Androgen Receptor: A Complex Therapeutic Target for Breast Cancer
Abstract
:1. Introduction
2. Breast Cancer Classification
3. TNBC Sub-Classification
4. Androgen Receptor
5. Prognostic Value of the AR in Breast Cancer
6. AR as Predictor of Therapeutic Response
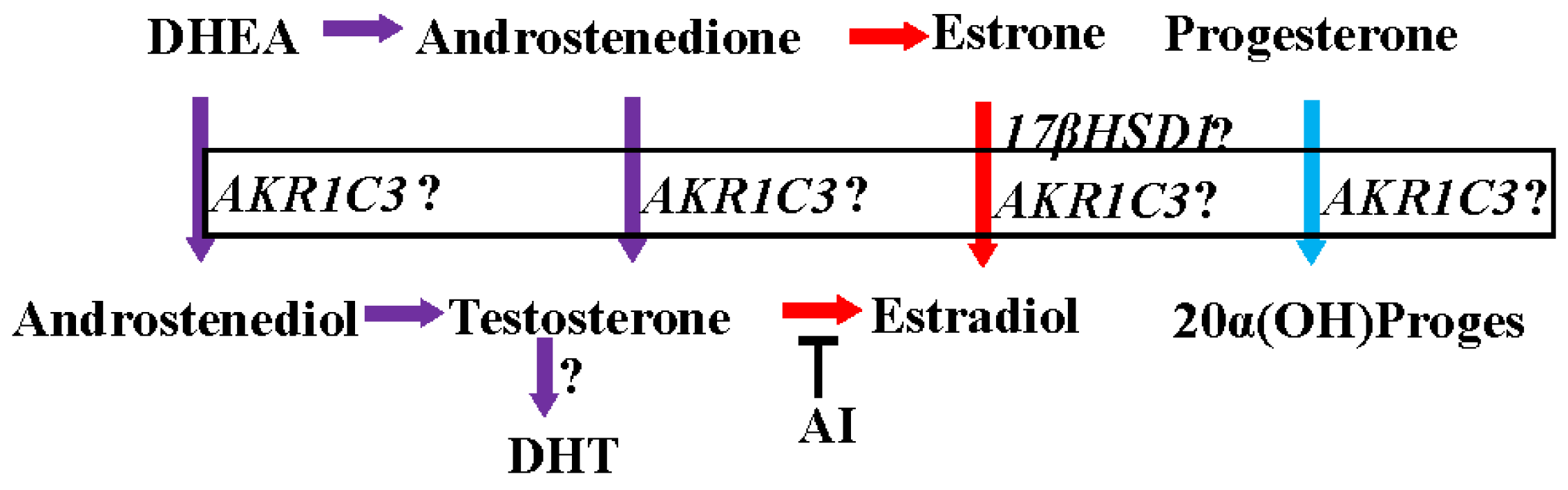
7. Role of Intracrine Androgen Synthesis in Breast Cancer
8. AR as Therapeutic Target for Breast Cancer
9. Preclinical Evidence Supporting the Beneficial Effects of AR agonists in Breast Cancer
10. Clinical Evidence Supporting the Use of AR Ligands in Hormone-Receptor-Positive Breast Cancer
11. Clinical Evidences Supporting the Use of AR Ligands in ER-Negative Breast Cancer
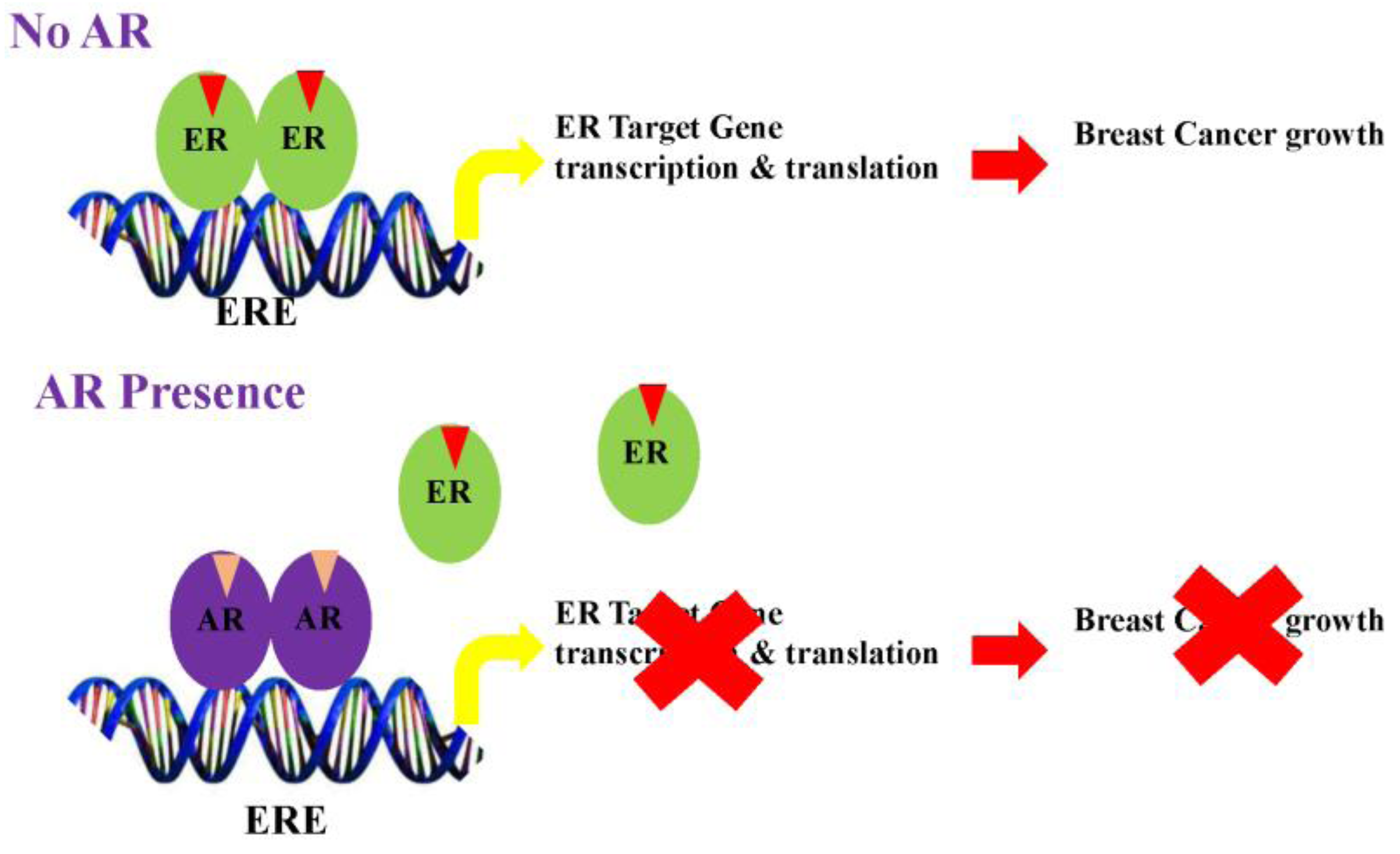
12. Mechanisms of Action of the AR in Breast Cancer
13. Conclusions
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2016. CA Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Sherry, M.M.; Greco, F.A.; Johnson, D.H.; Hainsworth, J.D. Metastatic breast cancer confined to the skeletal system. An indolent disease. Am. J. Med. 1986, 81, 381–386. [Google Scholar] [CrossRef]
- Perou, C.M.; Sorlie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; Rees, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A.; et al. Molecular portraits of human breast tumours. Nature 2000, 406, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, B.D.; Bauer, J.A.; Chen, X.; Sanders, M.E.; Chakravarthy, A.B.; Shyr, Y.; Pietenpol, J.A. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J. Clin. Investig. 2011, 121, 2750–2767. [Google Scholar] [CrossRef] [PubMed]
- Onitilo, A.A.; Engel, J.M.; Greenlee, R.T.; Mukesh, B.N. Breast cancer subtypes based on ER/PR and HER2 expression: Comparison of clinicopathologic features and survival. Clin. Med. Res. 2009, 7, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Cheang, M.C.; Chia, S.K.; Voduc, D.; Gao, D.; Leung, S.; Snider, J.; Watson, M.; Davies, S.; Bernard, P.S.; Parker, J.S.; et al. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J. Natl. Cancer Inst. 2009, 101, 736–750. [Google Scholar] [CrossRef] [PubMed]
- Sotiriou, C.; Pusztai, L. Gene-expression signatures in breast cancer. N. Engl. J. Med. 2009, 360, 790–800. [Google Scholar] [CrossRef] [PubMed]
- Loi, S.; Sotiriou, C.; Haibe-Kains, B.; Lallemand, F.; Conus, N.M.; Piccart, M.J.; Speed, T.P.; McArthur, G.A. Gene expression profiling identifies activated growth factor signaling in poor prognosis (Luminal-B) estrogen receptor positive breast cancer. BMC Med. Genom. 2009. [Google Scholar] [CrossRef] [PubMed]
- Wirapati, P.; Sotiriou, C.; Kunkel, S.; Farmer, P.; Pradervand, S.; Haibe-Kains, B.; Desmedt, C.; Ignatiadis, M.; Sengstag, T.; Schutz, F.; et al. Meta-analysis of gene expression profiles in breast cancer: Toward a unified understanding of breast cancer subtyping and prognosis signatures. Breast Cancer Res. 2008. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.L.; Taghian, A.G.; Katz, M.S.; Niemierko, A.; Abi Raad, R.F.; Boon, W.L.; Bellon, J.R.; Wong, J.S.; Smith, B.L.; Harris, J.R. Breast cancer subtype approximated by estrogen receptor, progesterone receptor, and HER-2 is associated with local and distant recurrence after breast-conserving therapy. J. Clin. Oncol. 2008, 26, 2373–2378. [Google Scholar] [CrossRef] [PubMed]
- Nam, B.H.; Kim, S.Y.; Han, H.S.; Kwon, Y.; Lee, K.S.; Kim, T.H.; Ro, J. Breast cancer subtypes and survival in patients with brain metastases. Breast Cancer Res. 2008. [Google Scholar] [CrossRef] [PubMed]
- Tran, B.; Bedard, P.L. Luminal-B breast cancer and novel therapeutic targets. Breast Cancer Res. 2011. [Google Scholar] [CrossRef] [PubMed]
