Non-Calorimetric Determination of the Adsorption Heat of Volatile Organic Compounds under Dynamic Conditions
Abstract
:1. Introduction
2. Results and Discussion
2.1. Materials
2.2. Textural and Chemical Characterization
2.3. Measurement of Dynamical Adsorption and Desorption Amounts
3. Results and Discussion
3.1. Textural and Chemical Characterization
3.1.1. BET Surface Area and Porosity
| Solids | SBET(m2/g) | Vt a(cm3(STP)/g) | Vmeso b(cm3(STP)/g) | Vmic c(cm3(STP)/g) | Sext d(m2/g) | Smic e(m2/g) |
|---|---|---|---|---|---|---|
| Bentonite | 83.5 | 0.213 | 0.2113 | 0.0017 | 81.02 | 2.469 |
| SiO2 | 201.7 | 0.430 | 0.4298 | 0.0002 | 198.53 | 1.47 |

3.1.2. Chemical Composition
| Solids | SiO2 | Al2O3 | CaO | MgO | Fe2O3 | Na2O | K2O | SO3 | Cl | CuO | TiO2 | ZnO |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bentonite | 74 | 13.1 | 5.36 | 2.9 | 1.88 | 1.1 | 0.52 | 0.38 | 0.32 | 0.13 | 0.12 | 0.1 |
| SiO2 | 99.4 | 0.053 | 0.025 | - | 0.017 | - | 0.023 | 0.2 | 0.059 | - | - | - |
3.2. Dynamic Adsorption/Desorption
3.2.1. Determination of Adsorbed and Desorbed Amounts

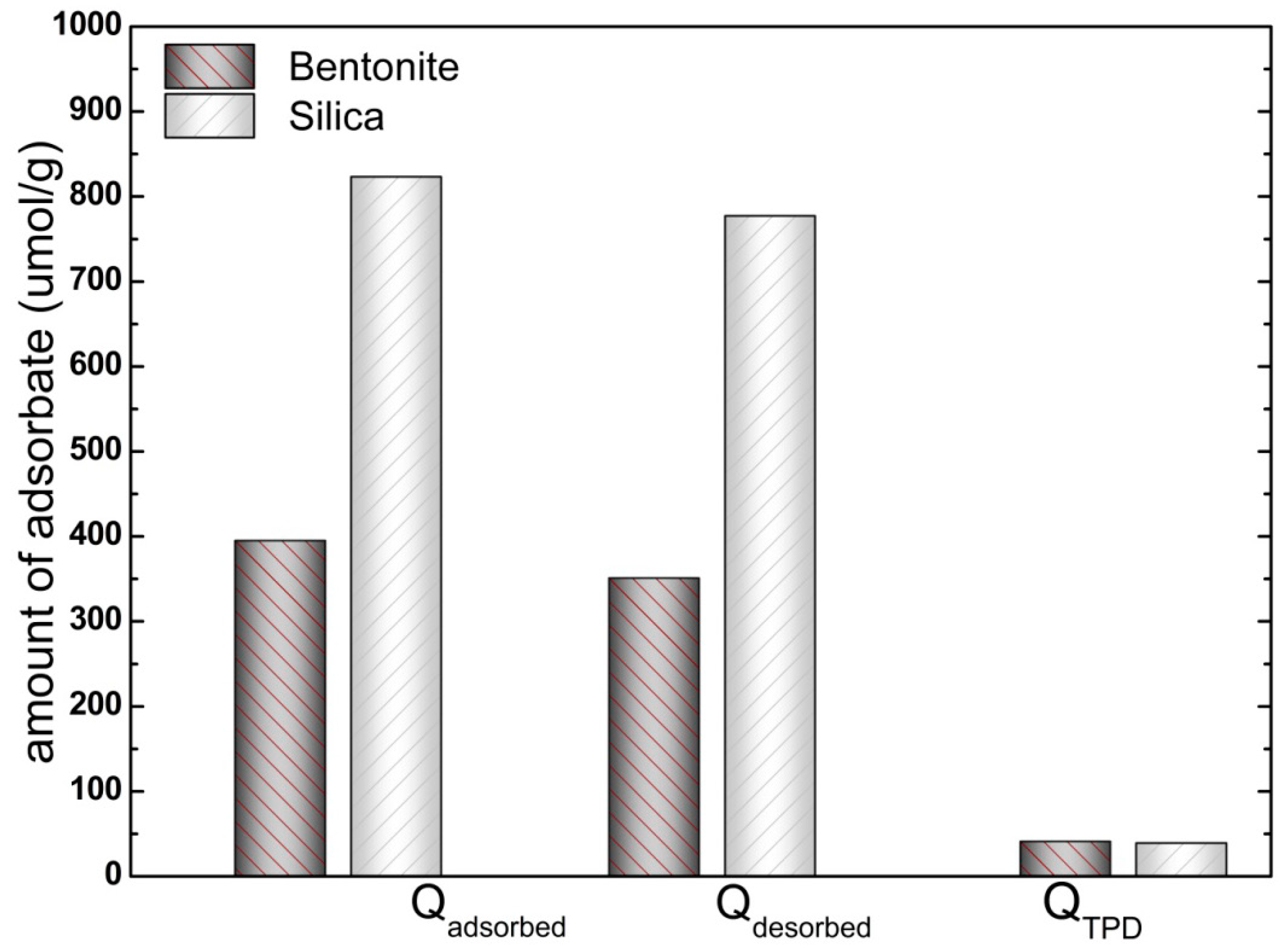

3.2.2. Adsorption Isotherms

| Solids | Adsorption Temperatures | Adsorption amounts | Langmuir parameters | Freundlich parameters | Temkin parameters | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| T(K) | Qm exp | Qm | b | R2 | k | N | R2 | a | c | R2 | |
