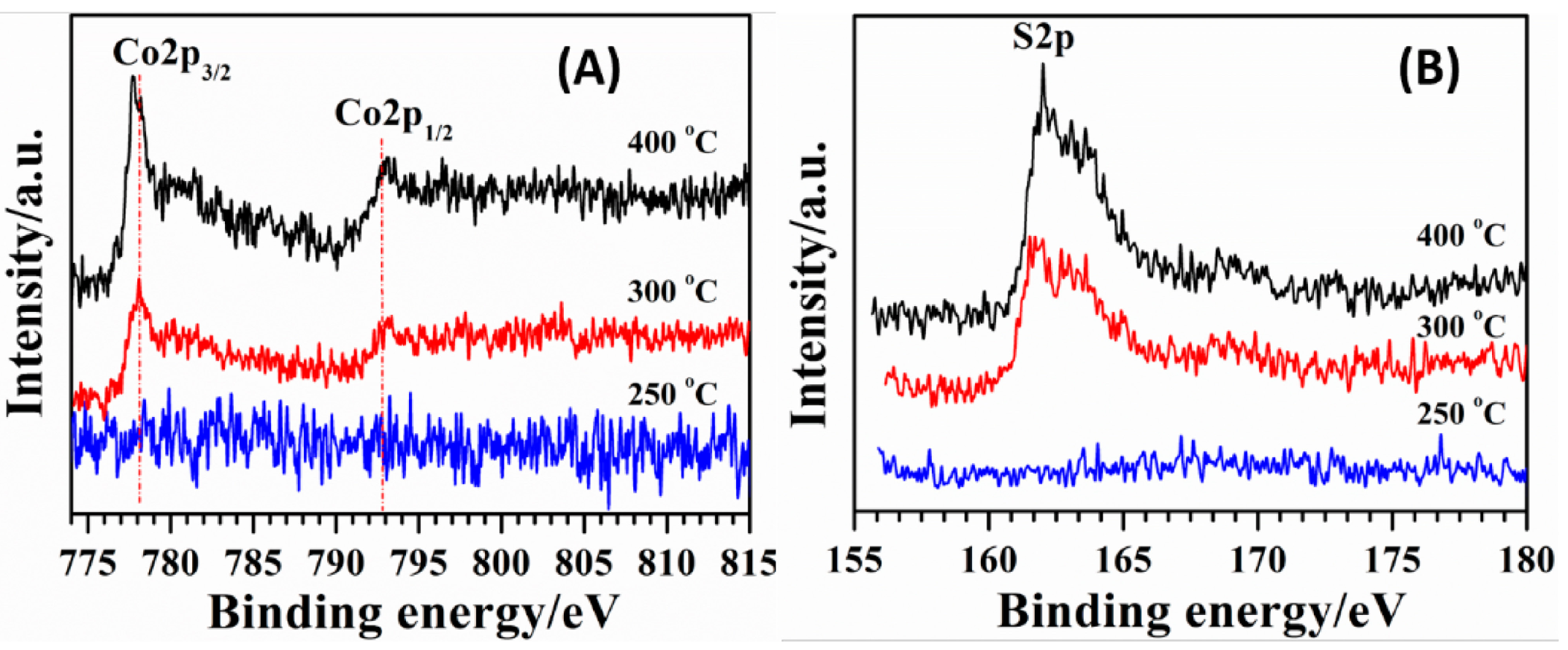
2.2. Influence of Heat-Treatment on Electrocatalytic Activity towards ORR
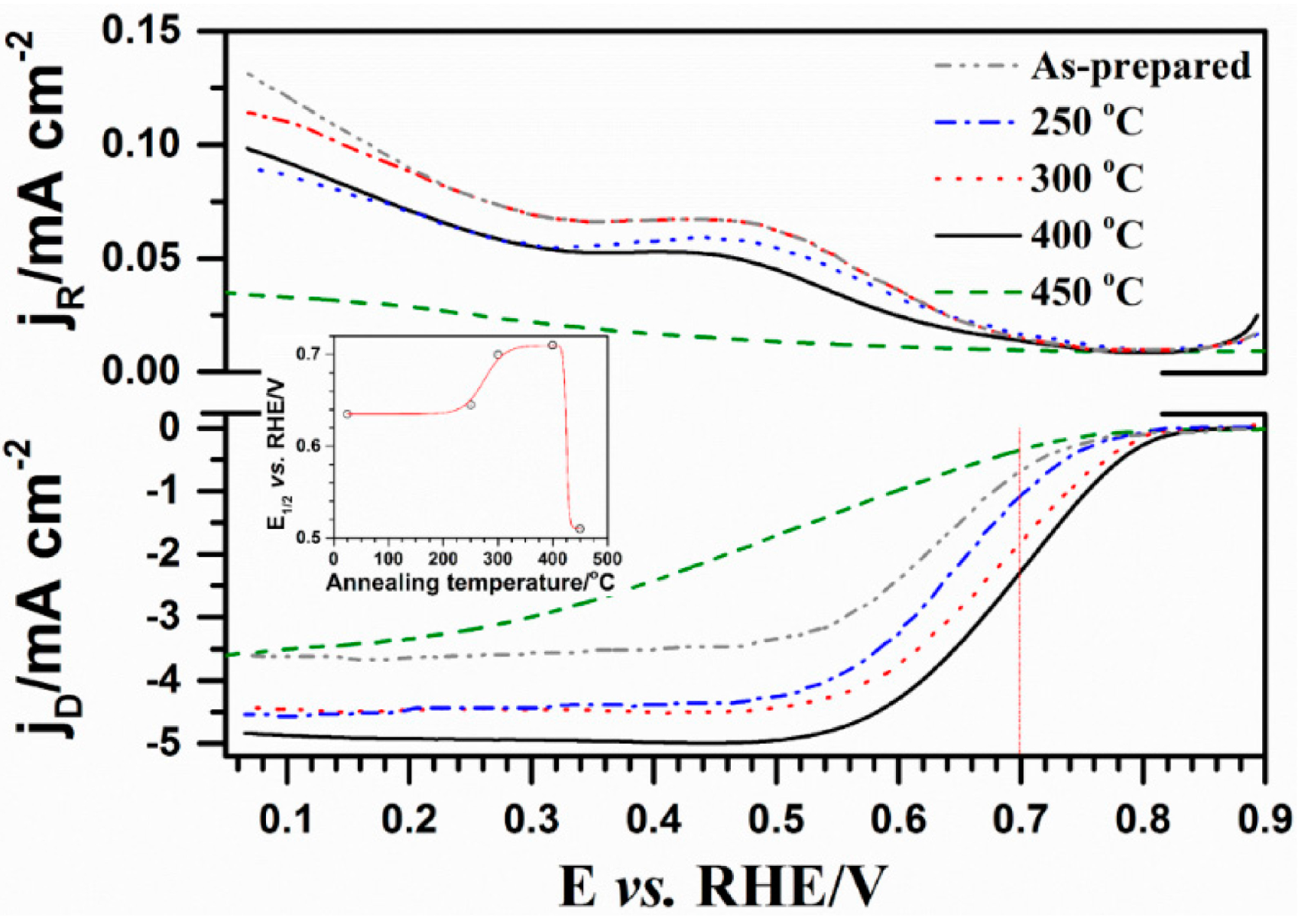

2.3. Electrocatalytic Stability
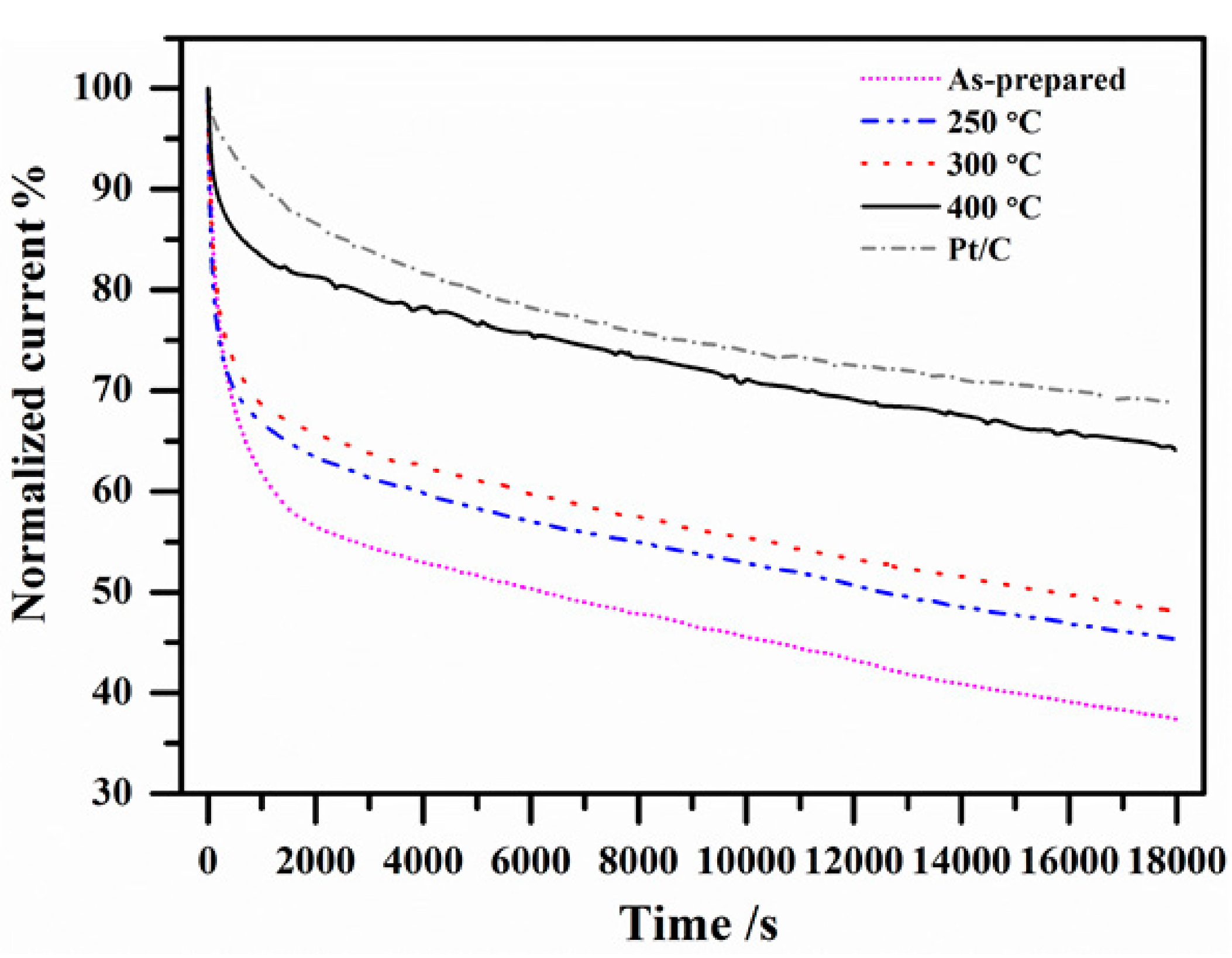
3. Materials and Methods
3.1. Chemicals
3.2. Synthesis of Carbon-Supported CoS Nanocatalyst
3.3. Structural Characterization and Electrochemical Measurements
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, Y.; Chen, K.S.; Mishler, J.; Cho, S.C.; Adroher, X.C. A review of polymer electrolyte membrane fuel cells: Technology, applications, and needs on fundamental research. Appl. Energy 2011, 88, 981–1007. [Google Scholar] [CrossRef]
- Miller, M.; Bazylak, A. A review of polymer electrolyte membrane fuel cell stack testing. J. Power Sources 2011, 196, 601–613. [Google Scholar] [CrossRef]
- Feng, Y.; Gago, A.; Timperman, L.; Alonso-Vante, N. Chalcogenide metal centers for oxygen reduction reaction: Activity and tolerance. Electrochim. Acta 2011, 56, 1009–1022. [Google Scholar] [CrossRef]
- Guo, J.; Li, H.; He, H.; Chu, D.; Chen, R. CoPc- and CoPcF16-modified Ag nanoparticles as novel catalysts with tunable oxygen reduction activity in alkaline media. J. Phys. Chem. C 2011, 115, 8494–8502. [Google Scholar] [CrossRef]