- Leyland-Jones, B. Human epidermal growth factor receptor 2-positive breast cancer and central nervous system metastases. J. Clin. Oncol. 2009, 27, 5278–5286. [Google Scholar] [CrossRef] [PubMed]
- Prat, A.; Adamo, B.; Cheang, M.C.; Anders, C.K.; Carey, L.A.; Perou, C.M. Molecular characterization of basal-like and non-basal-like triple-negative breast cancer. Oncologist 2013, 18, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Prat, A.; Ellis, M.J.; Perou, C.M. Practical implications of gene-expression-based assays for breast oncologists. Nat. Rev. Clin. Oncol. 2012, 9, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature 2012, 490, 61–70. [Google Scholar]
- Jenster, G.; van der Korput, H.A.; Trapman, J.; Brinkmann, A.O. Identification of two transcription activation units in the N-terminal domain of the human androgen receptor. J. Biol. Chem. 1995, 270, 7341–7346. [Google Scholar] [PubMed]
- Verrijdt, G.; Tanner, T.; Moehren, U.; Callewaert, L.; Haelens, A.; Claessens, F. The androgen receptor DNA-binding domain determines androgen selectivity of transcriptional response. Biochem. Soc. Trans. 2006, 34, 1089–1094. [Google Scholar] [CrossRef] [PubMed]
- Matias, P.M.; Donner, P.; Coelho, R.; Thomaz, M.; Peixoto, C.; Macedo, S.; Otto, N.; Joschko, S.; Scholz, P.; Wegg, A.; et al. Structural evidence for ligand specificity in the binding domain of the human androgen receptor. Implications for pathogenic gene mutations. J. Biol. Chem. 2000, 275, 26164–26171. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Li, W.; Zhang, Y.; Yuan, X.; Xu, K.; Yu, J.; Chen, Z.; Beroukhim, R.; Wang, H.; Lupien, M.; et al. Androgen receptor regulates a distinct transcription program in androgen-independent prostate cancer. Cell 2009, 138, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Collins, L.C.; Cole, K.S.; Marotti, J.D.; Hu, R.; Schnitt, S.J.; Tamimi, R.M. Androgen receptor expression in breast cancer in relation to molecular phenotype: Results from the Nurses’ Health Study. Mod. Pathol. 2011, 24, 924–931. [Google Scholar] [CrossRef] [PubMed]
- Vera-Badillo, F.E.; Templeton, A.J.; de Gouveia, P.; Diaz-Padilla, I.; Bedard, P.L.; Al-Mubarak, M.; Seruga, B.; Tannock, I.F.; Ocana, A.; Amir, E. Androgen receptor expression and outcomes in early breast cancer: A systematic review and meta-analysis. J. Natl. Cancer Inst. 2014. [Google Scholar] [CrossRef] [PubMed]
- Sultana, A.; Idress, R.; Naqvi, Z.A.; Azam, I.; Khan, S.; Siddiqui, A.A.; Lalani, E.N. Expression of the Androgen Receptor, pAkt, and pPTEN in Breast Cancer and Their Potential in Prognostication. Transl. Oncol. 2014, 7, 355–362. [Google Scholar] [CrossRef] [PubMed]
- McNamara, K.M.; Yoda, T.; Nurani, A.M.; Shibahara, Y.; Miki, Y.; Wang, L.; Nakamura, Y.; Suzuki, K.; Yang, Y.; Abe, E.; et al. Androgenic pathways in the progression of triple-negative breast carcinoma: A comparison between aggressive and non-aggressive subtypes. Breast Cancer Res. Treat. 2014, 145, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Shi, Y.X.; Li, Z.M.; Jiang, W.Q. Expression and clinical significance of androgen receptor in triple negative breast cancer. Chin. J. Cancer 2010, 29, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Qu, Q.; Mao, Y.; Fei, X.C.; Shen, K.W. The impact of androgen receptor expression on breast cancer survival: A retrospective study and meta-analysis. PLoS ONE 2013, 8, e82650. [Google Scholar] [CrossRef] [PubMed]
- Agoff, S.N.; Swanson, P.E.; Linden, H.; Hawes, S.E.; Lawton, T.J. Androgen receptor expression in estrogen receptor-negative breast cancer. Immunohistochemical, clinical, and prognostic associations. Am. J. Clin. Pathol. 2003, 120, 725–731. [Google Scholar] [CrossRef] [PubMed]