| Bentonite | 300 | 831 | 1261 | 0.29 | 0.999 | 256 | 0.55 | 0.997 | 296 | 167 | 0.991 |
| 313 | 385 | 526 | 0.285 | 0.998 | 143 | 0.459 | 0.993 | 128 | 100 | 0.998 | |
| 323 | 292 | 408 | 0.281 | 0.998 | 110 | 0.453 | 0.985 | 95 | 80 | 0.997 | |
| 348 | 192 | 264 | 0.276 | 0.998 | 70 | 0.474 | 0.99 | 65 | 47 | 0.998 | |
| SiO2 | 300 | 1298 | 2807 | 0.31 | 0.990 | 851.50 | 0.713 | 0.978 | 857.66 | 1044 | 0.996 |
| 308 | 971 | 2387 | 0.29 | 0.997 | 500.70 | 0.694 | 0.986 | 493.71 | 581.1 | 0.993 | |
| 318 | 633 | 1132 | 0.28 | 0.994 | 230.90 | 0.690 | 0.986 | 225.80 | 267.42 | 0.990 | |
| 328 | 402 | 631 | 0.267 | 0.994 | 130.45 | 0.679 | 0.991 | 127.49 | 149 | 0.986 | |
3.3. Heat of Adsorption Determination
3.3.1. Application of the Isosteric Heat Method
| Surface coverage | Bentonite | Silica |
|---|---|---|
| Recovery rate | Isosteric heat (kJ/mol) | Isosteric heat (kJ/mol) |
| θ = 0.15 | 45.672 | 49.395 |
| θ = 0.17 | 43.475 | 48.572 |
| θ = 0.22 | 42.73 | 48.057 |
| θ = 0.27 | 41.567 | 47.974 |
3.3.2. Application of the Temperature-Programmed Desorption (TPD) Method
3.3.3. Application of Temperature-Programmed Adsorption Equilibrium (TPAE) Method

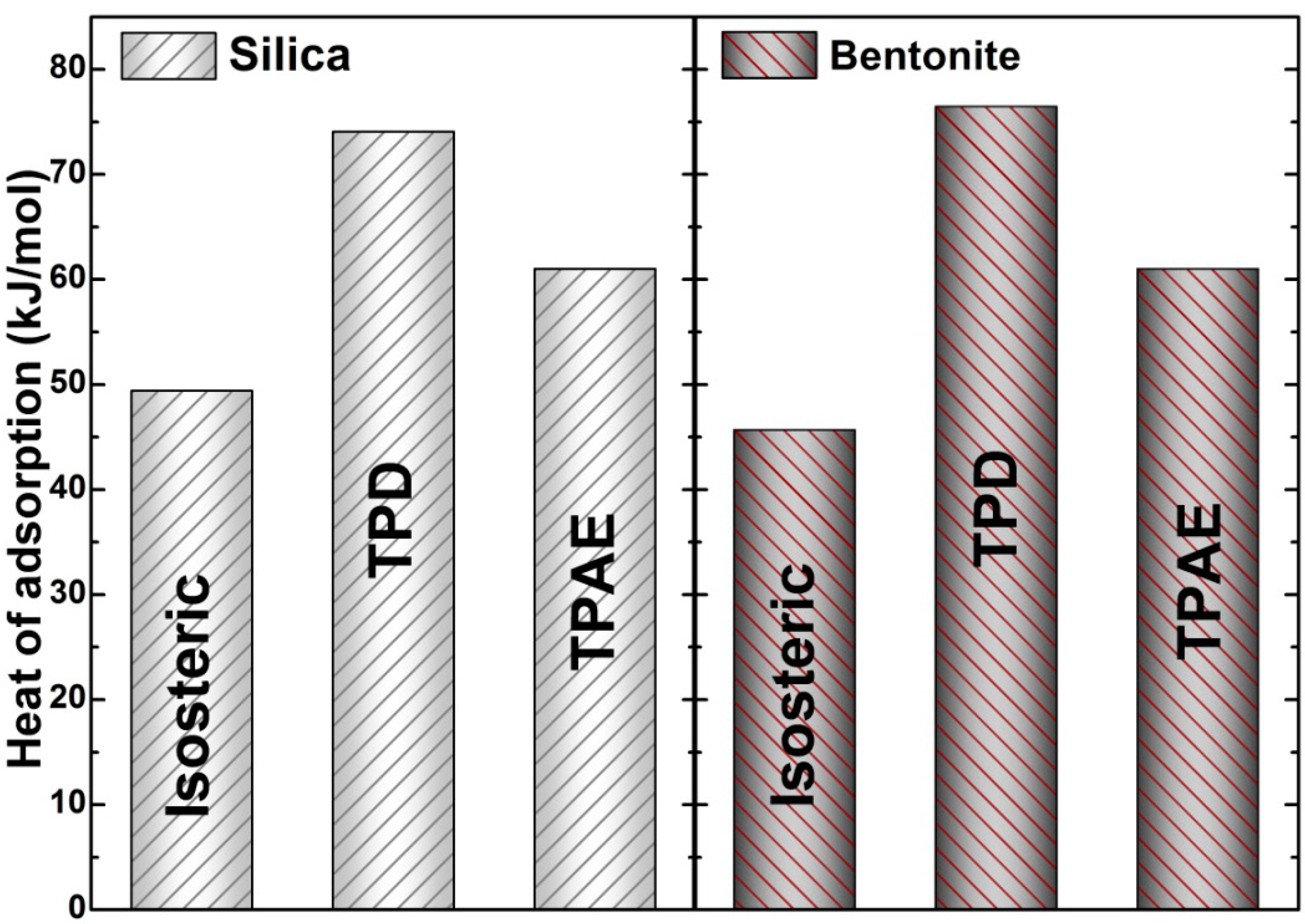
4. Conclusions
Acknowledgement
Author Contributions
Conflicts of Interest
References
- Chafik, T.; Zaitan, H.; Harti, S.; Darir, A.; Achak, O. Determination of the Heat of Adsorption and Desorption of a Volatile Organic Compound under Dynamic Conditions Using Fourier-Transform Infrared Spectroscopy. Spectrosc. Lett. 2007, 40, 763–775. [Google Scholar] [CrossRef]
