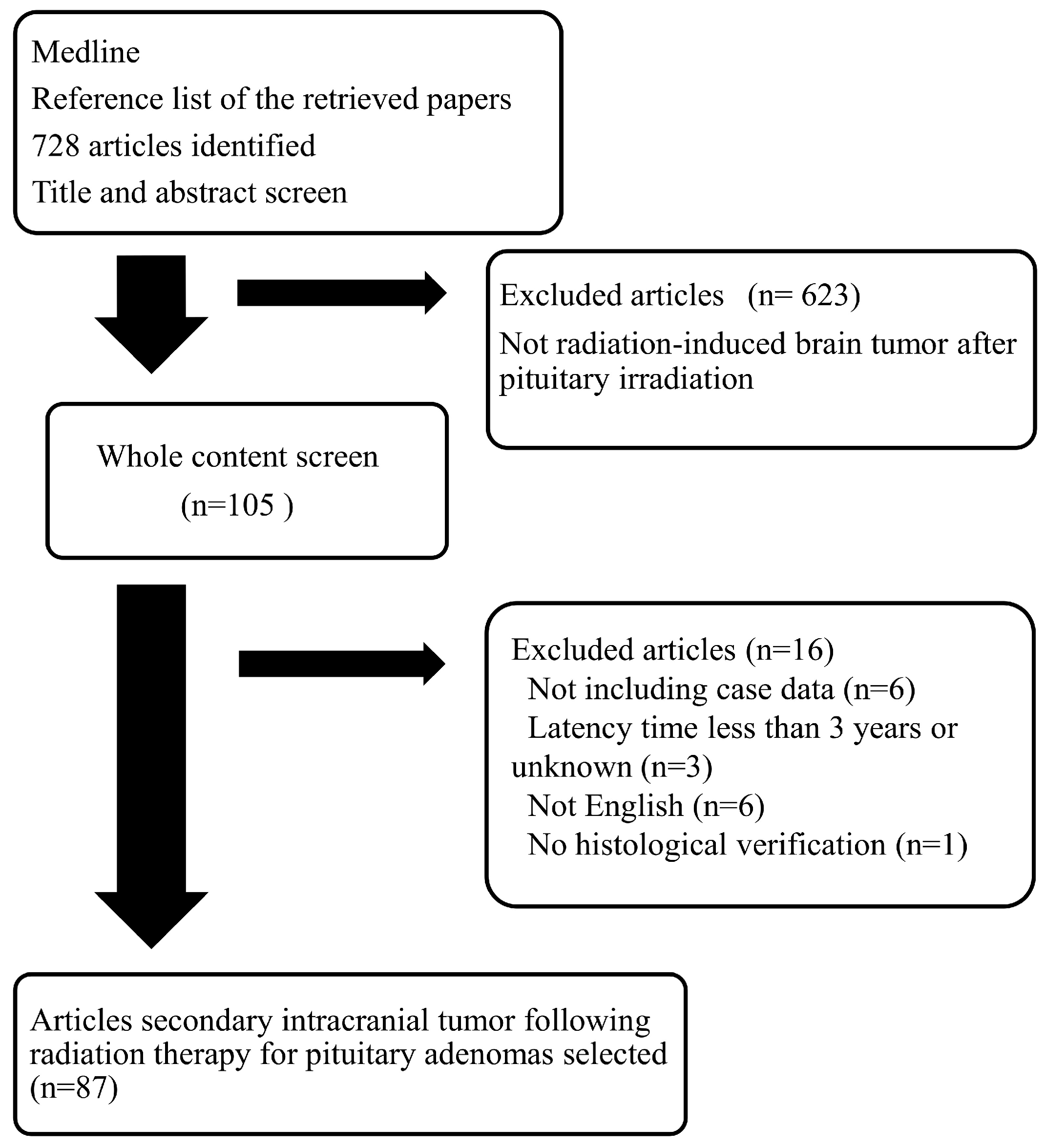
Secondary Intracranial Tumors Following Radiotherapy for Pituitary Adenomas: A Systematic Review
Abstract
:1. Introduction
2. Results
2.1. The Incidence of Intracranial Tumors Following Radiotherapy to Pituitary Adenomas Reported in the Literature
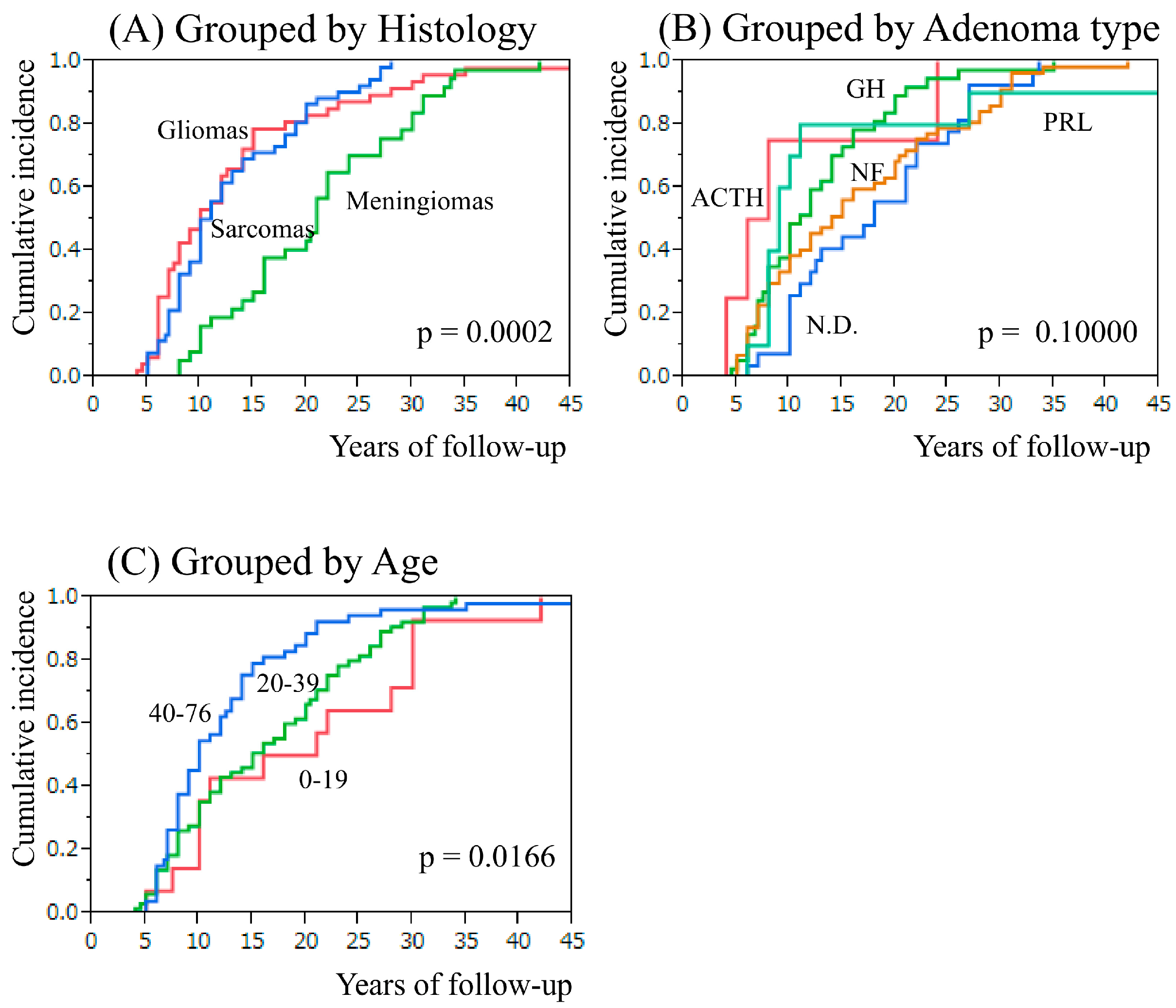
2.2. Secondary Intracranial Tumor and Adenoma Type or Age at Pituitary Irradiation
2.3. Irradiation Method and Secondary Tumor Location
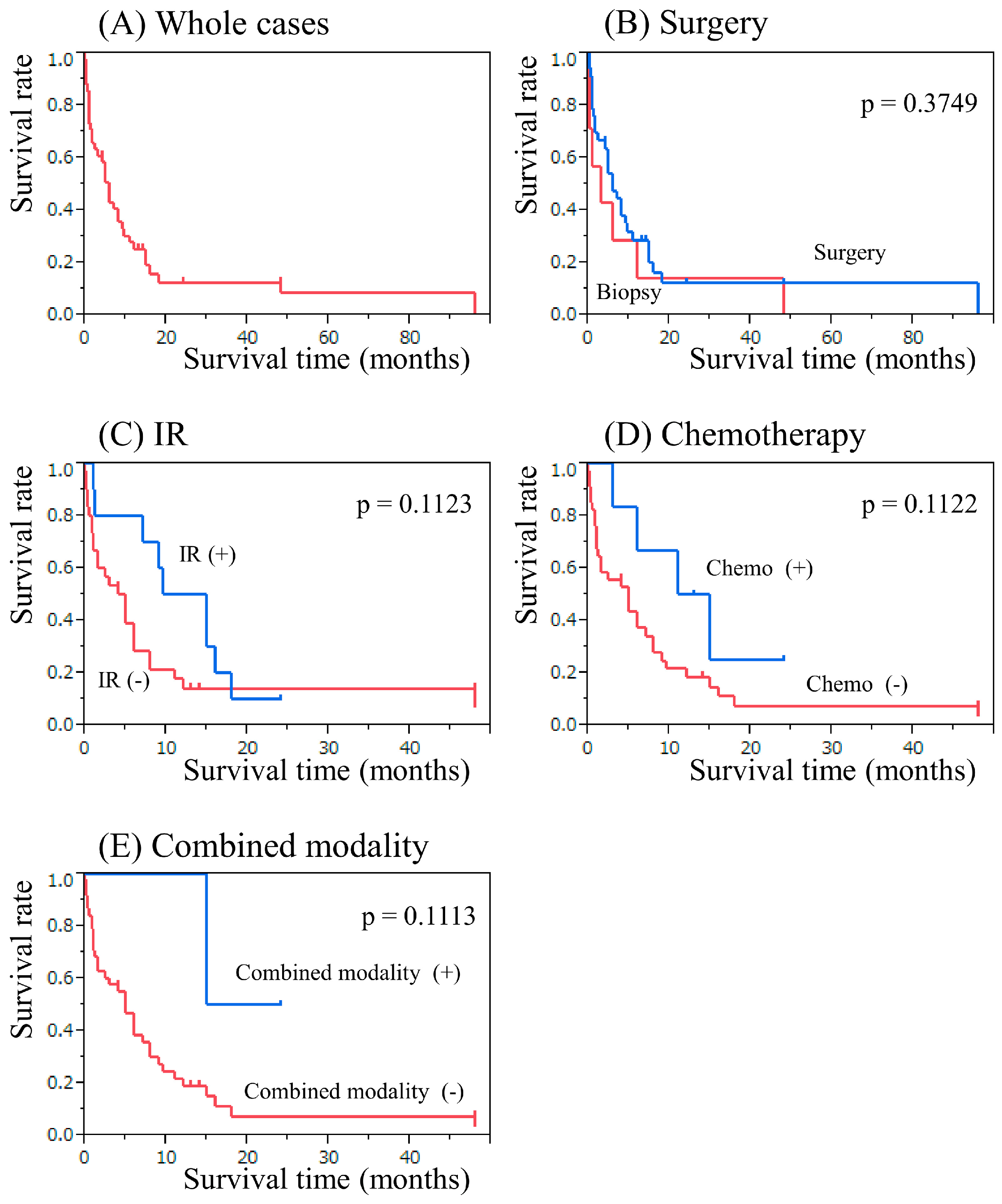
2.4. The Result of Secondary Neuroepithelial Tumor Therapy
2.5. The Results of Secondary Meningioma and Sarcoma Therapy
3. Discussion
4. Conclusions
Supplementary Materials
Acknowledgments
Conflicts of Interest
References
- Knosp, E.; Perneczky, A.; Kitz, K.; Grunert, P.; Wild, A. The need for adjunctive focused radiation therapy in pituitary adenomas. Acta Neurochir. Suppl. 1995, 63, 81–84. [Google Scholar] [PubMed]
- Kobayashi, T.; Tanaka, T.; Kida, Y. Stereotactic gamma radiosurgery of craniopharyngiomas. Pediatr. Neurosurg. 1994, 21, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Pui, C.H.; Pei, D.; Sandlund, J.T.; Campana, D.; Ribeiro, R.C.; Razzouk, B.I.; Rubnitz, J.E.; Howard, S.C.; Hijiya, N.; Jeha, S.; et al. Risk of adverse events after completion of therapy for childhood acute lymphoblastic leukemia. J. Clin. Oncol. 2005, 23, 7936–7941. [Google Scholar] [CrossRef] [PubMed]
- Reddick, W.E.; Glass, J.O.; Helton, K.J.; Langston, J.W.; Xiong, X.; Wu, S.; Pui, C.H. Prevalence of leukoencephalopathy in children treated for acute lymphoblastic leukemia with high-dose methotrexate. AJNR Am. J. Neuroradiol. 2005, 26, 1263–1269. [Google Scholar] [PubMed]
- Spiegler, B.J.; Kennedy, K.; Maze, R.; Greenberg, M.L.; Weitzman, S.; Hitzler, J.K.; Nathan, P.C. Comparison of long-term neurocognitive outcomes in young children with acute lymphoblastic leukemia treated with cranial radiation or high-dose or very high-dose intravenous methotrexate. J. Clin. Oncol. 2006, 24, 3858–3864. [Google Scholar] [CrossRef] [PubMed]
- Ushio, Y.; Arita, N.; Yoshimine, T.; Nagatani, M.; Mogami, H. Glioblastoma after radiotherapy for craniopharyngioma: Case report. Neurosurgery 1987, 21, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Relling, M.V.; Rubnitz, J.E.; Rivera, G.K.; Boyett, J.M.; Hancock, M.L.; Felix, C.A.; Kun, L.E.; Walter, A.W.; Evans, W.E.; Pui, C.H. High incidence of secondary brain tumours after radiotherapy and antimetabolites. Lancet 1999, 354, 34–39. [Google Scholar] [CrossRef]
- Walter, A.W.; Hancock, M.L.; Pui, C.H.; Hudson, M.M.; Ochs, J.S.; Rivera, G.K.; Pratt, C.B.; Boyett, J.M.; Kun, L.E. Secondary brain tumors in children treated for acute lymphoblastic leukemia at St Jude Children’s Research Hospital. J. Clin. Oncol. 1998, 16, 3761–3767. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, R.; Hayano, A.; Kanayama, T. Radiation-induced gliomas: A comprehensive review and meta-analysis. Neurosurg. Rev. 2016. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, R.; Hayano, A.; Kanayama, T. Radiation-induced meningiomas: An exhaustive review of the literature. World Neurosurg. 2016. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, R.; Hayano, A. Radiation-Induced Sarcomas of the central nervous system: A systematic review. World Neurosurg. 2016. [Google Scholar] [CrossRef] [PubMed]
- Cahan, W.G.; Woodard, H.Q.; Higinbotham, N.L.; Stewart, F.W.; Coley, B.L. Sarcoma arising in irradiated bone: Report of eleven cases. 1948. Cancer 1998, 82, 8–34. [Google Scholar] [CrossRef]
- Schrantz, J.L.; Araoz, C.A. Radiation induced meningeal fibrosarcoma. Arch. Pathol. 1972, 93, 26–31. [Google Scholar] [PubMed]
- Terry, R.D.; Hyams, V.J.; Davidoff, L.M. Combined nonmetastasizing fibrosarcoma and chromophobe tumor of the pituitary. Cancer 1959, 12, 791–798. [Google Scholar] [CrossRef]