- Robertson, N.J.; Kostalik, H.A.; Clark, T.J.; Mutolo, P.F.; Abruña, H.D.; Coates, G.W. Tunable High Performance Cross-Linked Alkaline Anion Exchange Membranes for Fuel Cell Applications. J. Am. Chem. Soc. 2010, 132, 3400–3404. [Google Scholar] [CrossRef] [PubMed]
- Tamain, C.; Poynton, S.D.; Slade, R.C.T.; Carroll, B.; Varcoe, J.R. Development of cathode architectures customized for H2/O2 metal-cation-free alkaline membrane fuel cells. J. Phys. Chem. C 2007, 111, 18423–18430. [Google Scholar] [CrossRef]
- Wu, H.; Chen, W. Copper nitride nanocubes: Size-controlled synthesis and application as cathode catalyst in alkaline fuel cells. J. Am. Chem. Soc. 2011, 133, 15236–15239. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.S.; Bhattacharjya, D.; Inamdar, S.; Park, J.; Yu, J.S. Phosphorus-doped ordered mesoporous carbons with different lengths as efficient metal-free electrocatalysts for oxygen reduction reaction in alkaline media. J. Am. Chem. Soc. 2012, 134, 16127–16130. [Google Scholar] [CrossRef] [PubMed]
- Susac, D.; Zhu, L.; Teo, M.; Sode, A.; Wong, K.C.; Wong, P.C.; Parsons, R.R.; Bizzotto, D.; Mitchell, K.A.R.; Campbell, S.A. Characterization of FeS2-Based Thin Films as Model Catalysts for the oxygen reduction reaction. J. Phys. Chem. C 2007, 111, 18715–18723. [Google Scholar] [CrossRef]