- Gupta, V.K.; Suhas. Application of low-cost adsorbents for dye removal—A review. J. Environ. Manage. 2009, 90, 2313–2342. [Google Scholar] [CrossRef] [PubMed]
- Zaitan, H.; Korrir, A.; Chafik, T.; Bianchi, D. Evaluation of the potential of volatile organic compound (di-methyl benzene) removal using adsorption on natural minerals compared to commercial oxides. J. Hazard. Mater. 2013, 262, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Kane, A.; Giraudet, S.; Vilmain, J.-B.; Le Cloirec, P. Intensification of the temperature-swing adsorption process with a heat pump for the recovery of dichloromethane. J. Environ. Chem. Eng. 2015, in press. [Google Scholar]
- Dunne, J.A.; Rao, M.; Sircar, S.; Gorte, R.J.; Myers, A.L. Calorimetric Heats of Adsorption and Adsorption Isotherms. 3. Mixtures of CH4 and C2H6 in Silicalite and Mixtures of CO2 and C2H6 in NaX. Langmuir 1997, 13, 4333–4341. [Google Scholar] [CrossRef]
- Rouquerol, J.; Rouquerol, F.; Llewellyn, P.; Maurin, G.; Sing, K.S.W. Adsorption by Powders and Porous Solids: Principles, Methodology and Applications; Academic Press: Waltham, MA, USA, 2013. [Google Scholar]
- Calorimetry and Thermal Methods in Catalysis; Auroux, A. (Ed.) Springer: Berlin&Heidelberg, 2013; Volume 154.