- Meredith, J.M.; Mandeville, F.B.; Kay, S. Osteogenic sarcoma of the skull following roentgenray therapy for benign pituitary tumor. J. Neurosurg. 1960, 17, 792–799. [Google Scholar] [CrossRef]
- Newton, T.H.; Burhenne, H.J.; Palubinskas, A.J. Primary carcinoma of the pituitary. Am. J. Roentgenol. Radium. Ther. Nucl. Med. 1962, 87, 110–120. [Google Scholar] [PubMed]
- Goldberg, M.B.; Sheline, G.E.; Malamud, N. Malignant intracranial neoplasms, following radiation therapy for acromegaly. Radiology 1963, 80, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Greenhouse, A.H. Pituitary sarcoma: A possible consequence of radiation. JAMA 1964, 190, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Waltz, T.A.; Brownell, B. Sarcoma: A possible late result of effective radiation therapy for pituitary adenoma. Report of two cases. J. Neurosurg. 1966, 24, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.H.; Pool, J.L. The radiotherapy of pituitary chromophobe adenomas: An evaluation of indication, technic, and result. Radiology 1967, 89, 1005–1016. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.D.; Hill, D.M.; Lowy, C.; Fraser, T.R. Mortality in acromegaly. Q. J. Med. 1970, 39, 1–16. [Google Scholar] [PubMed]
- Lawrence, J.H.; Tobias, C.A.; Linfoot, J.A.; Born, J.L.; Lyman, J.T.; Chong, C.Y.; Manougian, E.; Wei, W.C. Successful treatment of acromegaly: Metabolic and clinical studies in 145 patients. J. Clin. Endocrinol. Metab. 1970, 31, 180–198. [Google Scholar] [CrossRef] [PubMed]
- Miura, K.; Iino, M.; Demura, H.; Demura, E.; Sasaki, C. Diagnosis and management of Cushing’ syndrome based on our 28 cases, with special reference to combined therapy of 60Co irradiation of the hypothalamus and reserpine. Nihon Rinsho 1970, 28, 1366–1378. [Google Scholar] [PubMed]
- Sparagana, M.; Eells, R.W.; Stefani, S.; Jablokow, V. Osteogenic sarcoma of the skull: A rare sequela of pituitary irradition. Cancer 1972, 29, 1376–1379. [Google Scholar] [CrossRef]
- Bogdanowicz, W.M.; Sachs, E., Jr. The possible role of radiation in oncogenesis of meningioma. Surg. Neurol. 1974, 2, 379–383. [Google Scholar] [PubMed]
- Stock, J.M.; Ghatak, N.R.; Oppenheimer, J.H. Unususpected meningioma in a patient with pituitary gigantism. Case report with autopsy findings. Metabolism 1975, 24, 767–775. [Google Scholar] [CrossRef]
- Amine, A.R.; Sugar, O. Suprasellar osteogenic sarcoma following radiation for pituitary adenoma. Case report. J. Neurosurg. 1976, 44, 88–91. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Vitale, J.C.; Slavin, R.E.; McQueen, J.D. Radiation-induced intracranial malignant fibrous histiocytoma. Cancer 1976, 37, 2960–2963. [Google Scholar] [CrossRef]
- Powell, H.C.; Marshall, L.F.; Ignelzi, R.J. Post-irradiation pituitary sarcoma. Acta Neuropathol. 1977, 39, 165–167. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, K.; Fayos, J.V. Pituitary fibrosarcoma secondary to radiation therapy. Cancer 1978, 42, 107–110. [Google Scholar] [CrossRef]
- Coppeto, J.R.; Roberts, M. Fibrosarcoma after proton-beam pituitary ablation. Arch. Neurol. 1979, 36, 380–381. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.; Namba, H.; Ishige, N.; Ohsato, K.; Nakamura, T.; Yamaura, A.; Makino, H. Pituitary fibrosarcoma secondary to radiation therapy for the treatment of chromophobe adenoma (author’s transl.). No Shinkei Geka 1980, 8, 605–614. (In Japanese) [Google Scholar] [PubMed]
- Martin, W.H.; Cail, W.S.; Morris, J.L.; Constable, W.C. Fibrosarcoma after high energy radiation therapy for pituitary adenoma. AJR Am. J. Roentgenol. 1980, 135, 1087–1090. [Google Scholar] [CrossRef] [PubMed]
- Vikhert, T.M.; Kasumova, S. Radiation fibrosarcoma of the pituitary. Zh. Vopr. Neirokhir. Im. N. N. Burdenko 1980, 57–58. [Google Scholar]
