Dosimetric Characteristics of a Two-Dimensional Diode Array Detector Irradiated with Passively Scattered Proton Beams
Abstract
:1. Introduction
2. Materials and Methods
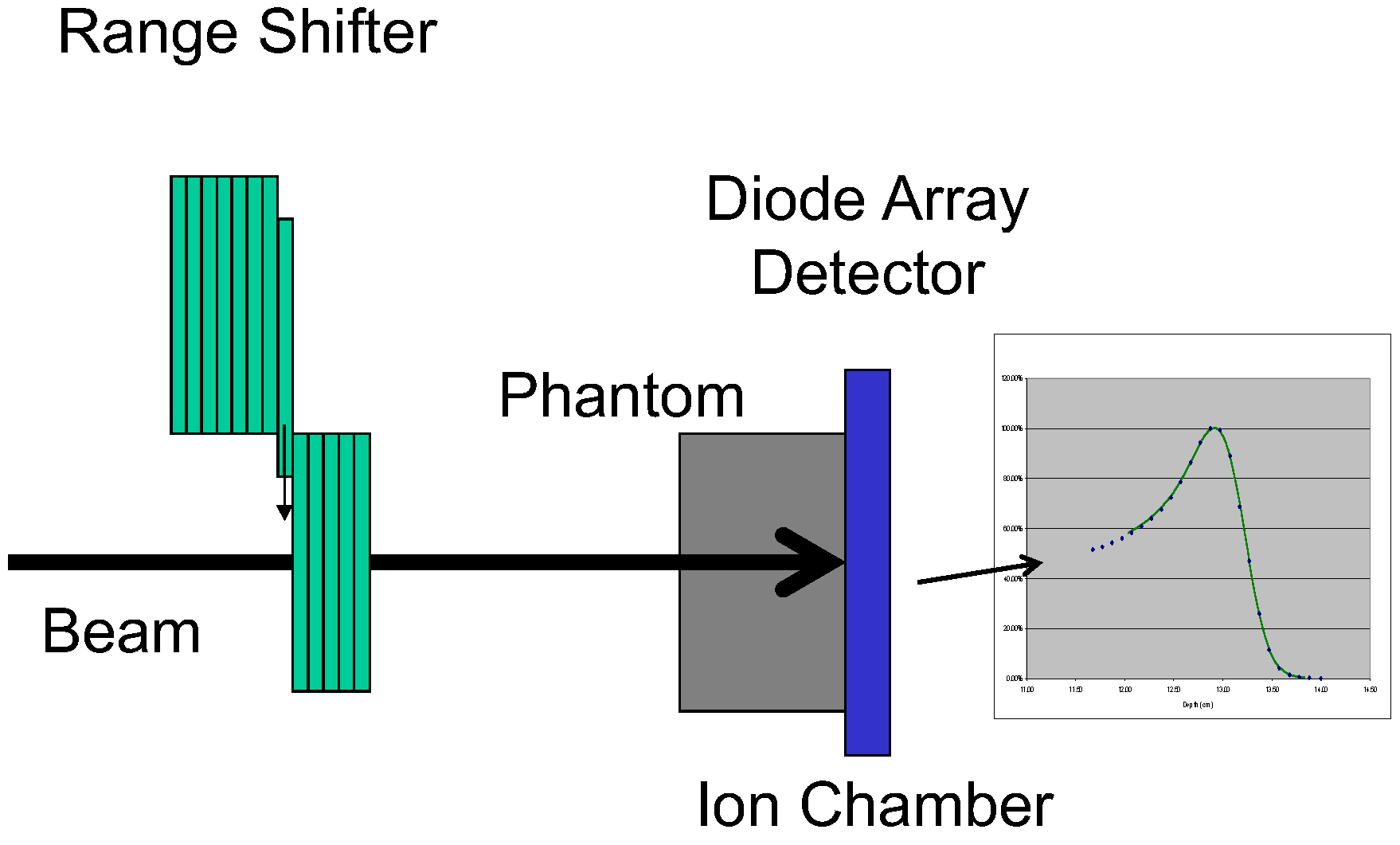
3. Results and Discussion
3.1. Results
| Energy (MeV) | Output Factor Ion Chamber | Output Factor MapCHECK | Diff (%) |
|---|---|---|---|
| 250 | 1.000 | 1.00 | - |
| 180 | 0.886 | 0.881 | −0.6 |
| 160 | 0.809 | 0.802 | −0.9 |
| Energy (MeV) | SOBP (cm) | Ion Chamber | MapCHECK | Diff (%) |
|---|---|---|---|---|
| 250 | 4.0 | 1.256 | 1.241 | −1.2 |
| 16.0 | 0.901 | 0.896 | −0.6 | |
| 180 | 4.0 | 1.336 | 1.338 | −0.2 |
| 12.0 | 0.940 | 0.940 | 0.0 | |
| 160 | 4.0 | 1.394 | 1.382 | −0.9 |
| 12.0 | 0.928 | 0.926 | −0.3 |
| Energy (MeV) | Rang Shift (cm) | Ion Chamber | MapCHECK | Diff (%) |
|---|---|---|---|---|
| 250 | 4.8 | 0.962 | 0.965 | 0.3 |
| 180 | 3.0 | 0.920 | 0.919 | −0.1 |
| 160 | 2.9 | 0.878 | 0.881 | 0.4 |