- Otero Areán, C.; Manoilova, O.V.; Turnes Palomino, G.; Rodriguez Delgado, M.; Tsyganenko, A.A.; Bonelli, B.; Garrone, E. Variable-temperature infrared spectroscopy: An access to adsorption thermodynamics of weakly interacting systems. Phys. Chem. Chem. Phys. 2002, 4, 5713–5715. [Google Scholar] [CrossRef]
- Chafik, T.; Darir, A.; Achak, O.; Carvalho, A.P.; Pires, J. Determination of the heat effects involved during toluene vapor adsorption and desorption from microporous activated carbon. Comptes Rendus Chim. 2012, 15, 474–481. [Google Scholar] [CrossRef]
- Ruthven, D.M. Principles of Adsorption and Adsorption Processes; John Wiley & Sons: Weinheim, Germany, 1984. [Google Scholar]
- Eley, D.D.; Pines, H.; Weisz, P.B. Advances in Catalysis; Academic Press: Waltham, MA, USA, 1967. [Google Scholar]
- Niwa, M.; Katada, N.; Sawa, M.; Murakami, Y. Temperature-programmed desorption of ammonia with readsorption based on the derived theoretical equation. J. Phys. Chem. 1995, 99, 8812–8816. [Google Scholar] [CrossRef]
- Hachimi, A.; Chafik, T.; Bianchi, D. Adsorption models and heat of adsorption of adsorbed ortho di-methyl benzene species on silica by using Temperature Programmed Adsorption Equilibrium methods. Appl. Catal. A 2008, 335, 220–229. [Google Scholar] [CrossRef]
- Chabanel, C.; Nerriere, L.; Pean, A. Composés organiques volatils : réduction des émissions de COV dans l’industrie. Avalable online: http://www.ademe.fr/composes-organiques-volatils-reduction-emissions-cov-lindustrie (accessed on 28 March 2015).