- Pieterse, S.; Dinning, T.A.; Blumbergs, P.C. Postirradiation sarcomatous transformation of a pituitary adenoma: A combined pituitary tumor. Case report. J. Neurosurg. 1982, 56, 283–286. [Google Scholar] [CrossRef] [PubMed]
- Spallone, A. Meningioma as a sequel of radiotherapy for pituitary adenoma. Neurochirurgia (Stuttg) 1982, 25, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.T. Intracranial malignant meningioma occurring after radiotherapy. Zhonghua Shen Jing Jing Shen Ke Za Zhi 1982, 15, 245–248. [Google Scholar] [PubMed]
- Piatt, J.H., Jr.; Blue, J.M.; Schold, S.C., Jr.; Burger, P.C. Glioblastoma multiforme after radiotherapy for acromegaly. Neurosurgery 1983, 13, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Farrell, M.A.; Kaufmann, J.C. Fibrosarcoma complicating irradiated pituitary adenoma. Surg. Neurol. 1984, 22, 277–284. [Google Scholar] [CrossRef]
- Kolodny, J.; Dluhy, R.G. Recurrent prolactinoma and meningioma following irradiation and bromocriptine treatment. Am. J. Med. 1985, 78, 153–155. [Google Scholar] [CrossRef]
- Okamoto, S.; Handa, H.; Yamashita, J.; Tokuriki, Y.; Abe, M. Post-irradiation brain tumors. Neurol. Med. Chir. (Tokyo) 1985, 25, 528–533. [Google Scholar] [CrossRef] [PubMed]
- Pages, A.; Pages, M.; Ramos, J.; Benezech, J. Radiation-induced intracranial fibrochondrosarcoma. J. Neurol. 1986, 233, 309–310. [Google Scholar] [CrossRef] [PubMed]
- Marus, G.; Levin, C.V.; Rutherfoord, G.S. Malignant glioma following radiotherapy for unrelated primary tumors. Cancer 1986, 58, 886–894. [Google Scholar] [CrossRef]
- Huang, C.I.; Chiou, W.H.; Ho, D.M. Oligodendroglioma occurring after radiation therapy for pituitary adenoma. J. Neurol. Neurosurg. Psychiatry 1987, 50, 1619–1624. [Google Scholar] [CrossRef] [PubMed]
- Kasantikul, V.; Shuangshoti, S.; Phonprasert, C. Intrasellar meningioma after radiotherapy for prolactinoma. J. Med. Assoc. Thai. 1988, 71, 524–527. [Google Scholar] [PubMed]
- Hufnagel, T.J.; Kim, J.H.; Lesser, R.; Miller, J.M.; Abrahams, J.J.; Piepmeier, J.; Manuelidis, E.E. Malignant glioma of the optic chiasm eight years after radiotherapy for prolactinoma. Arch. Ophthalmol. 1988, 106, 1701–1705. [Google Scholar] [CrossRef] [PubMed]
- Dierssen, G.; Alvarez, G.; Figols, J. Anaplastic astrocytomas associated with previous radiotherapy: Report of three cases. Neurosurgery 1988, 22, 1095–1097. [Google Scholar] [CrossRef] [PubMed]
- Romero, F.J.; Ortega, A.; Ibarra, B.; Piqueras, J.; Rovira, M. Post-radiation cranial malignant fibrous histiocytoma studied by CT. Comput. Med. Imaging Graph. 1989, 13, 191–194. [Google Scholar] [CrossRef]
- Amendola, B.E.; Amendola, M.A.; McClatchey, K.D.; Miller, C.H., Jr. Radiation-associated sarcoma: A review of 23 patients with postradiation sarcoma over a 50-year period. Am. J. Clin. Oncol. 1989, 12, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, K. Radiation-induced meningioma. Neurosurgery 1991, 28, 482. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, S.; Mealey, J., Jr.; Sartorius, C. Radiation-induced intracranial malignant gliomas. J. Neurosurg. 1989, 71, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Zampieri, P.; Zorat, P.L.; Mingrino, S.; Soattin, G.B. Radiation-associated cerebral gliomas. A report of two cases and review of the literature. J. Neurosurg. Sci. 1989, 33, 271–279. [Google Scholar] [PubMed]
- Suda, Y.; Mineura, K.; Kowada, M.; Ohishi, H. Malignant astrocytoma following radiotherapy in pituitary adenoma: Case report. No Shinkei Geka 1989, 17, 783–788. (In Japanese) [Google Scholar] [PubMed]
- Sato, K.; Hayashi, M.; Komai, T.; Kubota, T.; Kawano, H.; Handa, Y. Clinical and histological study of pituitary fibrosarcoma following radiotherapy for pituitary adenoma. Case report. Neurol. Med. Chir. (Tokyo) 1990, 30, 888–892. [Google Scholar] [CrossRef] [PubMed]
