Cancer-Related Constituents of Strawberry Jam as Compared with Fresh Fruit
Abstract
:1. Introduction
2. Experimental Section
2.1. Samples and Materials
2.2. Strawberry Jam Homemade Preparation
2.3. Determination of Antioxidant Phenolic Content
2.3.1. Extraction and Hydrolysis
2.3.2. HPLC Analysis
2.4. Determination of Carcinogenic Heat-Induced Compounds
2.4.1. Solid Phase Microextraction (SPME)
2.4.2. GC-MS Analysis
2.5. Statistical Analyses
3. Results and Discussion
3.1. Determination of Antioxidant Phenolic Content
| Samples | Ellagic Acid | Myricetin | Quercetin | Kaempferol |
|---|---|---|---|---|
| Fresh Fruit | 138.4 ± 0.23 a | 75.7 ± 0.15 a | 106.8 ± 0.33 a | 26.8 ± 0.25 a |
| Home-Made Jam | 107.0 ± 0.21 b | 3.2 ± 0.11 b | 16.5 ± 0.13 b | 10.5 ± 0.11 b |
| Commercial Jam | 86.4 ± 0.17 b | 2.8 ± 0.08 b | 1.7 ± 0.10 b | 7.7 ± 0.08 b |
3.2. Determination of Carcinogenic Heat-Induced Compounds
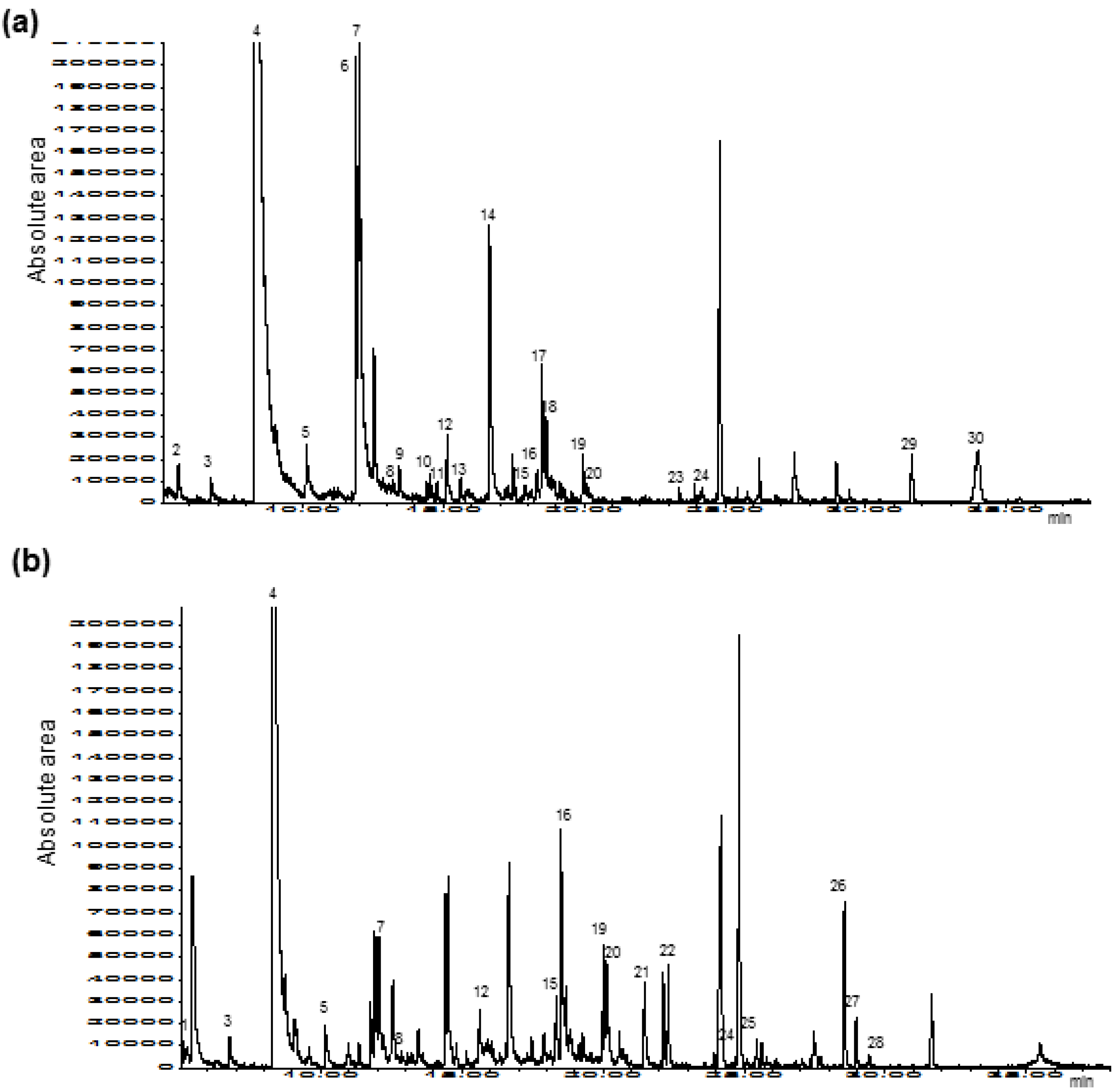
| Numbers | Compounds | Home-Made Strawberry Jam | Commercial Strawberry Jam |
|---|---|---|---|
| 1 | 2-Methyl-1-penten-3-one | n.d. a | 6,060,266 ± 0.103 |
| 2 | Butyric acid | 798,148 ± 0.231 | n.d. |
| 3 | Hexanal | 667,617 ± 0.213 a | 600,082 ± 0.111 a |
| 4 | Furfural | 105,220,694 ± 0.105 a | 39,931,568 ± 0.206 b |
| 5 | 2,5-Dimethyl-4-methoxy-3-(2H)-furanone (mesifurane) | 2,080,383 ± 0.182 a | 1,328,358 ± 0.232 b |
| 6 | 5-Methylfurfural | 7,981,259 ± 0.098 | n.d. |
| 7 | Benzaldehyde | 18,199,786 ± 0.114 a | 2,818,282 ± 0.157 b |
| 8 | 2-Acetylfuran | 1,132,193 ± 0.098 a | 25,701 ± 0.121 b |
| 9 | Acetophenone | 328,488 ± 0.214 | n.d. |
| 10 | Nonanal | 468,206 ± 0.036 | n.d. |
| 11 | Phenylacetaldehyde | 398,036 ± 0.213 | n.d. |
| 12 | 2-Methylbutyric acid | 464,182 ± 0.086 a | 426,165 ± 0.145 a |
| 13 | 2-Heptanone | 1,735,403 ± 0.201 | n.d. |
| 14 | Methyl 3-hydroxyhexanoate | 578,543 ± 0.193 | n.d. |
| 15 | Benzyl alcohol | 6,830,691 ± 0.231 a | 1 574,617 ± 0.254 b |
| 16 | Hexanoic acid | 521,249 ± 0.087 a | 5,347,177 ± 0.113 b |
| 17 | 2-Hexenylhexanoate | 853,129 ± 0.151 | n.d. |
| 18 | Ethyl 3-hydroxyhexanoate | 3,201,276 ± 0.183 | n.d. |
| 19 | (-)-α-Terpineol | 717,332 ± 0.075 a | 1,854,972 ± 0.165 b |
| 20 | (+)-α-Terpineol | 983,539 ± 0.189 a | 2,165,148 ± 0196 b |
| 21 | 2,4-Hexadienoic acid | n.d. | 813,206 ± 0.118 |
| 22 | Isobutyric acid | n.d. | 2,252,812 ± 0.126 |
| 23 | Furan | 717,332 ± 0.105 | n.d. |
| 24 | 5-Hydroxymethyl-2-furfural | 683,539 ± 0.143 a | 6,528,233 ± 0.177 b |
| 25 | Benzyl acetate | n.d. | 414,871 ± 0.123 |
| 26 | 2,4-Bis (1,1-dimethylethyl)-phenol (BHT) | n.d. | 2,501,944 ± 0.169 |