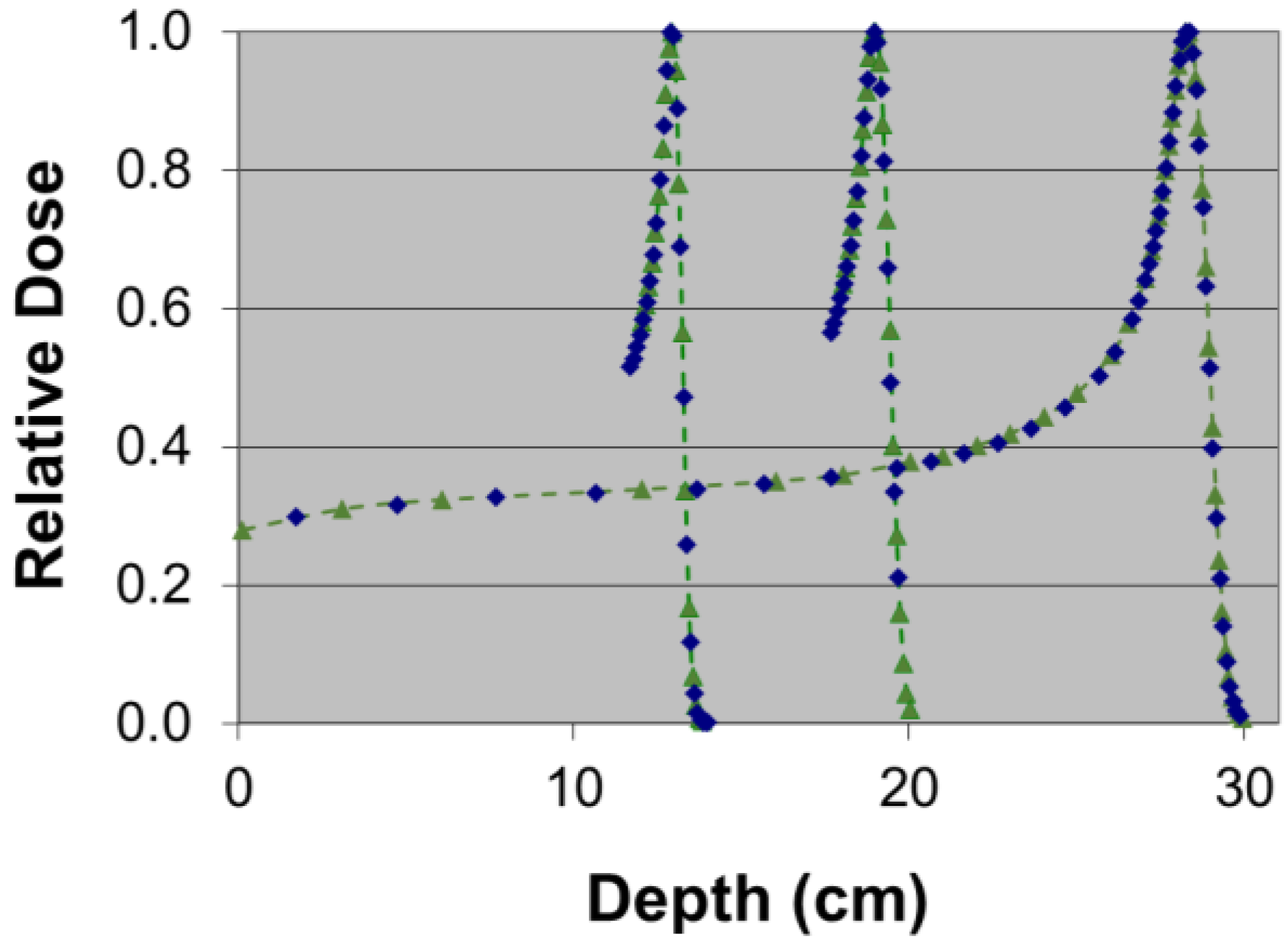

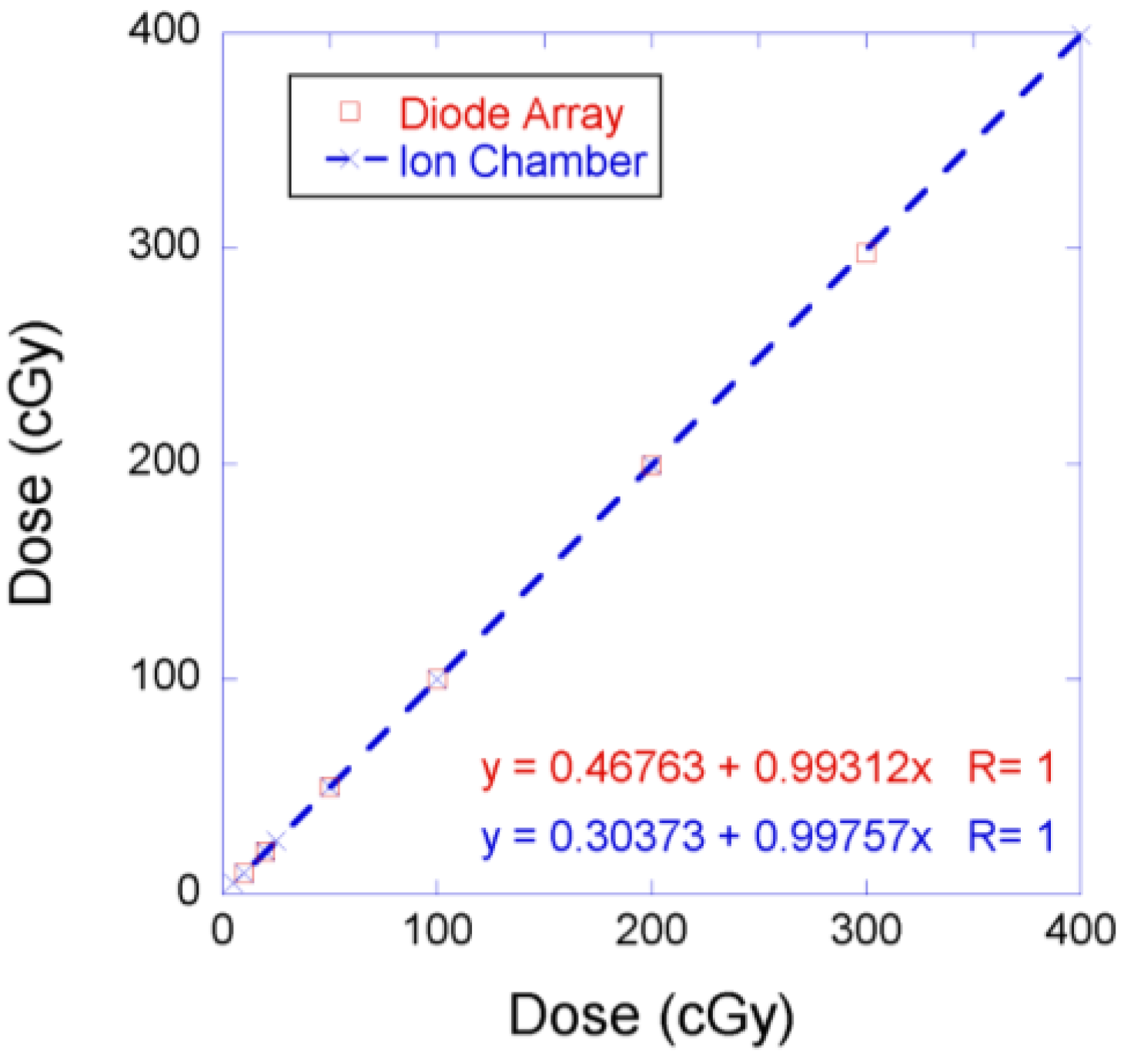

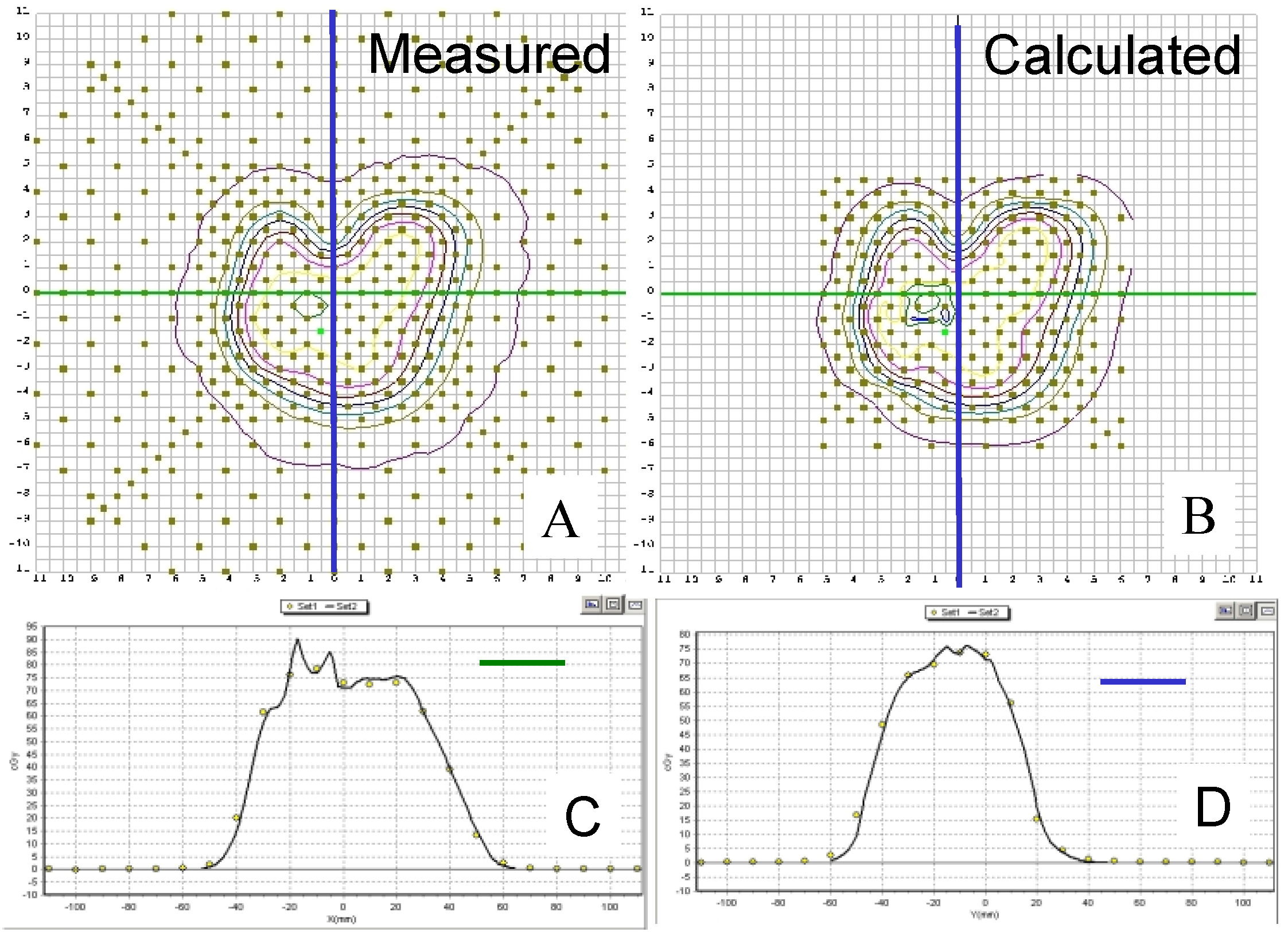
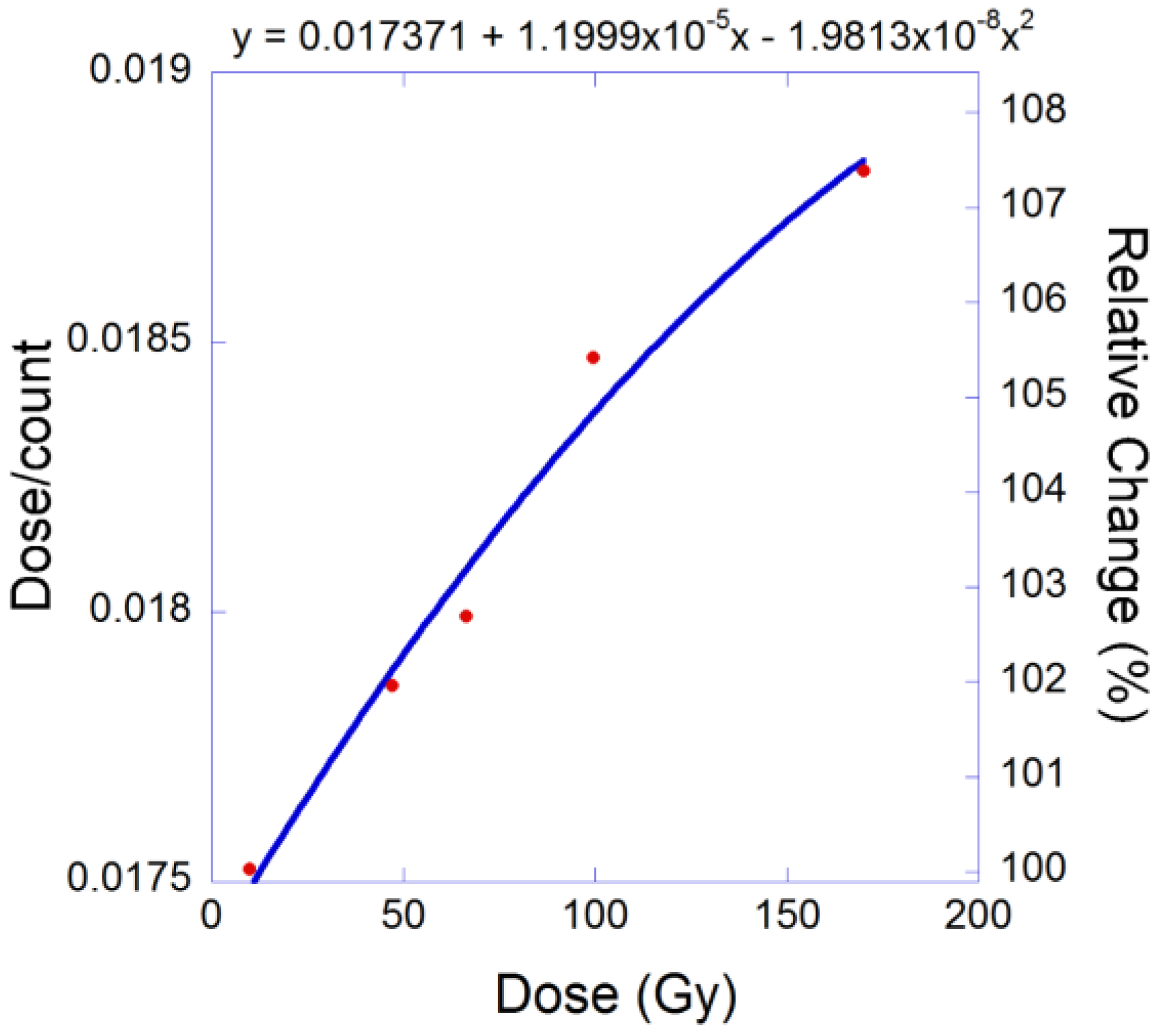
3.2. Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Delaney, T.F.; Kooy, H.M. Proton and Charged Particle Radiotherapy; Wolters Kluwer Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2008. [Google Scholar]
- Koehler, A.M.; Schneider, R.J.; Sisterson, J.M. Flattening of proton dose distributions for large-field radiotherapy. Med. Phys. 1977, 4, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Koehler, A.M.; Schneider, R.J.; Sisterson, J.M. Range modulations for protons and heavy ions. Nucl. Instrum. Methods 1975, 131, 437–440. [Google Scholar] [CrossRef]
- Urie, M.; Goitein, M.; Wagner, M. Compensating for heterogeneities in proton radiation therapy. Phys. Med. Biol. 1984, 29, 553–566. [Google Scholar] [CrossRef] [PubMed]