- Zhu, L.; Susac, D.; Teo, M.; Wong, K.C.; Wong, P.C.; Parsons, R.R.; Bizzotto, D.; Mitchell, K.A.R.; Campbell, S.A. Investigation of CoS2-based Thin Films as Model Catalysts for the Oxygen Reduction Reaction. J. Catal. 2008, 258, 235–242. [Google Scholar] [CrossRef]
- Zhao, C.; Li, D.; Feng, Y. Size-controlled hydrothermal synthesis and high electrocatalytic performance of CoS2 nanocatalysts as non-precious metal cathode materials for fuel cells. J. Mater. Chem. A 2013, 1, 5741–5746. [Google Scholar] [CrossRef]
- Feng, Y.J.; He, T.; Alonso-Vante, N. In situ Surfactant-Free Synthesis and ORR- Electrochemistry of Carbon-Supported Co3S4 and CoSe2 Nanoparticles. Chem. Mater. 2008, 20, 26–28. [Google Scholar] [CrossRef]
- Feng, Y.J.; He, T.; Alonso-Vante, N. Carbon-Supported CoSe2 Nanoparticles for Oxygen Reduction Reaction in Acid Medium. Fuel Cells 2010, 10, 77–83. [Google Scholar]
- Wang, H.; Liang, Y.; Li, Y.; Dai, H. Co1−xS-Graphene Hybrid: A High-Performance Metal Chalcogenide Electrocatalyst for Oxygen Reduction. Angew. Chem. Int. Ed. 2011, 50, 10969–10972. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, C.W.B.; Zhang, L.; Liu, H.; Lee, K.; Marques, A.L.B.; Marques, E.P.; Wang, H.; Zhang, J. A review of heat-treatment effects on activity and stability of PEM fuel cell catalysts for oxygen reduction reaction. J. Power Sources 2007, 173, 891–908. [Google Scholar] [CrossRef]