| 27 | ɣ-Butyrolactone | n.d. | 866,534 ± 0.088 |
| 28 | Methyl 3-hydroxyoctanoate | n.d. | 159,336 ± 0.092 |
| 29 | Farnesol | 451,790 ± 0.099 | n.d. |
| 30 | (−)-Furaneol | 6,019,384 ± 0.116 | n.d. |
4. Concluding Remarks
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Van Duyn, M.A.; Pivonka, E. Overview of the health benefits of fruits and vegetable consumption for the dietetics professional: Selected literature. J. Am. Diet Assoc. 2000, 100, 1511–1521. [Google Scholar] [CrossRef]
- Bjelke, E. Dietary vitamin A and human lung cancer. Int. J. Cancer 1975, 15, 561–565. [Google Scholar] [CrossRef] [PubMed]
- Steinmetz, K.A.; Potter, J.D. Vegetables, fruit, and cancer. II. Mechanisms. Cancer Causes Control 1991, 2, 427–442. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Joshi, V.K. An overview on strawberry [Fragaria x ananassa (Weston) Duchesne ex Rozier] wine production technology, composition, maturation and quality evaluation. Nat. Product. Rad. 2009, 8, 356–365. [Google Scholar]
- Losso, N.; Bansode, R.R.; Trappey, A.; Bawadi, H.A.; Truax, R. In vitro anti-proliferative actitivities of ellagic acid. J. Nutr. Biochem. 2004, 15, 672–678. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, B.A.; Geoffroy, O.; Willinghanm, M.C.; Re, G.G.; Nixon, D.W. p53/p21(WAF1/CIP1) expression and its possible role in G1 arrest and apoptosis in ellagic acid treated cancer cells. Cancer Letters 1999, 136, 215–221. [Google Scholar] [CrossRef]
- Sporn, M.B.; Liby, K.T. Cancer chemoprevention: Scientific promise, clinical uncertainty. Nat. Clin. Pract. Oncol. 2005, 2, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Russo, GL. Ins and outs of dietary phytochemicals in cancer chemoprevention. Biochem. Pharmacol. 2007, 74, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Orsolic, N.; Knezevic, A.H.; Sver, L.; Terzic, S.; Basic, I. Immunomodulatory and antimetastatic action of propolis and related polyphenolic compounds. J. Ethnopharm. 2004, 94, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Elattar, T.M.A.; Virji, AS. The inhibitory effects of curcumin, genistein, quercetin and cisplatin on the growth of oral cancer cells in vitro. Antic. Res. 2000, 20, 1733–1738. [Google Scholar]
- Pérez-Vizcaino, F.; Bishop-Bailley, D.; Lodi, F.; Duarte, J.; Cogolludo, A.; Moreno, L.; Bosca, L.; Mitchell, J.A.; Warner, T.D. The flavonoid quercetin induces apoptosis and inhibits JNK activation in intimal vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 2006, 346, 919–925. [Google Scholar] [CrossRef] [PubMed]