- Pistelli, M.; Caramanti, M.; Biscotti, T.; Santinelli, A.; Pagliacci, A.; De Lisa, M.; Ballatore, Z.; Ridolfi, F.; Maccaroni, E.; Bracci, R.; et al. Androgen receptor expression in early triple-negative breast cancer: Clinical significance and prognostic associations. Cancers 2014, 6, 1351–1362. [Google Scholar] [CrossRef] [PubMed]
- Noh, S.; Kim, J.Y.; Koo, J.S. Metabolic differences in estrogen receptor-negative breast cancer based on androgen receptor status. Tumour Biol. 2014, 35, 8179–8192. [Google Scholar] [CrossRef] [PubMed]
- Doane, A.S.; Danso, M.; Lal, P.; Donaton, M.; Zhang, L.; Hudis, C.; Gerald, W.L. An estrogen receptor-negative breast cancer subset characterized by a hormonally regulated transcriptional program and response to androgen. Oncogene 2006, 25, 3994–4008. [Google Scholar] [CrossRef] [PubMed]
- Cha, Y.J.; Jung, W.H.; Koo, J.S. The clinicopathologic features of molecular apocrine breast cancer. Korean J. Pathol. 2012, 46, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Cochrane, D.R.; Bernales, S.; Jacobsen, B.M.; Cittelly, D.M.; Howe, E.N.; D’Amato, N.C.; Spoelstra, N.S.; Edgerton, S.M.; Jean, A.; Guerrero, J.; et al. Role of the Androgen Receptor in Breast Cancer and Preclinical Analysis of Enzalutamide. Breast Cancer Res. 2014. [Google Scholar] [CrossRef] [PubMed]
- Moore, N.L.; Buchanan, G.; Harris, J.M.; Selth, L.A.; Bianco-Miotto, T.; Hanson, A.R.; Birrell, S.N.; Butler, L.M.; Hickey, T.E.; Tilley, W.D. An androgen receptor mutation in the MDA-MB-453 cell line model of molecular apocrine breast cancer compromises receptor activity. Endocr. Relat. Cancer 2012, 19, 599–613. [Google Scholar] [CrossRef] [PubMed]
- Mills, A.M.; Chelsea, E.G.; Scott, M.W.; Christiana, M.B.; Atkins, K.A. Pure Apocrine Carcinomas Represent a Clinicopathologically Distinct Androgen Receptor-Positive Subset of Triple-Negative Breast Cancers. Am. J. Surg. Pathol. 2016, 40, 1109–1116. [Google Scholar] [CrossRef] [PubMed]
- Yepuru, M.; Wu, Z.; Kulkarni, A.; Yin, F.; Barrett, C.M.; Kim, J.; Steiner, M.S.; Miller, D.D.; Dalton, J.T.; Narayanan, R. Steroidogenic enzyme AKR1C3 is a novel androgen receptor-selective coactivator that promotes prostate cancer growth. Clin. Cancer Res. 2013, 19, 5613–5625. [Google Scholar] [CrossRef] [PubMed]
- McNamara, K.M.; Yoda, T.; Miki, Y.; Chanplakorn, N.; Wongwaisayawan, S.; Incharoen, P.; Kongdan, Y.; Wang, L.; Takagi, K.; Mayu, T.; et al. Androgenic pathway in triple negative invasive ductal tumors: Its correlation with tumor cell proliferation. Cancer Sci. 2013, 104, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Aleskandarany, M.A.; Abduljabbar, R.; Ashankyty, I.; Elmouna, A.; Jerjees, D.; Ali, S.; Buluwela, L.; Diez-Rodriguez, M.; Caldas, C.; Green, A.R.; et al. Prognostic significance of androgen receptor expression in invasive breast cancer: Transcriptomic and protein expression analysis. Breast Cancer Res. Treat. 2016, 159, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Safarpour, D.; Pakneshan, S.; Tavassoli, F.A. Androgen receptor (AR) expression in 400 breast carcinomas: Is routine AR assessment justified? Am. J. Cancer Res. 2014, 4, 353–368. [Google Scholar] [PubMed]
- Choi, J.E.; Kang, S.H.; Lee, S.J.; Bae, Y.K. Androgen receptor expression predicts decreased survival in early stage triple-negative breast cancer. Ann. Surg. Oncol. 2015, 22, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Elebro, K.; Borgquist, S.; Simonsson, M.; Markkula, A.; Jirstrom, K.; Ingvar, C.; Rose, C.; Jernstrom, H. Combined Androgen and Estrogen Receptor Status in Breast Cancer: Treatment Prediction and Prognosis in a Population-Based Prospective Cohort. Clin. Cancer Res. 2015, 21, 3640–3650. [Google Scholar] [CrossRef] [PubMed]