- Cheng, H.; Yuan, W.; Scott, K. Influence of Thermal Treatment on RuSe Cathode Materials for Direct Methanol Fuel Cells. Fuel Cells 2007, 7, 16–20. [Google Scholar] [CrossRef]
- Cheng, H.; Yuan, W.; Scott, K. The influence of a new fabrication procedure on the catalytic of ruthenium-selenium catalysts. Electrochim. Acta 2006, 52, 466–473. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Jorio, A.; Hofmann, M.; Dresselhaus, G.; Saito, R. Perspectives on carbon nanotubes and graphene Raman spectroscopy. Nano Lett. 2010, 10, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Bao, S.-J.; Li, Y.B.; Li, C.M.; Bao, Q.L.; Lu, Q.; Guo, J. Shape evolution and magnetic properties of cobalt sulfide. Cryst. Growth Des. 2008, 8, 3745–3749. [Google Scholar] [CrossRef]
- Delacôte, C.; Bonakdarpour, A.; Johnston, C.M.; Zelenay, P.; Wieckowski, A. Aqueous-based synthesis of ruthenium-selenium catalyst for oxygen reduction reaction. Faraday Discuss. 2008, 140, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Li, Y.; Wang, H.; Zhou, J.; Wang, J.; Regier, T.; Dai, H. Co3O4 nanocrystals on graphene as a synergistic catalyst for oxygen reduction reaction. Nat. Mater. 2011, 10, 1780–1786. [Google Scholar] [CrossRef] [PubMed]
- Tuci, G.; Zafferoni, C.; D’Ambrosio, P.; Caporali, S.; Ceppatelli, M.; Rossin, A.; Tsoufis, T.; Innocenti, M.; Giambastiani, G. Tailoring carbon nanotube N-dopants while designing metal-free electrocatalys for the oxygen reduction reaction in alkaline medium. ACS Catal. 2013, 3, 2108–2111. [Google Scholar] [CrossRef]