- De Ancos, B.; González, E.M.; Cano, M.P. Ellagic acid, vitamin C, and total phenolic contents and radical scavenging capacity affected by freezing and frozen storage in raspberry fruit. J. Agric. Food Chem. 2000, 48, 4565–4570. [Google Scholar] [CrossRef] [PubMed]
- Häkkinen, S.A.; Heinonen, M.; Kärenlampi, S.; Mykkänen, H.; Ruuskanen, J.; Törronën, A.R. Screening of selected flavonoids and phenolic acids in 19 berries. Food. Res. Internat. 1999, 32, 345–353. [Google Scholar] [CrossRef]
- Häkkinen, S.A.; Kärenlampi, S.; Mykkänen, H.; Heinonen, M.; Törronen, A.R. Ellagic acid content in berries: Influence of domestic processing and storage. Eur. Food Res. Technol. 2000, 212, 75–80. [Google Scholar]
- Häkkinen, S.A.; Kärenlampi, S.; Mykkänen, H.; Törronen, A.R. Influence of domestic processing and storage on flavonol contents in berries. J. Agric. Food Chem. 2000, 48, 2960–2965. [Google Scholar] [CrossRef] [PubMed]
- Schreier, P. Quantitative composition of volatile constituents in cultivated strawberries, Fragaria ananassa cv. Senga sengana, senga litessa and senga gourmella. J. Sci. Food Agric. 1980, 31, 487–494. [Google Scholar] [CrossRef]
- Hirvi, T. Mass fragmentographic and sensory analysis in the evaluation of the aroma of some strawberry varieties. Lebensm Wiss & Technol 1983, 16, 157–161. [Google Scholar]
- Latrasse, A. Fruit III. In Volatile Compounds in Foods and Beverages; Maarse, H., Marcel, D., Eds.; CRC Press: New York, NY, USA, 1991; pp. 329–387. [Google Scholar]
- Zabetakis, I.; Holden, M.A. Strawberry Flavor: Analysis and biosynthesis. J. Sci. Food Agric. 1997, 74, 421–434. [Google Scholar] [CrossRef]
- Schulbach, K.F.; Ruseff, R.L.; Sims, C.A. Changes in volatile sulphur compounds in strawberry puree during heating. J. Food Sci. 2004, 69, FCT268–FCT272. [Google Scholar]
- Jetti, R.R.; Yang, E.; Kurnianta, A.; Finn, C.; Qian, M.C. Quantification of selected aroma-active compounds in strawberries by headspace solid-phase microextraction gas chromatography and correlation with sensory descriptive analysis. J. Food Sci. 2007, 72, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Pérez, A.G.; Olías, R.; Sanz, C.; Olías, J.M. Furanones in strawberries: Evolution during ripening and postharvest shelf life. J. Agric. Food Chem. 1996, 44, 3620–3624. [Google Scholar] [CrossRef]
- Pyysalo, T.; Honkanen, E.; Hirvi, T. Volatiles of wild strawberries, Fragaria vesca l., compared to those of cultivated berries, Fragaria ananassa cv Senga sengana. J. Agric. Food Chem. 1979, 27, 19–22. [Google Scholar] [CrossRef]