- Delage, F.; Pré, P.; Le Cloirec, P. Mass Transfer and Warming during Adsorption of High Concentrations of VOCs on an Activated Carbon Bed: Experimental and Theoretical Analysis. Environ. Sci. Technol. 2000, 34, 4816–4821. [Google Scholar] [CrossRef]
- Le Cloirec, P.; Pré, P.; Delage, F.; Giraudet, S. Visualization of the exothermal VOC adsorption in a fixed-bed activated carbon adsorber. Environ. Technol. 2012, 33, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Zaitan, H.; Chafik, T. FTIR determination of adsorption characteristics for volatile organic compounds removal on diatomite mineral compared to commercial silica. Comptes Rendus Chim. 2005, 8, 1701–1708. [Google Scholar] [CrossRef]
- Zaitan, H.; Feronnato, C.; Bianchi, D.; Achak, O.; Chafik, T. Etude des propriétés texturales et adsorbantes d’une diatomite marocaine : Application au traitement d’air charge d’un polluant de type compose organique volatil. Ann. Chim. 2006, 31, 183–196. (In French) [Google Scholar] [CrossRef]
- Bianchi, D.; Gass, J.L.; Bouly, C.; Maret, D. Determination of Efficiency of Exhaust Gas Catalyst by F.T.I.R. Spectroscopy; SAE International: Warrendale, PA, USA, 1991. [Google Scholar]
- Kim, D.J.; Kim, J.M.; Yie, J.E.; Seo, S.G.; Kim, S.-C. Adsorption and conversion of various hydrocarbons on monolithic hydrocarbon adsorber. J. Colloid Interface Sci. 2004, 274, 538–542. [Google Scholar] [CrossRef] [PubMed]
- Chafik, T.; Dulaurent, O.; Gass, J.L.; Bianchi, D. Heat of Adsorption of Carbon Monoxide on a Pt/Rh/CeO 2/Al2O3, Three-Way Catalyst Using in-Situ Infrared Spectroscopy at High Temperatures. J. Catal. 1998, 179, 503–514. [Google Scholar] [CrossRef]
- Zeradine, S.; Bourane, A.; Bianchi, D. Comparison of the Coverage of the Linear CO Species on Cu/Al2O3 Measured under Adsorption Equilibrium Conditions by Using FTIR and Mass Spectroscopy. J. Phys. Chem. B 2001, 105, 7254–7257. [Google Scholar] [CrossRef]
- Rouquerol, F.; Rouquerol, J.; Sing, K.S.W. Adsorption by Powders and Porous Solids Principles, Methodology, and Applications; Academic Press: Waltham, MA, USA, 1999. [Google Scholar]
- Unger, K.K.; Rodríguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Characterization of Porous Solids II; Elsevier: Amsterdam, The Netherlands, 1991. [Google Scholar]
- Gregg, S.J.; Sing, K.S.W. Adsorption, Surface Area and Porosity, 2nd ed.; Academic Press: London, UK, 1982. [Google Scholar]
- Groen, J.C.; Peffer, L.A.A.; Pérez-Ramı́rez, J. Pore size determination in modified micro- and mesoporous materials. Pitfalls and limitations in gas adsorption data analysis. Microporous Mesoporous Mater. 2003, 60, 1–17. [Google Scholar] [CrossRef]
- Chevalier, S.; Franck, R.; Lambert, J.F.; Barthomeuf, D.; Suquet, H. Characterization of the porous structure and cracking activity of Al-pillared saponites. Appl. Catal. A 1994, 110, 153–165. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Corma, A.; Corell, C.; Pérez-Pariente, J.; Guil, J.M.; Guil-López, R.; Nicolopoulos, S.; Calbet, J.G.; Vallet-Regi, M. Adsorption and catalytic properties of MCM-22: The influence of zeolite structure. Zeolites 1996, 16, 7–14. [Google Scholar] [CrossRef]
- Pennell, K.D.; Rhue, R.D.; Rao, P.S.C.; Johnston, C.T. Vapor-phase sorption of p-xylene and water on soils and clay minerals. Environ. Sci. Technol. 1992, 26, 756–763. [Google Scholar] [CrossRef]