- Hilborn, E.; Gacic, J.; Fornander, T.; Nordenskjold, B.; Stal, O.; Jansson, A. Androgen receptor expression predicts beneficial tamoxifen response in oestrogen receptor-alpha-negative breast cancer. Br. J. Cancer 2016, 114, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Loibl, S.; Muller, B.M.; von Minckwitz, G.; Schwabe, M.; Roller, M.; Darb-Esfahani, S.; Ataseven, B.; du Bois, A.; Fissler-Eckhoff, A.; Gerber, B.; et al. Androgen receptor expression in primary breast cancer and its predictive and prognostic value in patients treated with neoadjuvant chemotherapy. Breast Cancer Res. Treat. 2011, 130, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Parker, J.S.; Mullins, M.; Cheang, M.C.; Leung, S.; Voduc, D.; Vickery, T.; Davies, S.; Fauron, C.; He, X.; Hu, Z.; et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J. Clin. Oncol. 2009, 27, 1160–1167. [Google Scholar] [CrossRef] [PubMed]
- Prat, A.; Lluch, A.; Albanell, J.; Barry, W.T.; Fan, C.; Chacon, J.I.; Parker, J.S.; Calvo, L.; Plazaola, A.; Arcusa, A.; et al. Predicting response and survival in chemotherapy-treated triple-negative breast cancer. Br. J. Cancer 2014, 111, 1532–1541. [Google Scholar] [CrossRef] [PubMed]
- Labrie, F. Intracrinology. Mol. Cell Endocrinol. 1991, 78, C113–C118. [Google Scholar] [CrossRef]
- Labrie, F. All sex steroids are made intracellularly in peripheral tissues by the mechanisms of intracrinology after menopause. J. Steroid Biochem. Mol. Biol. 2015, 145, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Labrie, F.; Luu-The, V.; Labrie, C.; Belanger, A.; Simard, J.; Lin, S.X.; Pelletier, G. Endocrine and intracrine sources of androgens in women: Inhibition of breast cancer and other roles of androgens and their precursor dehydroepiandrosterone. Endocr. Rev. 2003, 24, 152–182. [Google Scholar] [CrossRef] [PubMed]
- Labrie, F.; Belanger, A.; Luu-The, V.; Labrie, C.; Simard, J.; Cusan, L.; Gomez, J.L.; Candas, B. DHEA and the intracrine formation of androgens and estrogens in peripheral target tissues: Its role during aging. Steroids 1998, 63, 322–328. [Google Scholar] [CrossRef]
- Luu-The, V.; Labrie, F. The intracrine sex steroid biosynthesis pathways. Prog. Brain Res. 2010, 181, 177–192. [Google Scholar] [PubMed]
- Miyoshi, Y.; Ando, A.; Shiba, E.; Taguchi, T.; Tamaki, Y.; Noguchi, S. Involvement of up-regulation of 17beta-hydroxysteroid dehydrogenase type 1 in maintenance of intratumoral high estradiol levels in postmenopausal breast cancers. Int. J. Cancer 2001, 94, 685–689. [Google Scholar] [CrossRef] [PubMed]
- Chetrite, G.S.; Cortes-Prieto, J.; Philippe, J.C.; Wright, F.; Pasqualini, J.R. Comparison of estrogen concentrations, estrone sulfatase and aromatase activities in normal, and in cancerous, human breast tissues. J. Steroid Biochem. Mol. Biol. 2000, 72, 23–27. [Google Scholar] [CrossRef]
- Recchione, C.; Venturelli, E.; Manzari, A.; Cavalleri, A.; Martinetti, A.; Secreto, G. Testosterone, dihydrotestosterone and oestradiol levels in postmenopausal breast cancer tissues. J. Steroid Biochem. Mol. Biol. 1995, 52, 541–546. [Google Scholar] [CrossRef]
- Sharifi, N. Minireview: Androgen metabolism in castration-resistant prostate cancer. Mol. Endocrinol. 2013, 27, 708–714. [Google Scholar] [CrossRef] [PubMed]
- Fukami, M.; Homma, K.; Hasegawa, T.; Ogata, T. Backdoor pathway for dihydrotestosterone biosynthesis: Implications for normal and abnormal human sex development. Dev. Dyn. 2013, 242, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Kamrath, C.; Hochberg, Z.; Hartmann, M.F.; Remer, T.; Wudy, S.A. Increased activation of the alternative “backdoor” pathway in patients with 21-hydroxylase deficiency: Evidence from urinary steroid hormone analysis. J. Clin. Endocrinol. Metab. 2012, 97, E367–E375. [Google Scholar] [CrossRef] [PubMed]