- Moyers, M.F.; Miller, D.W.; Bush, D.A.; Slater, J.D. Methodologies and tools for proton beam design for lung tumors. Int. J. Radiat. Oncol. Biol. Phys. 2001, 49, 1429–1438. [Google Scholar] [CrossRef]
- Grusell, E.; Medin, J. General characteristics of the use of silicon diode detectors for clinical dosimetry in proton beams. Phys. Med. Biol. 2000, 45, 2573–2582. [Google Scholar] [CrossRef] [PubMed]
- Newhauser, W.D.; Myers, K.D.; Rosenthal, S.J.; Smith, A.R. Proton beam dosimetry for radiosurgery: Implementation of the icru report 59 at the harvard cyclotron laboratory. Phys. Med. Biol. 2002, 47, 1369–1389. [Google Scholar] [CrossRef] [PubMed]
- Oozeer, R.; Mazal, A.; Rosenwald, J.C.; Belshi, R.; Nauraye, C.; Ferrand, R.; Biensan, S. A model for the lateral penumbra in water of a 200 mev proton beam devoted to clinical applications. Med. Phys. 1997, 24, 1599–1604. [Google Scholar] [CrossRef] [PubMed]
- Pacilio, M.; De Angelis, C.; Onori, S.; Azario, L.; Fidanzio, A.; Miceli, R.; Piermattei, A.; Kacperek, A. Characteristics of silicon and diamond detectors in a 60 mev proton beam. Phys. Med. Biol. 2002, 47. [Google Scholar] [CrossRef]
- Vatnitsky, S.M.; Miller, D.W.; Moyers, M.F.; Levy, R.P.; Schulte, R.W.; Slater, J.D.; Slater, J.M. Dosimetry techniques for narrow proton beam radiosurgery. Phys. Med. Biol. 1999, 44, 2789–2801. [Google Scholar] [CrossRef] [PubMed]
- ICRU. Clinical Proton Dosimetry Part 1: Beam Production, Beam Delivery and Measurements of Absorbed Dose Report 59; International Commission on Radiation Units and Measurements: Washington, DC, USA, 1998. [Google Scholar]
- Arjomandy, B.; Sahoo, N.; Ding, X.; Gillin, M. Use of a two-dimensional ionization chamber array for proton therapy beam quality assurance. Med. Phys. 2008, 35, 3889–3894. [Google Scholar] [CrossRef] [PubMed]