- Benkhedda, J.; Jaubert, J.-N.; Barth, D.; Perrin, L.; Bailly, M. Adsorption isotherms of m-xylene on activated carbon: measurements and correlation with different models. J. Chem. Thermodyn. 2000, 32, 401–411. [Google Scholar] [CrossRef]
- Wang, C.-M.; Chang, K.-S.; Chung, T.-W.; Wu, H. Adsorption Equilibria of Aromatic Compounds on Activated Carbon, Silica Gel, and 13X Zeolite. J. Chem. Eng. Data 2004, 49, 527–531. [Google Scholar] [CrossRef]
- Huang, Q.; Vinh-Thang, H.; Malekian, A.; Eić, M.; Trong-On, D.; Kaliaguine, S. Adsorption of n-heptane, toluene and o-xylene on mesoporous UL-ZSM5 materials. Microporous Mesoporous Mater. 2006, 87, 224–234. [Google Scholar] [CrossRef]
- Chiang, A.S.T.; Lee, C.-K.; Chang, Z.-H. Adsorption and diffusion of aromatics in AIPO4-5. Zeolites 1991, 11, 380–386. [Google Scholar] [CrossRef]
- Derrouiche, S.; Bianchi, D. Heats of Adsorption Using Temperature Programmed Adsorption Equilibrium: Application to the Adsorption of CO on Cu/Al2O3 and H2 on Pt/Al2O3. Langmuir 2004, 20, 4489–4497. [Google Scholar] [CrossRef] [PubMed]
- Langmuir, I. The Constitution and Fundamental Properties of Solids and Liquids. Part I. Solids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef]
- Freundlich, H. Ueber die Adsorption in Loesungen; Wilhelm Engelmann: Leipzig, Germany, 1906. [Google Scholar]
- Gundry, P.M.; Tompkins, F.C. Chemisorption of gases on metals. Q Rev. Chem. Soc. 1960, 14, 257–291. [Google Scholar] [CrossRef]
- Dulaurent, O.; Bianchi, D. Adsorption isobars for CO on a Pt/Al2O3 catalyst at high temperatures using FTIR spectroscopy: isosteric heat of adsorption and adsorption model. Appl. Catal. A 2000, 196, 271–280. [Google Scholar] [CrossRef]
- Yoshimoto, R.; Hara, K.; Okumura, K.; Katada, N.; Niwa, M. Analysis of Toluene Adsorption on Na-Form Zeolite with a Temperature-Programmed Desorption Method. J. Phys. Chem. C 2007, 111, 1474–1479. [Google Scholar] [CrossRef]
- Joly, J.-P.; Perrard, A. Determination of the Heat of Adsorption of Ammonia on Zeolites from Temperature-Programmed Desorption Experiments. Langmuir 2001, 17, 1538–1542. [Google Scholar] [CrossRef]
- Kanervo, J.; Keskitalo, T.; Slioor, R.; Krause, A. Temperature-programmed desorption as a tool to extract quantitative kinetic or energetic information for porous catalysts. J. Catal. 2006, 238, 382–393. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korrir, A.; Kasmi, A.E.; Assebban, M.; Souikny, A.; Haffane, S.; Achak, O.; Chafik, T. Non-Calorimetric Determination of the Adsorption Heat of Volatile Organic Compounds under Dynamic Conditions. Catalysts 2015, 5, 653-670. https://doi.org/10.3390/catal5020653
Korrir A, Kasmi AE, Assebban M, Souikny A, Haffane S, Achak O, Chafik T. Non-Calorimetric Determination of the Adsorption Heat of Volatile Organic Compounds under Dynamic Conditions. Catalysts. 2015; 5(2):653-670. https://doi.org/10.3390/catal5020653
Chicago/Turabian StyleKorrir, Abdelhamid, Achraf El Kasmi, Mhamed Assebban, Ahmed Souikny, Soukaina Haffane, Ouafae Achak, and Tarik Chafik. 2015. "Non-Calorimetric Determination of the Adsorption Heat of Volatile Organic Compounds under Dynamic Conditions" Catalysts 5, no. 2: 653-670. https://doi.org/10.3390/catal5020653