- Sloan, J.L.; Bills, D.D.; Libbey, L.M. Heat-induced compounds in strawberries. J. Agric. Food Chem. 1969, 17, 1370–1372. [Google Scholar] [CrossRef]
- Barron, D.; Etiévant, P.X. The volatile constituents of strawberry jam. Z. Lebensm. Unter. Forsch A 1990, 191, 279–285. [Google Scholar] [CrossRef]
- Ames, J.M. Dietary Maillard reaction products: Implications for human health and disease. Czech. J. Food Sci. 2009, 27, S66–S69. [Google Scholar]
- Makris, D.P.; ROSSITER, J.T. Heat-induced, metal-catalyzed oxidative degradation of quercetin and rutin (quercetin 3-O-rhamnosylglucoside) in aqueous model systems. J. Agric. Food Chem. 2000, 48, 3830–3838. [Google Scholar] [CrossRef] [PubMed]
- Price, K.R.; Bacon, J.R.; Rhodes, M.J.C. Effect of storage and domestic processing on the content and composition of flavonol glucosides in onion (Allium cepa). J. Agric. Food Chem. 1997, 45, 938–942. [Google Scholar] [CrossRef]
- Amakura, Y.; Umino, Y.; Tsuji, S.; Tonogay, Y. Influence of jam processing on the radical scavenging activity and phenolic content in berries. J. Agric. Food Chem. 2001, 48, 6292–6297. [Google Scholar] [CrossRef]
- Zafrilla, P.; Ferreres, F.; Tomás-Barberán, F.A. Effect of processing and storage on the antioxidante ellagic acid derivatives and flavonoids in red raspberry (Rubus idaeus) jams. J. Agric. Food Chem. 2001, 49, 3651–3655. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, E.; Ito, T.; Odagirl, S. Sweet aroma components in three kinds of jam. Nippon Nogeïkogaku Kaishi 1982, 56, 101–108. [Google Scholar] [CrossRef]
- Hodge, J.E. Nonenzymatic Browning Reactions. In Symposium of Foods: The Chemistry and Physiology of Flavors; Schultz, H.W., Day, E.A., Libbey, L.M., Eds.; The AVI Publishing Co.: Mohan Garden, Near Nawada Metro Station, New Delhi, India, 1967; pp. 465–491. [Google Scholar]
- Georgilopoulos, D.N.; Gallois, A.N. Volatile flavour compounds in heated blackberry juices. Z. Lebensm. Unter. Forsch A 1987, 185, 299–306. [Google Scholar] [CrossRef]
- Mayerl, F.; Näf, R.; Thomas, A.F. 2,5-Dimethyl-4-hydroxy-3(2H)-furanone glucoside: Isolation from strawberries and synthesis. Phytochemistry 1989, 28, 631–633. [Google Scholar] [CrossRef]
- Hodge, J.E.; Fisher, B.E.; Nelson, E.C. Dicarbonyls, reductones, and heterocyclics produced by reactions of reducing sugars with secondary amine salts. Am. Soc. Brewing Chem. 1963, 84–92. [Google Scholar]
- Hayase, F.; Kim, S.B.; Kato, H. Maillard reaction products formed from d-glucose and glycine and the formation mechanisms of amides as major components. Agric. Biol. Chem. 1985, 49, 2337–2341. [Google Scholar] [CrossRef]