- Partington, M.D.; Davis, D.H. Radiation-induced meningioma after treatment for pituitary adenoma: Case report and literature review. Neurosurgery 1990, 26, 329–331. [Google Scholar] [CrossRef] [PubMed]
- Jones, A. Radiation oncogenesis in relation to the treatment of pituitary tumours. Clin. Endocrinol. (Oxf) 1991, 35, 379–397. [Google Scholar] [CrossRef] [PubMed]
- Sugita, Y.; Shigemori, M.; Miyagi, J.; Ochiai, S.; Lee, S.; Watanabe, T.; Abe, H.; Morimatsu, M. Radiation-induced osteosarcoma of the calvaria—Case report. Neurol. Med. Chir. (Tokyo) 1992, 32, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Brada, M.; Ford, D.; Ashley, S.; Bliss, J.M.; Crowley, S.; Mason, M.; Rajan, B.; Traish, D. Risk of second brain tumour after conservative surgery and radiotherapy for pituitary adenoma. BMJ 1992, 304, 1343–1346. [Google Scholar] [CrossRef] [PubMed]
- Tamura, M.; Misumi, S.; Kurosaki, S.; Shibasaki, T.; Ohye, C. Anaplastic astrocytoma 14 years after radiotherapy for pituitary adenoma. No Shinkei Geka 1992, 20, 493–497. (In Japanese) [Google Scholar] [PubMed]
- Hodges, L.C.; Smith, J.L.; Garrett, A.; Tate, S. Prevalence of glioblastoma multiforme in subjects with prior therapeutic radiation. J. Neurosci. Nurs. 1992, 24, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Tsang, R.W.; Laperriere, N.J.; Simpson, W.J.; Brierley, J.; Panzarella, T.; Smyth, H.S. Glioma arising after radiation therapy for pituitary adenoma. A report of four patients and estimation of risk. Cancer 1993, 72, 2227–2233. [Google Scholar] [CrossRef]
- Salvati, M.; Ciappetta, P.; Capoccia, G.; Capone, R.; Raco, A. Osteosarcoma of the skull. Report of a post-Paget and post-radiation case in an elderly woman. Neurosurg. Rev. 1994, 17, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.M.; Barker, F.G., 2nd; Larson, D.A.; Bollen, A.W.; Prados, M.D. Sarcomas subsequent to cranial irradiation. Neurosurgery 1995, 36, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Musa, B.S.; Pople, I.K.; Cummins, B.H. Intracranial meningiomas following irradiation—A growing problem? Br. J. Neurosurg. 1995, 9, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Niwa, J.; Hashi, K.; Minase, T. Radiation induced intracranial leiomyosarcoma: Its histopathological features. Acta Neurochir. (Wien) 1996, 138, 1470–1471. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Kanaan, I.; Rifai, A.; Tulbah, A.; Ghannam, N. An unusual treatment-related complication in a patient with growth hormone-secreting pituitary tumor. J. Clin. Endocrinol. Metab. 1997, 82, 2816–2820. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.B.; Rout, D.; Radhakrishnan, V.V. Suprasellar meningioma subsequent to treatment for a pituitary adenoma: Case report. Surg. Neurol. 1997, 47, 443–446. [Google Scholar] [CrossRef]
- Chauveinc, L.; Ricoul, M.; Sabatier, L.; Gaboriaud, G.; Srour, A.; Bertagna, X.; Dutrillaux, B. Dosimetric and cytogenetic studies of multiple radiation-induced meningiomas for a single patient. Radiother. Oncol. 1997, 43, 285–288. [Google Scholar] [CrossRef]
- Salvati, M.; Cervoni, L.; Puzzilli, F.; Bristot, R.; Delfini, R.; Gagliardi, F.M. High-dose radiation-induced meningiomas. Surg. Neurol. 1997, 47, 435–441, discussion 441–432. [Google Scholar] [CrossRef]
- Nishio, S.; Morioka, T.; Inamura, T.; Takeshita, I.; Fukui, M.; Sasaki, M.; Nakamura, K.; Wakisaka, S. Radiation-induced brain tumours: Potential late complications of radiation therapy for brain tumours. Acta Neurochir. (Wien) 1998, 140, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Simmons, N.E.; Laws, E.R., Jr. Glioma occurrence after sellar irradiation: Case report and review. Neurosurgery 1998, 42, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Alexander, M.J.; DeSalles, A.A.; Tomiyasu, U. Multiple radiation-induced intracranial lesions after treatment for pituitary adenoma. Case report. J. Neurosurg. 1998, 88, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Dallasta, L.M.; Martinez, J.; Nichols, L.; Hanzlick, R. Pathologic findings in a transplant donor. Arch. Intern. Med. 1999, 159, 2115–2116. [Google Scholar] [CrossRef] [PubMed]