- Mohler, J.L.; Titus, M.A.; Bai, S.; Kennerley, B.J.; Lih, F.B.; Tomer, K.B.; Wilson, E.M. Activation of the androgen receptor by intratumoral bioconversion of androstanediol to dihydrotestosterone in prostate cancer. Cancer Res. 2011, 71, 1486–1496. [Google Scholar] [PubMed]
- Mohler, J.L.; Titus, M.A.; Wilson, E.M. Potential prostate cancer drug target: Bioactivation of androstanediol by conversion to dihydrotestosterone. Clin. Cancer Res. 2011, 17, 5844–5849. [Google Scholar] [CrossRef] [PubMed]
- Lukacik, P.; Kavanagh, K.L.; Oppermann, U. Structure and function of human 17beta-hydroxysteroid dehydrogenases. Mol. Cell. Endocrinol. 2006, 248, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Moeller, G.; Adamski, J. Multifunctionality of human 17beta-hydroxysteroid dehydrogenases. Mol. Cell. Endocrinol. 2006, 248, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Penning, T.M. Hydroxysteroid dehydrogenases and pre-receptor regulation of steroid hormone action. Hum. Reprod. Update 2003, 9, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Walker, E.A.; Stewart, P.M. 11beta-hydroxysteroid dehydrogenase: Unexpected connections. Trends Endocrinol. Metab. 2003, 14, 334–339. [Google Scholar] [CrossRef]
- Nobel, S.; Abrahmsen, L.; Oppermann, U. Metabolic conversion as a pre-receptor control mechanism for lipophilic hormones. Eur. J. Biochem. 2001, 268, 4113–4125. [Google Scholar] [CrossRef] [PubMed]
- Nagasaki, S.; Miki, Y.; Akahira, J.; Suzuki, T.; Sasano, H. 17beta-hydroxysteroid dehydrogenases in human breast cancer. Ann. N. Y. Acad. Sci. 2009, 1155, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.K.; Steckelbroeck, S.; Fung, K.M.; Jones, A.N.; Penning, T.M. Characterization of a monoclonal antibody for human aldo-keto reductase AKR1C3 (type 2 3alpha-hydroxysteroid dehydrogenase/type 5 17beta-hydroxysteroid dehydrogenase); immunohistochemical detection in breast and prostate. Steroids 2004, 69, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Penning, T.M.; Burczynski, M.E.; Jez, J.M.; Lin, H.K.; Ma, H.; Moore, M.; Ratnam, K.; Palackal, N. Structure-function aspects and inhibitor design of type 5 17beta-hydroxysteroid dehydrogenase (AKR1C3). Mol. Cell. Endocrinol. 2001, 171, 137–149. [Google Scholar] [CrossRef]
- Penning, T.M.; Byrns, M.C. Steroid hormone transforming aldo-keto reductases and cancer. Ann. N. Y. Acad. Sci. 2009, 1155, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Zumoff, B.; Levin, J.; Rosenfeld, R.S.; Markham, M.; Strain, G.W.; Fukushima, D.K. Abnormal 24-hr mean plasma concentrations of dehydroisoandrosterone and dehydroisoandrosterone sulfate in women with primary operable breast cancer. Cancer Res. 1981, 41, 3360–3363. [Google Scholar] [PubMed]
- Li, S.; Yan, X.; Belanger, A.; Labrie, F. Prevention by dehydroepiandrosterone of the development of mammary carcinoma induced by 7,12-dimethylbenz(a)anthracene (DMBA) in the rat. Breast Cancer Res. Treat. 1994, 29, 203–217. [Google Scholar] [CrossRef] [PubMed]
- Adair, F.E.; Herrmann, J.B. The Use of Testosterone Propionate in the Treatment of Advanced Carcinoma of the Breast. Ann. Surg. 1946, 123, 1023–1035. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, J.B.; Adair, F.E. The effect of testosterone propionate on carcinoma of the female breast with soft tissue metastases. J. Clin. Endocrinol. Metab. 1946, 6, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Huggins, C.; Briziarelli, G.; Sutton, H., Jr. Rapid induction of mammary carcinoma in the rat and the influence of hormones on the tumors. J. Exp. Med. 1959, 109, 25–42. [Google Scholar] [CrossRef] [PubMed]