- Jursinic, P.A.; Nelms, B.E. A 2-D diode array and analysis software for verification of intensity modulated radiation therapy delivery. Med. Phys. 2003, 30, 870–879. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.; Gillin, M.; Bues, M.; Zhu, X.R.; Suzuki, K.; Mohan, R.; Woo, S.; Lee, A.; Komaki, R.; Cox, J.; et al. The MD Anderson proton therapy system. Med. Phys. 2009, 36, 4068–4083. [Google Scholar] [CrossRef] [PubMed]
- ICRU. Prescribing, Recording, and Reporting Proton-Beam Therapy Report 78; International Commission on Radiation Units and Measurements: Bethesda, MD, USA, 2007. [Google Scholar]
- Sahoo, N.; Zhu, X.R.; Arjomandy, B.; Ciangaru, G.; Lii, M.; Amos, R.; Wu, R.; Gillin, M.T. A procedure for calculation of monitor units for passively scattered proton radiotherapy beams. Med. Phys. 2008, 35, 5088–5097. [Google Scholar] [CrossRef] [PubMed]
- Low, D.A.; Harms, W.B.; Mutic, S.; Purdy, J.A. A technique for the quantitative evaluation of dose distributions. Med. Phys. 1998, 25, 656–661. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liengsawangwong, P.; Sahoo, N.; Ding, X.; Lii, M.; Gillin, M.T.; Zhu, X.R. Dosimetric Characteristics of a Two-Dimensional Diode Array Detector Irradiated with Passively Scattered Proton Beams. Cancers 2015, 7, 1425-1435. https://doi.org/10.3390/cancers7030844
Liengsawangwong P, Sahoo N, Ding X, Lii M, Gillin MT, Zhu XR. Dosimetric Characteristics of a Two-Dimensional Diode Array Detector Irradiated with Passively Scattered Proton Beams. Cancers. 2015; 7(3):1425-1435. https://doi.org/10.3390/cancers7030844
Chicago/Turabian StyleLiengsawangwong, Praimakorn, Nanayan Sahoo, Xiaoning Ding, MingFwu Lii, Michale T. Gillin, and Xiaorong Ronald Zhu. 2015. "Dosimetric Characteristics of a Two-Dimensional Diode Array Detector Irradiated with Passively Scattered Proton Beams" Cancers 7, no. 3: 1425-1435. https://doi.org/10.3390/cancers7030844