- Mevissen, L.; Baltes, W. Model reactions on roast aroma formation: VI. Volatile reaction products from the reaction of phenylalanine with glucose during cooking and roasting. Z. Lebensm. Unter Forsch. A 1988, 187, 209–214. [Google Scholar]
- Rosatella, A.A.; Simeonov, S.P.; Frade, R.F.M.; Afonso, C.A.M. 5-Hydroxymethylfurfural (HMF) as a building block platform: Biological properties, synthesis and synthetic applications. Green Chem. 2011, 13, 754–793. [Google Scholar] [CrossRef]
- Berry, S.K.; Gramshaw, J.W. Some new volatile compounds from the non-enzymic browning reaction of glucose-glutamic acid system. Z. Lebensm. Unter. Forsch. A 1986, 182, 219–223. [Google Scholar] [CrossRef]
- Hidalgo, A.; Pompei, C. Hydroxymethylfurfural and furosine reaction kinectics in tomato products. J. Agric. Food Chem. 2000, 48, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.C.; Shlyankevich, M.; Jeong, H.K.; Douglas, J.S.; Surh, Y.J. Bioactivation of 5-hydroxymethyl-2-furaldehyde to an electrophilic and mutagenic allylic sulfuric acid ester. Biochem. Biophys. Res. Comm 1995, 209, 996–1002. [Google Scholar] [CrossRef] [PubMed]
- Habu, T.; Flath, R.A.; Mon, T.R.; Morton, J.F. Volatile components of Rooibos tea (Aspalathus linearis). J. Agric. Food Chem. 1985, 33, 249–254. [Google Scholar] [CrossRef]
- Tatum, J.H.; Shaw, P.E.; Berry, R.E. Degradation products from ascorbic acid. J. Agric. Food Chem. 1969, 17, 38–40. [Google Scholar] [CrossRef]
- National Toxicology Program (NTP). Toxicology and Carcinogenesis Studies of y-Butyrolactone (CAS No. 96-48-0) in F344fN1 Rats and B6C3F Mice (Gavage Studies), 1992; Technical Report Series No. 406. NIH Publication No. 92-3137; U.S. Department of Health and Human Services, Public Health Service, National Institutes of Health: Research Triangle Park, NC, USA, 1992.
- Motshakeri, M.; Ghazali, H.M. Nutritional, phytochemical and commercial quality of Noni fruit: A multi-beneficial gift from nature. Trends Food Sci. Technol. 2015, 45, 118–129. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flores, G.; Ruiz del Castillo, M.L. Cancer-Related Constituents of Strawberry Jam as Compared with Fresh Fruit. Cancers 2016, 8, 16. https://doi.org/10.3390/cancers8010016
Flores G, Ruiz del Castillo ML. Cancer-Related Constituents of Strawberry Jam as Compared with Fresh Fruit. Cancers. 2016; 8(1):16. https://doi.org/10.3390/cancers8010016
Chicago/Turabian StyleFlores, Gema, and Maria Luisa Ruiz del Castillo. 2016. "Cancer-Related Constituents of Strawberry Jam as Compared with Fresh Fruit" Cancers 8, no. 1: 16. https://doi.org/10.3390/cancers8010016