- Lubahn, D.B.; Joseph, D.R.; Sullivan, P.M.; Willard, H.F.; French, F.S.; Wilson, E.M. Cloning of human androgen receptor complementary DNA and localization to the X chromosome. Science 1988, 240, 327–330. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.S.; Kokontis, J.; Liao, S.T. Molecular cloning of human and rat complementary DNA encoding androgen receptors. Science 1988, 240, 324–326. [Google Scholar] [CrossRef] [PubMed]
- Poulin, R.; Baker, D.; Labrie, F. Androgens inhibit basal and estrogen-induced cell proliferation in the ZR-75–1 human breast cancer cell line. Breast Cancer Res. Treat. 1988, 12, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Dauvois, S.; Geng, C.S.; Levesque, C.; Merand, Y.; Labrie, F. Additive inhibitory effects of an androgen and the antiestrogen EM-170 on estradiol-stimulated growth of human ZR-75–1 breast tumors in athymic mice. Cancer Res. 1991, 51, 3131–3135. [Google Scholar] [PubMed]
- Cops, E.J.; Bianco-Miotto, T.; Moore, N.L.; Clarke, C.L.; Birrell, S.N.; Butler, L.M.; Tilley, W.D. Antiproliferative actions of the synthetic androgen, mibolerone, in breast cancer cells are mediated by both androgen and progesterone receptors. J. Steroid Biochem. Mol. Biol. 2008, 110, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Macedo, L.F.; Guo, Z.; Tilghman, S.L.; Sabnis, G.J.; Qiu, Y.; Brodie, A. Role of androgens on MCF-7 breast cancer cell growth and on the inhibitory effect of letrozole. Cancer Res. 2006, 66, 7775–7782. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, R.; Ahn, S.; Cheney, M.D.; Yepuru, M.; Miller, D.D.; Steiner, M.S.; Dalton, J.T. Selective androgen receptor modulators (SARMs) negatively regulate triple-negative breast cancer growth and epithelial:mesenchymal stem cell signaling. PLoS ONE 2014, 9, e103202. [Google Scholar] [CrossRef] [PubMed]
- Hall, R.E.; Birrell, S.N.; Tilley, W.D.; Sutherland, R.L. MDA-MB-453, an androgen-responsive human breast carcinoma cell line with high level androgen receptor expression. Eur. J. Cancer 1994, 30A, 484–490. [Google Scholar] [CrossRef]
- Barton, V.N.; D’Amato, N.C.; Gordon, M.A.; Lind, H.T.; Spoelstra, N.S.; Babbs, B.L.; Heinz, R.E.; Elias, A.; Jedlicka, P.; Jacobsen, B.M.; et al. Multiple molecular subtypes of triple-negative breast cancer critically rely on androgen receptor and respond to enzalutamide in vivo. Mol. Cancer Ther. 2015, 14, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, A.; Wang, B.; Picon-Ruiz, M.; Buchwald, P.; Ince, T.A. Vitamin D and androgen receptor-targeted therapy for triple-negative breast cancer. Breast Cancer Res. Treat. 2016, 157, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Santagata, S.; Thakkar, A.; Ergonul, A.; Wang, B.; Woo, T.; Hu, R.; Harrell, J.C.; McNamara, G.; Schwede, M.; Culhane, A.C.; et al. Taxonomy of breast cancer based on normal cell phenotype predicts outcome. J. Clin. Investig. 2014, 124, 859–870. [Google Scholar] [CrossRef] [PubMed]
- Adair, F.E.; Herrmann, J.B. The use of testosterone propionate in the treatment of advanced carcinoma of the breast. Ann Surg 1946, 123, 1023–1035. [Google Scholar] [CrossRef] [PubMed]
- Trams, G.; Maass, H. Specific binding of estradiol and dihydrotestosterone in human mammary cancers. Cancer Res. 1977, 37, 258–261. [Google Scholar] [PubMed]
- Bryan, R.M.; Mercer, R.J.; Bennett, R.C.; Rennie, G.C.; Lie, T.H.; Morgan, F.J. Androgen receptors in breast cancer. Cancer 1984, 54, 2436–2440. [Google Scholar] [CrossRef]
- Gordon, G. Anabolic-Androgenic Steroids; Springer-Verlag: New York, NY, USA, 1976. [Google Scholar]
- Bines, J.; Dienstmann, R.; Obadia, R.M.; Branco, L.G.; Quintella, D.C.; Castro, T.M.; Camacho, P.G.; Soares, F.A.; Costa, M.E. Activity of megestrol acetate in postmenopausal women with advanced breast cancer after nonsteroidal aromatase inhibitor failure: A phase II trial. Ann. Oncol. 2014, 25, 831–836. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, B.J. Fluoxymesterone therapy in advanced breast cancer. N. Engl. J. Med. 1958, 259, 673–675. [Google Scholar] [CrossRef] [PubMed]