- Brat, D.J.; James, C.D.; Jedlicka, A.E.; Connolly, D.C.; Chang, E.; Castellani, R.J.; Schmid, M.; Schiller, M.; Carson, D.A.; Burger, P.C. Molecular genetic alterations in radiation-induced astrocytomas. Am. J. Pathol. 1999, 154, 1431–1438. [Google Scholar] [CrossRef]
- Kato, N.; Kayama, T.; Sakurada, K.; Saino, M.; Kuroki, A. Radiation induced glioblastoma: A case report. No To Shinkei 2000, 52, 413–418. (In Japanese) [Google Scholar] [PubMed]
- Hill, M.D.; Mackenzie, I.; Mason, W.P. Radiation-induced glioma presenting as diffuse leptomeningeal gliomatosis: A case report. J. Neurooncol. 2001, 55, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Gnanalingham, K.K.; Chakraborty, A.; Galloway, M.; Revesz, T.; Powell, M. Osteosarcoma and fibrosarcoma caused by postoperative radiotherapy for a pituitary adenoma. Case report. J. Neurosurg. 2002, 96, 960–963. [Google Scholar] [CrossRef] [PubMed]
- Osipov, V.; Ho, K.C.; Krouwer, H.G.; Meyer, G.; Shidham, V.B. Post-radiation dedifferentiation of meningioma into osteosarcoma. BMC Cancer 2002, 2, 34. [Google Scholar] [CrossRef]
- Santoro, A.; Minniti, G.; Paolini, S.; Passacantilli, E.; Missori, P.; Frati, A.; Cantore, G.P. A typical tentorial meningioma 30 years after radiotherapy for a pituitary adenoma. Neurol. Sci. 2002, 22, 463–467. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, S.S.; Aldape, K.D.; Gagel, R.F.; Benjamin, R.S.; Trent, J.C.; McCutcheon, I.E. Sarcomatous change after sellar irradiation in a growth hormone-secreting pituitary adenoma. Can. J. Neurol. Sci. 2003, 30, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Loeffler, J.S.; Niemierko, A.; Chapman, P.H. Second tumors after radiosurgery: Tip of the iceberg or a bump in the road? Neurosurgery 2003, 52, 1436–1440, discussion 1440–1432. [Google Scholar] [CrossRef] [PubMed]
- Salvati, M.; Caroli, E.; Brogna, C.; Orlando, E.R.; Delfini, R. High-dose radiation-induced meningiomas. Report of five cases and critical review of the literature. Tumori 2003, 89, 443–447. [Google Scholar] [PubMed]
- Bembo, S.A.; Pasmantier, R.; Davis, R.P.; Xiong, Z.; Weiss, T.E. Osteogenic sarcoma of the sella after radiation treatment of a pituitary adenoma. Endocr. Pract. 2004, 10, 335–338. [Google Scholar] [CrossRef] [PubMed]
- Minniti, G.; Traish, D.; Ashley, S.; Gonsalves, A.; Brada, M. Risk of second brain tumor after conservative surgery and radiotherapy for pituitary adenoma: Update after an additional 10 years. J. Clin. Endocrinol. Metab. 2005, 90, 800–804. [Google Scholar] [CrossRef] [PubMed]
- Toh, C.H.; Wong, H.F.; Jung, S.M.; Wong, A.M. Radiation-induced skull base leiomyosarcoma presenting with intracerebral haemorrhage. Br. J. Radiol. 2007, 80, e212–e215. [Google Scholar] [CrossRef] [PubMed]
- Wu-Chen, W.Y.; Jacobs, D.A.; Volpe, N.J.; Dalmau, J.O.; Moster, M.L. Intracranial malignancies occurring more than 20 years after radiation therapy for pituitary adenoma. J. Neuroophthalmol. 2009, 29, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Kondziolka, D.; Kano, H.; Kanaan, H.; Madhok, R.; Mathieu, D.; Flickinger, J.C.; Lunsford, L.D. Stereotactic radiosurgery for radiation-induced meningiomas. Neurosurgery 2009, 64, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Alexandru, D.; Van Horn, D.K.; Bota, D.A. Secondary fibrosarcoma of the brain stem treated with cyclophosphamide and Imatinib. J. Neurooncol. 2010, 99, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Berkmann, S.; Tolnay, M.; Hanggi, D.; Ghaffari, A.; Gratzl, O. Sarcoma of the sella after radiotherapy for pituitary adenoma. Acta Neurochir. (Wien) 2010, 152, 1725–1735. [Google Scholar] [CrossRef] [PubMed]