- Lee, J.S.; Park, G.S.; Lee, H.I.; Kim, S.T.; Cao, R.; Liu, M.; Cho, J. Ketjenblack carbon supported amorphous manganese oxides nanowires as highly efficient electrocatalyst for oxygen reduction reaction in alkaline solution. Nano Lett. 2011, 11, 5362–5366. [Google Scholar] [CrossRef] [PubMed]
- Han, J.-T.; Huang, Y.-H.; Huang, W. Solvothermal synthesis and magnetic properties of pyrite Co1−xFexS2 with various morphologies. Mater. Lett. 2006, 60, 1805–1808. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, H.; Xi, J.; Tang, P.; Li, D.; Feng, Y. Positive Effect of Heat Treatment on Carbon-Supported CoS Nanocatalysts for Oxygen Reduction Reaction. Catalysts 2015, 5, 1211-1220. https://doi.org/10.3390/catal5031211
Zhong H, Xi J, Tang P, Li D, Feng Y. Positive Effect of Heat Treatment on Carbon-Supported CoS Nanocatalysts for Oxygen Reduction Reaction. Catalysts. 2015; 5(3):1211-1220. https://doi.org/10.3390/catal5031211
Chicago/Turabian StyleZhong, Haihong, Jingmin Xi, Pinggui Tang, Dianqing Li, and Yongjun Feng. 2015. "Positive Effect of Heat Treatment on Carbon-Supported CoS Nanocatalysts for Oxygen Reduction Reaction" Catalysts 5, no. 3: 1211-1220. https://doi.org/10.3390/catal5031211