- Tormey, D.C.; Lippman, M.E.; Edwards, B.K.; Cassidy, J.G. Evaluation of tamoxifen doses with and without fluoxymesterone in advanced breast cancer. Ann. Intern. Med. 1983, 98, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Muggia, F.M.; Cassieth, P.A.; Ochoa, M., Jr.; Flatow, F.A.; Gellhorn, A.; Hyman, G.A. Treatment of breast cancer with medroxyprogesterone acetate. Ann. Intern. Med. 1968, 68, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Ingle, J.N.; Twito, D.I.; Schaid, D.J.; Cullinan, S.A.; Krook, J.E.; Mailliard, J.A.; Tschetter, L.K.; Long, H.J.; Gerstner, J.G.; Windschitl, H.E.; et al. Combination hormonal therapy with tamoxifen plus fluoxymesterone versus tamoxifen alone in postmenopausal women with metastatic breast cancer. An updated analysis. Cancer 1991, 67, 886–891. [Google Scholar] [CrossRef]
- Birrell, S.N.; Roder, D.M.; Horsfall, D.J.; Bentel, J.M.; Tilley, W.D. Medroxyprogesterone acetate therapy in advanced breast cancer: The predictive value of androgen receptor expression. J. Clin. Oncol. 1995, 13, 1572–1577. [Google Scholar] [PubMed]
- Dalton, J.T.; Mukherjee, A.; Zhu, Z.; Kirkovsky, L.; Miller, D.D. Discovery of nonsteroidal androgens. Biochem. Biophys. Res. Commun. 1998, 244, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Dalton, J.T.; Barnette, K.G.; Bohl, C.E.; Hancock, M.L.; Rodriguez, D.; Dodson, S.T.; Morton, R.A.; Steiner, M.S. The selective androgen receptor modulator GTx-024 (enobosarm) improves lean body mass and physical function in healthy elderly men and postmenopausal women: Results of a double-blind, placebo-controlled phase II trial. J. Cachexia Sarcopenia Muscle 2011, 2, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.; Hwang, D.J.; Narayanan, R.; Miller, D.D.; Dalton, J.T. Effects of a novel selective androgen receptor modulator on dexamethasone-induced and hypogonadism-induced muscle atrophy. Endocrinology 2010, 151, 3706–3719. [Google Scholar] [CrossRef] [PubMed]
- Kearbey, J.D.; Gao, W.; Narayanan, R.; Fisher, S.J.; Wu, D.; Miller, D.D.; Dalton, J.T. Selective Androgen Receptor Modulator (SARM) treatment prevents bone loss and reduces body fat in ovariectomized rats. Pharm. Res. 2007, 24, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, R.; Coss, C.C.; Yepuru, M.; Kearbey, J.D.; Miller, D.D.; Dalton, J.T. Steroidal androgens and nonsteroidal, tissue-selective androgen receptor modulator, S-22, regulate androgen receptor function through distinct genomic and nongenomic signaling pathways. Mol. Endocrinol. 2008, 22, 2448–2465. [Google Scholar] [CrossRef] [PubMed]
- O’Shaughnessy, J.; Campone, M.; Brain, E.; Neven, P.; Hayes, D.; Bondarenko, I.; Griffin, T.W.; Martin, J.; De Porre, P.; Kheoh, T.; et al. Abiraterone acetate, exemestane or the combination in postmenopausal patients with estrogen receptor-positive metastatic breast cancer. Ann. Oncol. 2016, 27, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, H.; Russell, I.A.; Stark, R.; Rueda, O.M.; Hickey, T.E.; Tarulli, G.A.; Serandour, A.A.; Birrell, S.N.; Bruna, A.; Saadi, A.; et al. Progesterone receptor modulates ERalpha action in breast cancer. Nature 2015, 523, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Gucalp, A.; Tolaney, S.; Isakoff, S.J.; Ingle, J.N.; Liu, M.C.; Carey, L.A.; Blackwell, K.; Rugo, H.; Nabell, L.; Forero, A.; et al. Phase II Trial of Bicalutamide in Patients with Androgen Receptor-Positive, Estrogen Receptor-Negative Metastatic Breast Cancer. Clin. Cancer Res. 2013, 19, 5505–5512. [Google Scholar] [CrossRef] [PubMed]