- Sedney, C.L.; Morris, J.M.; Giannini, C.; Link, M.J.; Swetz, K.M. Radiation-associated sarcoma of the skull base after irradiation for pituitary adenoma. Rare Tumors 2012, 4, e7. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.M.; Ishii, Y.; Yamada, S.; Kuribayashi, S.; Kumita, S.; Matsuno, A. Advanced therapeutic strategy for radiation-induced osteosarcoma in the skull base: A case report and review. Radiat. Oncol. 2012, 7, 136. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Song, L.; Meng, Y. Malignant peripheral nerve sheath tumour following radiotherapy for pituitary adenoma. J. Clin. Neurosci. 2014, 21, 184–185. [Google Scholar] [CrossRef] [PubMed]
- Sasagawa, Y.; Tachibana, O.; Iizuka, H. Undifferentiated sarcoma of the cavernous sinus after gamma knife radiosurgery for pituitary adenoma. J. Clin. Neurosci. 2013, 20, 1152–1154. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Chang, H.; Gao, Y.; Cui, L. Tumor-to-tumor metastasis from pituitary carcinoma to radiation-induced meningioma. Neuropathology 2013, 33, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Kon, T.; Natsumeda, M.; Takahashi, H.; Taki, T.; Fujii, Y.; Yamanaka, R. Radiation-induced glioblastoma following radiotherapy for pituitary adenomas: Marked response to chemotherapy. J. Neurol. Neurophysiol. 2013, 4. [Google Scholar] [CrossRef]
- Sarkar, S.; Rajaratnam, S.; Backianathan, S.; Chacko, G.; Chacko, A.G. Radiation-induced opticochiasmatic glioblastoma multiforme following conventional radiotherapy for Cushing’s disease. Br. J. Neurosurg. 2014, 28, 510–512. [Google Scholar] [CrossRef] [PubMed]
- Abboud, S.E.; Wolansky, L.J.; Manjila, S.V.; Lo, S.S.; Arafah, B.M.; Selman, W.R.; Couce, M.E.; Rogers, L.R. Histologically proven radiation-induced brainstem glioma 93 months after external beam radiotherapy for pituitary macroadenoma: Radiation treatment dose and volume correlation. J. Neuroimaging 2015, 25, 674–676. [Google Scholar] [CrossRef] [PubMed]
- Snead, F.E.; Amdur, R.J.; Morris, C.G.; Mendenhall, W.M. Long-term outcomes of radiotherapy for pituitary adenomas. Int. J. Radiat. Oncol. Biol. Phys. 2008, 71, 994–998. [Google Scholar] [CrossRef] [PubMed]
- Norberg, L.; Johansson, R.; Rasmuson, T. Intracranial tumours after external fractionated radiotherapy for pituitary adenomas in northern Sweden. Acta Oncol. 2010, 49, 1276–1282. [Google Scholar] [CrossRef] [PubMed]
- Sattler, M.G.; van Beek, A.P.; Wolffenbuttel, B.H.; van den Berg, G.; Sluiter, W.J.; Langendijk, J.A.; van den Bergh, A.C. The incidence of second tumours and mortality in pituitary adenoma patients treated with postoperative radiotherapy versus surgery alone. Radiother. Oncol. 2012, 104, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Van Varsseveld, N.C.; van Bunderen, C.C.; Ubachs, D.H.; Franken, A.A.; Koppeschaar, H.P.; van der Lely, A.J.; Drent, M.L. Cerebrovascular events, secondary intracranial tumors, and mortality after radiotherapy for nonfunctioning pituitary adenomas: A subanalysis from the Dutch National Registry of Growth Hormone Treatment in Adults. J. Clin. Endocrinol. Metab. 2015, 100, 1104–1112. [Google Scholar] [CrossRef] [PubMed]
- Burman, P.; van Beek, A.P.; Biller, B.M.; Camacho-Hubner, C.; Mattsson, A.F. Radiotherapy, especially at young age, increases the risk for de novo brain tumors in patients treated for pituitary/sellar lesions. J. Clin. Endocrinol. Metab. 2017, 2, 1051–1058. [Google Scholar] [CrossRef]
- Arlen, M.; Higinbotham, N.L.; Huvos, A.G.; Marcove, R.C.; Miller, T.; Shah, I.C. Radiation-induced sarcoma of bone. Cancer 1971, 28, 1087–1099. [Google Scholar] [CrossRef]
- Sheline, G.E. Role of conventional radiation therapy in treatment of functional pituitary tumours. In Recent Advances in the Diagnosis and Treatment of Pituitary Tumours; Linfoot, J.A., Ed.; Raven Press: New York, NY, USA, 1979; pp. 289–313. [Google Scholar]
- Sheline, G.E.; Goldberg, M.B.; Feldman, R. Pituitary irradiation in acromegaly. Radiology 1961, 76, 70–75. [Google Scholar] [CrossRef]
- Alexander, L.; Appleton, D.; Hall, R.; Ross, W.M.; Wilkinson, R. Epidemiology of acromegaly in the Newcastle region. Clin. Endocrinol. (Oxf) 1980, 12, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Bengtsson, B.A.; Eden, S.; Ernest, I.; Oden, A.; Sjogren, B. Epidemiology and long-term survival in acromegaly. A study of 166 cases diagnosed between 1955 and 1984. Acta Med. Scand. 1988, 223, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Boguszewski, C.L.; Ayuk, J. Management of endocrine disease: Acromegaly and cancer: An old debate revisited. Eur. J. Endocrinol. 2016, 175, R147–R156. [Google Scholar] [CrossRef] [PubMed]
- Crowe, F.L.; Key, T.J.; Allen, N.E.; Appleby, P.N.; Overvad, K.; Gronbaek, H.; Tjonneland, A.; Halkjaer, J.; Dossus, L.; Boeing, H.; et al. A cross-sectional analysis of the associations between adult height, BMI and serum concentrations of IGF-I and IGFBP-1 -2 and -3 in the European Prospective Investigation into Cancer and Nutrition (EPIC). Ann. Hum. Biol. 2011, 38, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Roberts, D.L.; Dive, C.; Renehan, A.G. Biological mechanisms linking obesity and cancer risk: New perspectives. Annu. Rev. Med. 2010, 61, 301–316. [Google Scholar] [CrossRef] [PubMed]
- Zumkeller, W. The effect of insulin-like growth factors on brain myelination and their potential therapeutic application in myelination disorders. Eur. J. Paediatr. Neurol. 1997, 1, 91–101. [Google Scholar] [CrossRef]
- Glick, R.P.; Unterman, T.G.; Lacson, R. Identification of insulin-like growth factor (IGF) and glucose transporter-1 and -3 mRNA in CNS tumors. Regul. Pept. 1993, 48, 251–256. [Google Scholar] [CrossRef]
- Zumkeller, W.; Westphal, M. The IGF/IGFBP system in CNS malignancy. Mol. Pathol. 2001, 54, 227–229. [Google Scholar] [CrossRef] [PubMed]
- Santosh, V.; Arivazhagan, A.; Sreekanthreddy, P.; Srinivasan, H.; Thota, B.; Srividya, M.R.; Vrinda, M.; Sridevi, S.; Shailaja, B.C.; Samuel, C.; et al. Grade-specific expression of insulin-like growth factor-binding proteins-2, -3, and -5 in astrocytomas: IGFBP-3 emerges as a strong predictor of survival in patients with newly diagnosed glioblastoma. Cancer Epidemiol. Biomarkers Prev. 2010, 19, 1399–1408. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, H.; Shen, W.; Huang, H.; Hu, L.; Ramdas, L.; Zhou, Y.H.; Liao, W.S.; Fuller, G.N.; Zhang, W. Insulin-like growth factor binding protein 2 enhances glioblastoma invasion by activating invasion-enhancing genes. Cancer Res. 2003, 63, 4315–4321. [Google Scholar] [PubMed]
- Glick, R.P.; Lichtor, T.; Unterman, T.G. Insulin-like growth factors in central nervous system tumors. J. Neurooncol. 1997, 35, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Ciccarelli, E.; Razzore, P.; Gaia, D.; Todaro, C.; Longo, A.; Forni, M.; Ghe, C.; Camanni, F.; Muccioli, G.; Faccani, G.; et al. Hyperprolactinaemia and prolactin binding in benign intracranial tumours. J. Neurosurg. Sci. 2001, 45, 70–74. [Google Scholar] [PubMed]
- Muccioli, G.; Ghe, C.; Faccani, G.; Lanotte, M.; Forni, M.; Ciccarelli, E. Prolactin receptors in human meningiomas: Characterization and biological role. J. Endocrinol. 1997, 153, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Ducret, T.; Boudina, S.; Sorin, B.; Vacher, A.M.; Gourdou, I.; Liguoro, D.; Guerin, J.; Bresson-Bepoldin, L.; Vacher, P. Effects of prolactin on intracellular calcium concentration and cell proliferation in human glioma cells. Glia 2002, 38, 200–214. [Google Scholar] [CrossRef] [PubMed]