- Arce-Salinas, C.; Riesco-Martinez, M.C.; Hanna, W.; Bedard, P.; Warner, E. Complete Response of Metastatic Androgen Receptor-Positive Breast Cancer to Bicalutamide: Case Report and Review of the Literature. J. Clin. Oncol. 2016, 34, e21–e24. [Google Scholar] [CrossRef] [PubMed]
- Tran, C.; Ouk, S.; Clegg, N.J.; Chen, Y.; Watson, P.A.; Arora, V.; Wongvipat, J.; Smith-Jones, P.M.; Yoo, D.; Kwon, A.; et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science 2009, 324, 787–790. [Google Scholar] [CrossRef] [PubMed]
- Bonnefoi, H.; Grellety, T.; Tredan, O.; Saghatchian, M.; Dalenc, F.; Mailliez, A.; L’Haridon, T.; Cottu, P.; Abadie-Lacourtoisie, S.; You, B.; et al. A phase II trial of abiraterone acetate plus prednisone in patients with triple-negative androgen receptor positive locally advanced or metastatic breast cancer (UCBG 12–1). Ann. Oncol. 2016, 27, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Peters, A.A.; Buchanan, G.; Ricciardelli, C.; Bianco-Miotto, T.; Centenera, M.M.; Harris, J.M.; Jindal, S.; Segara, D.; Jia, L.; Moore, N.L.; et al. Androgen receptor inhibits estrogen receptor-alpha activity and is prognostic in breast cancer. Cancer Res. 2009, 69, 6131–6140. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.L.; Macarthur, S.; Ross-Innes, C.S.; Tilley, W.D.; Neal, D.E.; Mills, I.G.; Carroll, J.S. Androgen receptor driven transcription in molecular apocrine breast cancer is mediated by FoxA1. EMBO J. 2011, 30, 3019–3027. [Google Scholar] [CrossRef] [PubMed]
- Adamczyk, A.; Niemiec, J.; Janecka, A.; Harazin-Lechowska, A.; Ambicka, A.; Grela-Wojewoda, A.; Domagala-Haduch, M.; Cedrych, I.; Majchrzyk, K.; Kruczak, A.; et al. Prognostic value of PIK3CA mutation status, PTEN and androgen receptor expression for metastasis-free survival in HER2-positive breast cancer patients treated with trastuzumab in adjuvant setting. Pol. J. Pathol. 2015, 66, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, B.D.; Bauer, J.A.; Schafer, J.M.; Pendleton, C.S.; Tang, L.; Johnson, K.C.; Chen, X.; Balko, J.M.; Gomez, H.; Arteaga, C.L.; et al. PIK3CA mutations in androgen receptor-positive triple negative breast cancer confer sensitivity to the combination of PI3K and androgen receptor inhibitors. Breast Cancer Res. 2014. [Google Scholar] [CrossRef] [PubMed]
- Hickey, T.E.; Irvine, C.M.; Dvinge, H.; Tarulli, G.A.; Hanson, A.R.; Ryan, N.K.; Pickering, M.A.; Birrell, S.N.; Hu, D.G.; Mackenzie, P.I.; et al. Expression of androgen receptor splice variants in clinical breast cancers. Oncotarget 2015, 6, 44728–44744. [Google Scholar] [PubMed]
| Reference | Ref | Summary |
|---|---|---|
| Pistelli et al., 2014 | [29] |
|
| Vera-Badillo et al., 2014 | [23] |
|
| Noh et al., 2014 | [30] |
|
| Sultana et al., 2014 | [24] |
|
| McNamara et al., 2014 | [25] |
|
| McNamara et al., 2013 | [37] |
|
| Luo et al., 2010 | [26] |
|
| Agoff et al., 2003 | [28] |
|
| Qu et al., 2013 | [27] |
|
| Reference | Ref | Summary |
|---|---|---|
| Hermann and Adair, 1947, 1946 | [71,84] |
|
| Bines et al., 2014 | [88] |
|
| Tormey et al.,1983 | [90] |
|
| Gucalp et al., 2013 | [101] |
|
| Arce-Salinas et al., 2016 | [102] |
|
| Bonnefoi et al., 2016 | [104] |
|
| O’Shaughnessy et al., 2016 | [99] |
|
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Narayanan, R.; Dalton, J.T. Androgen Receptor: A Complex Therapeutic Target for Breast Cancer. Cancers 2016, 8, 108. https://doi.org/10.3390/cancers8120108
Narayanan R, Dalton JT. Androgen Receptor: A Complex Therapeutic Target for Breast Cancer. Cancers. 2016; 8(12):108. https://doi.org/10.3390/cancers8120108
Chicago/Turabian StyleNarayanan, Ramesh, and James T. Dalton. 2016. "Androgen Receptor: A Complex Therapeutic Target for Breast Cancer" Cancers 8, no. 12: 108. https://doi.org/10.3390/cancers8120108