- Morioka, T.; Nishio, S.; Fukui, M.; Iwaki, T. Convexity meningioma with galactorrhea and hyperprolactinemia. A case report. Surg. Neurol. 1989, 31, 69–70. [Google Scholar] [CrossRef]
- Thorner, M.O.; Vance, M.L.; Horvarth, E.; Kovacs, K. The anterior pituitary. In Text book of Endocrinology; Wilson, J.D., Foster, D.W., Eds.; WB Saunders Co.: Philadelphia, PA, USA, 1992; pp. 221–311. [Google Scholar]
- Brachman, D.G.; Hallahan, D.E.; Beckett, M.A.; Yandell, D.W.; Weichselbaum, R.R. p53 gene mutations and abnormal retinoblastoma protein in radiation-induced human sarcomas. Cancer Res. 1991, 51, 6393–6396. [Google Scholar] [PubMed]
- Tada, M.; Sawamura, Y.; Abe, H.; Iggo, R. Homozygous p53 gene mutation in a radiation-induced glioblastoma 10 years after treatment for an intracranial germ cell tumor: Case report. Neurosurgery 1997, 40, 393–396. [Google Scholar] [CrossRef] [PubMed]
- Tarkkanen, M.; Wiklund, T.A.; Virolainen, M.J.; Larramendy, M.L.; Mandahl, N.; Mertens, F.; Blomqvist, C.P.; Tukiainen, E.J.; Miettinen, M.M.; Elomaa, A.I.; et al. Comparative genomic hybridization of postirradiation sarcomas. Cancer 2001, 92, 1992–1998. [Google Scholar] [CrossRef]
- Brassesco, M.S.; Valera, E.T.; Neder, L.; Pezuk, J.A.; Oliveira, R.S.; Scrideli, C.A.; Tone, L.G. Cytogenetic findings in pediatric radiation-induced atypical meningioma after treatment of medulloblastoma: Case report and review of the literature. J. Neurooncol. 2012, 110, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Shoshan, Y.; Chernova, O.; Juen, S.S.; Somerville, R.P.; Israel, Z.; Barnett, G.H.; Cowell, J.K. Radiation-induced meningioma: A distinct molecular genetic pattern? J. Neuropathol. Exp. Neurol. 2000, 59, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Mayer, R.; Sminia, P. Reirradiation tolerance of the human brain. Int. J. Radiat. Oncol. Biol. Phys. 2008, 70, 1350–1360. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.C.; Foote, R.L.; Coffey, R.J.; Gorman, D.A.; Earle, J.D.; Schomberg, P.J.; Kline, R.W. The role of stereotactic radiosurgery in the treatment of malignant skull base tumors. Int. J. Radiat. Oncol. Biol. Phys. 1997, 39, 977–981. [Google Scholar] [CrossRef]



| Secondary Tumor | Number of Cases | Age at Irradiation | Irradiation Dose (Gy) | Latency (Years) |
|---|---|---|---|---|
| Neuroepithelial Tumor | 48 | 39.7 ± 13.4 (35.7–43.6) | 51.3 ± 10.9 (47.5–55.2) | 13.3 ± 9.1 (10.6–15.9) |
| Meningioma | 37 | 31.3 ± 13.4 (26.7–36.0) | 50.9 ± 23.7 (41.7–60.1) | 20.9 ± 8.5 (18.1–23.8) |
| Sarcoma | 52 | 39.0 ± 15.0 (34.7–43.3) | 53.2 ± 22.0 (46.4–59.9) | 12.8 ± 6.5 (11.0–14.6) |
| Total | 137 | 37.2 ± 14.4 (34.7–39.7) | 52.0 ± 19.5 (48.2–55.8) | 15.2 ± 8.7 (13.7–16.6) |
| Radiation Technique | Neuroepithelial Tumor | Meningioma | Sarcoma | Total |
|---|---|---|---|---|
| Lateral opposing field | 10 (20.8) | 4 (10.8) | 9 (17.3) | 23 (16.7) |
| 3 or 4 field technique | 9 (18.7) | 5 (13.5) | 13 (25.0) | 27 (19.6) |
| Rotational technique | 2 (4.1) | 6 (16.2) | 2 (3.8) | 10 (7.2) |
| SRS | 1 (2.0) | 2 (5.4) | 3 (5.7) | 6 (4.3) |
| N.D. | 26 (54.1) | 20 (54.0) | 25 (48.0) | 71 (51.8) |
| Total | 48 | 37 | 52 | 137 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamanaka, R.; Abe, E.; Sato, T.; Hayano, A.; Takashima, Y. Secondary Intracranial Tumors Following Radiotherapy for Pituitary Adenomas: A Systematic Review. Cancers 2017, 9, 103. https://doi.org/10.3390/cancers9080103
Yamanaka R, Abe E, Sato T, Hayano A, Takashima Y. Secondary Intracranial Tumors Following Radiotherapy for Pituitary Adenomas: A Systematic Review. Cancers. 2017; 9(8):103. https://doi.org/10.3390/cancers9080103
Chicago/Turabian StyleYamanaka, Ryuya, Eisuke Abe, Toshiteru Sato, Azusa Hayano, and Yasuo Takashima. 2017. "Secondary Intracranial Tumors Following Radiotherapy for Pituitary Adenomas: A Systematic Review" Cancers 9, no. 8: 103. https://doi.org/10.3390/cancers9080103






